Abstract
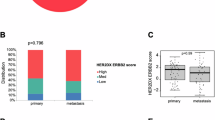
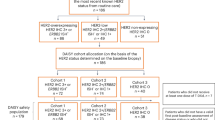
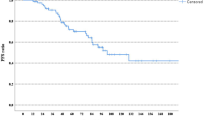
Trastuzumab, pertuzumab, and a taxane (THP) has been the standard first-line therapy for HER2+ advanced breast cancer for over a decade. With new regimens emerging, genomic tools like HER2DX may help identify patients who benefit durably from THP versus those requiring intensification. Here, baseline tumor tissue from 122 patients with HER2+ treated with THP in Poland was tested with HER2DX. A previously published Spanish real-world cohort (n = 93) was added to generate a combined cohort (n = 215). Univariable analyses were performed in the Polish cohort, and multivariable Cox and logistic regression models were applied to the combined cohort. A HER2DX metastatic prognostic score was trained on overall survival (OS) in the Spanish cohort and validated in the Polish cohort. In the Polish cohort, high ERBB2 mRNA scores were associated with significantly longer real-world progression-free survival (rwPFS) (33.8 vs. 17.9 months; hazard ratio [HR] 0.57; p = 0.022) and real-world overall survival (rwOS) (75.1 vs. 40.2; HR 0.48; p = 0.009). In the combined cohort, ERBB2 high-score tumors showed prolonged rwPFS (33.8 vs. 12.5; HR 0.50; p < 0.001) and rwOS (not reached vs. 37.1; HR 0.36; p < 0.001), and higher rwORR (84.4% vs. 52.0%; p < 0.001). Prognostic value was independent of clinical variables, including number of metastatic sites. Subgroup analyses showed particularly favorable outcomes in patients with <3 sites (median rwPFS 51.7 vs. 20.3 months). The HER2DX metastatic prognostic score outperformed ERBB2 alone in the validation cohort. In conclusion, the HER2DX ERBB2 mRNA score provides independent prognostic information in HER2+ advanced breast cancer treated with THP. The HER2DX metastatic prognostic score further improves prognostic accuracy.
Similar content being viewed by others
Data availability
The datasets analyzed during the current study are not publicly available as participants of this study did not agree for their data to be shared publicly. However, data can be made available from the corresponding author on reasonable request under a data transfer agreement and upon Ethics Committee approval.
References
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 21, 519–530 (2020).
Miles, D. et al. Final results from the PERUSE study of first-line pertuzumab plus trastuzumab plus a taxane for HER2-positive locally recurrent or metastatic breast cancer, with a multivariable approach to guide prognostication. Ann. Oncol. 32, 1245–1255 (2021).
Swain, S. M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov. 22, 101–126 (2022).
Tolaney, S. M. et al. Trastuzumab Deruxtecan plus Pertuzumab for HER2-Positive Metastatic Breast Cancer. New England J. Med. (2025).
Metzger, O. et al. AFT-38 PATINA: A randomized, open label, phase III trial to evaluate the efficacy and safety of palbociclib + anti-HER2 therapy + endocrine therapy vs anti-HER2 therapy + endocrine therapy after induction treatment for hormone receptor-positive/HER2-positive metastatic breast cancer. In: 2024 San Antonio Breast Cancer Symposium. Abstract P2-03-20 (SESS-18111). Presented December 10, 2024.; 2024.
Ding, N. et al. Prognostic value of baseline neutrophil/lymphocyte ratio in HER2-positive metastatic breast cancer: exploratory analysis of data from the CLEOPATRA trial. Breast Cancer Res. 26, (2024).
Arciero, C. A. et al. ER + /HER2+ breast cancer has different metastatic patterns and better survival than ER − /HER2+ breast cancer. Clin. Breast Cancer 19, 236–245 (2019).
Prat, A. et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 75, 103801 (2022).
Tolaney, S. M. et al. HER2DX genomic test in early-stage HER2-positive breast cancer. ESMO Open 9, 103987 (2024).
Villacampa, G. et al. HER2DX and survival outcomes in early-stage HER2-positive breast cancer: an individual patient-level meta-analysis. Lancet Oncol. 26, 1100–1112 (2025).
Villacampa, G. et al. Association of HER2DX with pathological complete response and survival outcomes in HER2-positive breast cancer. Ann. Oncol. 34, 783–795 (2023).
Bueno-Muiño, C. et al. Assessment of a genomic assay in patients with ERBB2-positive breast cancer following neoadjuvant trastuzumab-based chemotherapy with or without pertuzumab. JAMA Oncol. 9, 841–846 (2023).
Llombart-Cussac, A. et al. HER2DX genomic assay in HER2-positive early breast cancer treated with trastuzumab and pertuzumab: a correlative analysis from the PHERGain phase II trial. Clin. Cancer Res. 30, OF1–OF8 (2024).
