Abstract
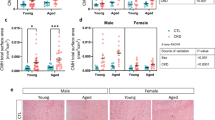
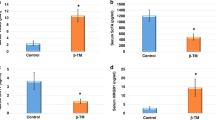
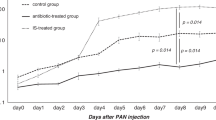
Renal injury is a common complication of hyperuricemia (HUA), which has been recognized as an independent risk factor for chronic kidney disease (CKD). The gut-kidney axis theory suggests that targeting the gut microbiota may be a potential treatment option for kidney disease. In this study, we utilized a spontaneous HUA rat model to demonstrate that Simiao decoction (SMD), a traditional Chinese medicine formula, can effectively alleviate HUA-induced renal injury by modulating gut microbiota and bacterial metabolism of tryptophan and tyrosine, thereby reducing gut-derived uremic toxins such as indoxyl sulfate (IS) and p-Cresol (PC). Fecal microbiota transplantation (FMT) further confirmed that the therapeutic effect of SMD was mediated by gut microbiota. Finally, in vitro studies revealed that IS promotes epithelial-mesenchymal transition (EMT) while PC induces cellular senescence in tubular cells. Collectively, our findings suggest that SMD can effectively alleviate HUA-induced renal injury through regulating gut dysbiosis and decreasing gut-derived uremic toxins. This study sheds light on a novel mechanism by which SMD exerts its effects on HUA-induced renal injury.

Similar content being viewed by others
Data availability
The shallow genome sequencing is available from the NCBI Sequence Read Archive (SRA) database (Bioproject: *PRJNA1135458*). The data for this study were available by contacting the corresponding author upon reasonable request.
References
Zhang, M. et al. Prevalence of hyperuricemia among chinese adults: findings from two nationally representative cross-sectional surveys in 2015-16 and 2018-19. Front. Immunol. 12, 791983 (2021).
Mortada, I. Hyperuricemia, type 2 diabetes mellitus, and hypertension: an emerging association. Curr. Hypertens. Rep. 19(9), 69 (2017).
Kuwabara, M. et al. Uric acid is a strong risk marker for developing hypertension from prehypertension: a 5-Year Japanese Cohort Study. Hypertension 71(1), 78–86 (2018).
Shi, Q. et al. Association between serum uric acid and cardiovascular disease risk factors in adolescents in America: 2001-2018. Plos ONE 16(8), e254590 (2021).
Srivastava, A., Kaze, A. D., Mcmullan, C. J., Isakova, T. & Waikar, S. S. Uric acid and the risks of kidney failure and death in individuals with CKD. Am. J. Kidney Dis. 71(3), 362–70 (2018).
Chou, Y. C. et al. Elevated uric acid level as a significant predictor of chronic kidney disease: a cohort study with repeated measurements. J. Nephrol. 28(4), 457–62 (2015).
El, R. R. & Tallima, H. Physiological functions and pathogenic potential of uric acid: a review. J. Adv. Res. 8(5), 487–93 (2017).
Jung, S. W., Kim, S. M., Kim, Y. G., Lee, S. H. & Moon, J. Y. Uric acid and inflammation in kidney disease. Am. J. Physiol. Ren. Physiol. 318(6), F1327–40 (2020).
Yang, T., Richards, E. M., Pepine, C. J. & Raizada, M. K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 14(7), 442–56 (2018).
Chen, Y. Y. et al. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J. Transl. Med. 17(1), 5 (2019).
Huang, Y. et al. The intestinal microbiota and metabolites in the gut-kidney-heart axis of chronic kidney disease. Front. Pharm. 13, 837500 (2022).
Wang, H. et al. Perturbed gut microbiome and fecal and serum metabolomes are associated with chronic kidney disease severity. Microbiome 11(1), 3 (2023).
Graboski, A.L. & Redinbo, M.R. Gut-derived protein-bound uremic toxins. Toxins. 12, 590 (2020).
Wei, J. et al. Association between gut microbiota and elevated serum urate in two independent cohorts. Arthritis Rheumatol. 74(4), 682–91 (2022).
Zhou, X. et al. Gut microbiota dysbiosis in hyperuricaemia promotes renal injury through the activation of NLRP3 inflammasome. Microbiome 12(1), 109 (2024).
Guo, X. L. et al. Amelioration effects of alpha-viniferin on hyperuricemia and hyperuricemia-induced kidney injury in mice. Phytomedicine 116, 154868 (2023).
Lu, M. et al. Fuling-Zexie formula attenuates hyperuricemia-induced nephropathy and inhibits JAK2/STAT3 signaling and NLRP3 inflammasome activation in mice. J. Ethnopharmacol. 319(Pt 2), 117262 (2024).
Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C. & Mollace, V. Regulation of uric acid metabolism and excretion. Int J. Cardiol. 213, 8–14 (2016).
Piani, F., Agnoletti, D. & Borghi, C. Advances in pharmacotherapies for hyperuricemia. Expert Opin. Pharmacother. 24(6), 737–45 (2023).
Chen, L. et al. The efficacy and mechanism of chinese herbal medicines in lowering serum uric acid levels: a systematic review. Front. Pharm. 11, 578318 (2020).
Peng, B. et al. Quercetin ameliorates hyperuricemic nephropathy through improving gut dysfunctions and decreasing gut bacteria-derived uremic toxins. Phytomedicine 143, 156801 (2025).
Lin, X. et al. Simiao decoction alleviates gouty arthritis by modulating proinflammatory cytokines and the gut ecosystem. Front. Pharm. 11, 955 (2020).
