Abstract
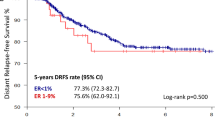
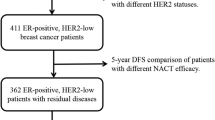
This study aimed to investigate the clinicopathological characteristics, response to chemotherapy, and clinical outcomes according to estrogen receptor (ER) expression levels in breast cancer (BC) patients treated with neoadjuvant chemotherapy (NAC). ER expression levels were categorized as ER-negative (expression in <1% of tumor cells), ER-low positive (1-10%), ER-intermediate positive (11-50%), and ER- high positive (>50%). Of the 1,365 cases, 647 (47.4%) were classified as ER-negative, 49 (3.6%) as ER- low positive, 48 (3.5%) as ER-intermediate positive, and 621 (45.5%) as ER-high positive BCs in pre-NAC biopsies. ER-intermediate positive tumors as well as ER-low positive tumors showed no differences in clinicopathological characteristics compared to ER-negative tumors with the exception of progesterone receptor positivity. While ER-low positive and ER-negative tumors showed similar chemo-responsiveness, ER-intermediate positive tumors were less responsive to NAC compared to ER-negative tumors. In patients with residual disease, pre- and post-NAC ER expression levels were found to be independent prognostic factors, but with no significant differences among ER-negative, ER-low positive, and ER-intermediate positive tumors. Our study indicates that ER-low positive BCs are similar to ER-negative BCs and that ER-intermediate positive BCs exhibit characteristics heterogeneous between ER-negative and ER-high positive BCs in terms of clinicopathological characteristics, chemo-responsiveness, and clinical outcomes.
Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy concerns and institutional policy, but are available from the corresponding author on reasonable request.
References
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Sørlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 98, 10869–10874 (2001).
Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Korde, L. A. et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J. Clin. Oncol. 39, 1485–1505 (2021).
Allison, K. H. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J. Clin. Oncol. 38, 1346–1366 (2020).
Hwang, K. T. et al. Impact of breast cancer subtypes on prognosis of women with operable invasive breast cancer: a population-based study using SEER database. Clin. Cancer Res. 25, 1970–1979 (2019).
Łukasiewicz, S. et al. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers 13, 4287 (2021).
Faneyte, I. F. et al. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br. J. Cancer 88, 406–412 (2003).
Berry, D. A. et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA 295, 1658–1667 (2006).
Harvey, J. M., Clark, G. M., Osborne, C. K. & Allred, D. C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 17, 1474–1481 (1999).
Hammond, M. E. et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 28, 2784–2795 (2010).
Raghav, K. P. et al. Impact of low estrogen/progesterone receptor expression on survival outcomes in breast cancers previously classified as triple negative breast cancers. Cancer 118, 1498–1506 (2012).
Chen, T. et al. Borderline ER-positive primary breast cancer gains no significant survival benefit from endocrine therapy: a systematic review and meta-analysis. Clin. Breast Cancer 18, 1–8 (2018).
Iwamoto, T. et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J. Clin. Oncol. 30, 729–734 (2012).
Bari, S. et al. A real-world data retrospective cohort study of low estrogen receptor-positive early breast cancer: natural history and treatment outcomes. Breast Cancer 14, 199–210 (2022).
Voorwerk, L. et al. Immune landscape of breast tumors with low and intermediate estrogen receptor expression. NPJ Breast Cancer 9, 39 (2023).
Massa, D. et al. Immune and gene-expression profiling in estrogen receptor low and negative early breast cancer. J. Natl. Cancer Inst. 116, 1914–1927 (2024).
Ohara, A. M. et al. PAM50 for prediction of response to neoadjuvant chemotherapy for ER-positive breast cancer. Breast Cancer Res. Treat. 173, 533–543 (2019).
Paakkola, N. M., Karakatsanis, A., Mauri, D., Foukakis, T. & Valachis, A. The prognostic and predictive impact of low estrogen receptor expression in early breast cancer: a systematic review and meta-analysis. ESMO Open 6, 100289 (2021).
Reinert, T. et al. Clinical implication of low estrogen receptor (ER-low) expression in breast cancer. Front. Endocrinol. 13, 1015388 (2022).
Osako, T., Nishimura, R., Okumura, Y., Toyozumi, Y. & Arima, N. Predictive significance of the proportion of ER-positive or PgR-positive tumor cells in response to neoadjuvant chemotherapy for operable HER2-negative breast cancer. Exp. Ther. Med. 3, 66–71 (2012).
Raphael, J., Gandhi, S., Li, N., Lu, F. I. & Trudeau, M. The role of quantitative estrogen receptor status in predicting tumor response at surgery in breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 164, 285–294 (2017).
Yi, M. et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann. Oncol. 25, 1004–1011 (2014).
Zhang, Z. et al. Pathological features and clinical outcomes of breast cancer according to levels of oestrogen receptor expression. Histopathology 65, 508–516 (2014).
Choi, J. E. et al. Breast cancer statistics in Korea, 2019. J. Breast Cancer 26, 207–220 (2023).
Kim, J. et al. Survival outcomes of young-age female patients with early breast cancer: an international multicenter cohort study. ESMO Open 9, 103732 (2024).
Fujii, T. et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann. Oncol. 28, 2420–2428 (2017).
Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365, 1273–1283 (2011).
Gradishar, W. J. et al. Breast cancer, version 3.2024, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 22, 331–357 (2024).
Chen, H. L., Huang, F. B., Chen, Q. & Deng, Y. C. Impact of estrogen receptor expression level on response to neoadjuvant chemotherapy and prognosis in HER2-negative breast cancers. BMC Cancer 23, 841 (2023).
Dieci, M. V. et al. Quantitative expression of estrogen receptor on relapse biopsy for ER-positive breast cancer: prognostic impact. Anticancer Res. 34, 3657–3662 (2014).
Choong, G. M., Hoskin, T. L., Boughey, J. C., Ingle, J. N. & Goetz, M. P. Endocrine therapy omission in estrogen receptor-low (1%-10%) early-stage breast cancer. J. Clin. Oncol. 43, 1875–1885 (2025).
Taparra, K. et al. Disaggregation of Asian American and Pacific Islander Women With Stage 0-II breast cancer unmasks disparities in survival and surgery-to-radiation intervals: a national cancer database analysis from 2004 to 2017. JCO Oncol. Pr. 18, e1255–e1264 (2022).
Landmann, A. et al. Low estrogen receptor (ER)-positive breast cancer and neoadjuvant systemic chemotherapy: is response similar to typical ER-positive or ER-negative disease?. Am. J. Clin. Pathol. 150, 34–42 (2018).
Moldoveanu, D. et al. Clinical behavior, management, and treatment response of estrogen receptor low (1-10%) breast cancer. Ann. Surg. Oncol. 30, 6475–6483 (2023).
Symmans, W. F. et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J. Clin. Oncol. 25, 4414–4422 (2007).
Ogston, K. N. et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast 12, 320–327 (2003).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804 (2012).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36, 2105–2122 (2018).
Goldhirsch, A. et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann. Oncol. 24, 2206–2223 (2013).
Acknowledgements
This study was funded by National Research Foundation of Korea (NRF)’s Basic Science Research Program to Park SY by the Ministry of Science and ICT (Grant No. 2022R1F1A1065468). The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
K.B. participated in the interpretation and analysis of data and drafted the manuscript. H.J.S. and Y.R.C. participated in the acquisition and interpretation of pathological data. H.C.S., E.K.K., K.J.S., S.H.K., and J.H.K. participated in the acquisition of clinical data. H.J.K. and S.Y.P. conceived of the study, participated in its design and interpretation, and were responsible for the preparation of the manuscript. All authors read and approved of the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bai, K., Sung, HJ., Chung, Y.R. et al. Impact of estrogen receptor expression levels on chemo-responsiveness and prognosis of breast cancer patients treated with neoadjuvant chemotherapy. npj Breast Cancer (2026). https://doi.org/10.1038/s41523-026-00907-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-026-00907-2