Hu, Q. H., Jiao, R. Q., Wang, X., Lv, Y. Z. & Kong, L. D. Simiao pill ameliorates urate underexcretion and renal dysfunction in hyperuricemic mice. J. Ethnopharmacol. 128(3), 685–92 (2010).
Zhang, Y. et al. Simiao San alleviates hyperuricemia and kidney inflammation by inhibiting NLRP3 inflammasome and JAK2/STAT3 signaling in hyperuricemia mice. J. Ethnopharmacol. 312, 116530 (2023).
Zeng, L. et al. Simiao pills alleviates renal injury associated with hyperuricemia: a multi-omics analysis. J. Ethnopharmacol. 333, 118492 (2024).
Gong, W. et al. Brahma-related gene-1 promotes tubular senescence and renal fibrosis through Wnt/beta-catenin/autophagy axis.Clin. Sci.135(15), 1873–95 (2021).
Luo, C. et al. Wnt9a promotes renal fibrosis by accelerating cellular senescence in tubular epithelial cells. J. Am. Soc. Nephrol. 29(4), 1238–56 (2018).
Wang, W. J., Chen, X. M. & Cai, G. Y. Cellular senescence and the senescence-associated secretory phenotype: potential therapeutic targets for renal fibrosis. Exp. Gerontol. 151, 111403 (2021).
Xu, J., Zhou, L. & Liu, Y. Cellular senescence in kidney fibrosis: pathologic significance and therapeutic strategies. Front. Pharm. 11, 601325 (2020).
Liu, X. et al. Fecal microbiota transplantation restores normal fecal composition and delays malignant development of mild chronic kidney disease in rats. Front. Microbiol. 13, 1037257 (2022).
Chang, H. W. et al. Prevotella copri and microbiota members mediate the beneficial effects of a therapeutic food for malnutrition. Nat. Microbiol. 9(4), 922–37 (2024).
Kleerebezem, M. et al. Lifestyle, metabolism and environmental adaptation in Lactococcus lactis. Fems Microbiol. Rev. 44(6), 804–20 (2020).
Li, G. & Young, K. D. Indole production by the tryptophanase TnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 159(Pt 2), 402–10 (2013).
Pan, L. et al. Berberine ameliorates chronic kidney disease through inhibiting the production of gut-derived uremic toxins in the gut microbiota. Acta Pharm. Sin. B 13(4), 1537–53 (2023).
Xu, Y. X. et al. Alistipes indistinctus-derived hippuric acid promotes intestinal urate excretion to alleviate hyperuricemia. Cell Host Microbe 32(3), 366–81 (2024).
Zou, Z. P., Li, J. L., Zhang, Y. F., Zhou, Y. & Ye, B. C. Empowering probiotics with high xanthine transport for effective hyperuricemia management. Gut Microbes 16(1), 2399213 (2024).
Lim, Y.J., Sidor, N.A., Tonial, N.C., Che, A. & Urquhart, B.L. Uremic toxins in the progression of chronic kidney disease and cardiovascular disease: mechanisms and therapeutic targets. Toxins 13, 142 (2021).
Meijers, B. K. et al. p-Cresol and cardiovascular risk in mild-to-moderate kidney disease. Clin. J. Am. Soc. Nephrol. 5(7), 1182–9 (2010).
Niwa, T. & Shimizu, H. Indoxyl sulfate induces nephrovascular senescence. J. Ren. Nutr. 22(1), 102–6 (2012).
Huang, Y. et al. Indoxyl sulfate induces intestinal barrier injury through the IRF1-DRP1 axis-mediated mitophagy impairment. Theranostics 10(16), 7384–400 (2020).
Gryp, T., Vanholder, R., Vaneechoutte, M. & Glorieux, G. p-Cresyl Sulfate. Toxins. 9, 52 (2017).
Poveda, J. et al. p-cresyl sulphate has pro-inflammatory and cytotoxic actions on human proximal tubular epithelial cells. Nephrol. Dial. Transpl. 29(1), 56–64 (2014).
Zhou, X. et al. Ginger extract decreases susceptibility to dextran sulfate sodium-induced colitis in mice following early antibiotic exposure. Front. Med. 8, 755969 (2021).
Acknowledgements
This work was supported by the Joint Funds of National Natural Science Foundation of China [grant number U22A20365], National Natural Science Foundation of China [grant number 82274499, T2341019, 82405279], the Key Project of National Natural Science Foundation of China [grant number 81830117], the Guangzhou Science and Technology Plan Project [grant number 2024B03J1343], the Major scientific and technological project of Guangzhou Municipal Health Commission [grant number 20252D003], Guangdong Basic and Applied Basic Research Foundation, China [grant number 2023A1515110757], Dongguan social development technology program (High level hospital constructon project), China [grant number 20231800913372].
Author information
Authors and Affiliations
Contributions
Xiaoshan Zhao and Yanyan Liu designed the study and supervised the project; Xinghong Zhou, Xiaoyu Liu, Baizhao Peng and Ying Yang completed the animal experiments, analyzed data and wrote the orignial manuscript; Hanqi Lu, Dexian Li, Yijian Deng and Zihao Jiang carried out metagenomic and metabolomic analysis. Chuanghai Wu, Wen Fang and Yanting You completed the in vitro experiments and histological analysis. Hiu Yee Kwan provided technical assistance and revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, X., Liu, X., Peng, B. et al. Simiao Decoction alleviates hyperuricemia-induced renal injury through regulating gut dysbiosis and decreasing gut-derived uremic toxins. npj Biofilms Microbiomes (2026). https://doi.org/10.1038/s41522-026-00923-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-026-00923-x