Marín-Aguilera, M. et al. Analytical validation of HER2DX genomic test for early-stage HER2-positive breast cancer. ESMO Open 9, 102903 (2024).
Martínez-Sáez, O. et al. Clinical decision impact of HER2DX, an algorithm-powered genomic diagnostic in early-stage HER2-positive breast cancer: results from a prospective real-world study. ESMO Real. World Data Digital Oncol. 8, 100123 (2025).
Tarantino, P. et al. Adjuvant TRastuzumab Emtansine Versus Paclitaxel plus Trastuzumab for Stage I human epidermal growth factor receptor 2-positive breast cancer: 5-year results and correlative analyses from ATEMPT. J Clin Oncol. 42, (2024).
Tolaney, S. M. et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 24, 273–285 (2023).
Villacampa, G. et al. Prognostic value of HER2DX in early-stage HER2-positive breast cancer: a comprehensive analysis of 757 patients in the Sweden Cancerome Analysis Network—Breast dataset (SCAN-B). ESMO Open 9, 102388 (2024).
Waks, A. G. et al. Assessment of the HER2DX Assay in Patients With ERBB2-positive breast cancer treated with neoadjuvant paclitaxel, trastuzumab, and pertuzumab. JAMA Oncol. 9, 835–840 (2023).
Sánchez-Bayona, R. et al. HER2DX ERBB2 mRNA score in first-line advanced HER2-positive breast cancer treated with chemotherapy, trastuzumab, and pertuzumab. npj Breast Cancer 11, 37 (2025).
Cortés, J. et al. Abstract PS4-06: HER2DX ERBB2 mRNA score in first-line metastatic HER2-positive breast cancer treated with docetaxel, trastuzumab and pertuzumab: correlative analysis from CLEOPATRA phase III trial. Clin. Cancer Res. 31, PS4–PS06 (2025).
zhang, M. et al. Patterns and prognostic implications of distant metastasis in breast Cancer based on SEER population data. Sci. Rep. 15, 26717 (2025).
Taskindoust, M. et al. Survival outcomes among patients with metastatic breast cancer: review of 47,000 patients. Ann. Surg. Oncol. 28, 7441 (2021).
Baselga, J. et al. Biomarker Analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol. 32, 3753–3761 (2014).
Brasó-Maristany, F. et al. HER2DX ERBB2 mRNA expression in advanced HER2-positive breast cancer treated with T-DM1. JNCI: J. National Cancer Institute. (2022).
Acknowledgements
The study was funded by Reveal Genomics, and it was designed and performed by investigators from Hospital Universitario 12 de Octubre (Madrid, Spain), Clínic Barcelona Comprehensive Cancer Center (Barcelona, Spain), Maria Sklodowska-Curie National Research Institute of Oncology (Gliwice, Poland), and Reveal Genomics. All authors had full access to the data and final responsibility for submission. FBM received funding from Fundación científica AECC Ayudas Investigador AECC 2021 (INVES21943BRAS). AP received funding from the Breast Cancer Research Foundation (BCRF-22-198, BCRF-23-198 and BCRF-24-198), Beca Marta Santamaría, Fundación CRIS contra el Cancer PR_EX_2021-14, Agència de Gestó d’Ajuts Universitaris I de Recerca 2021 SGR 01156, Fundación Fero BECA ONCOXXI21, Asociación Cáncer de Mama Metastásico IV Premios M. Chiara Giorgetti, PI22/01017: funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union and Fundación Científica Asociación Española Contra el Cáncer which is the entity responsible for the financial support of the Excellence Program, EPAEC246711CLIN. MBS received funding from Fundación BBVA Joan Rodés-Josep Baselga 2024-2027.
Author information
Authors and Affiliations
Contributions
M.K., A.P. and F.B-M. designed the study. M.K., S.C., R.S-B., B.P, J.S., E.Ch., E.S., M.R., F.P., A.A., O.C., A.L., M.O-W., E.Ca., B.A., M.V., M.B., J.M., G.V., L.P., P.V., E.Ci., M.J., A.P. and F.B-M. contributed to data collection and assembly, wrote and reviewed the article, and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
Potential conflicts of interest are the following: M.K. reports advisory board for Novartis; speaker’s honoraria from Novartis, Roche, MSD, Pfizer, Lilly, Teva, Amgen, Swixx Biopharma, Gilead, and AstraZeneca; clinical trials for Roche, MSD, Novartis, Seagen, and Gilead; conference fees for Pfizer, Roche, Novartis, Teva, Amgen, Gilead, MSD, Swixx Biopharma, and AstraZeneca; all outside the submitted work. M.J. reports conference fees for Gilead, Roche; clinical trials for Roche, MSD, Novartis, Seagen, and Gilead; speaker’s honoraria from Novartis, Roche, Lilly, Pfizer, Teva, Exact Sciences, Mammotome, and Gilead; advisory boards for Novartis and Pfizer; all outside the submitted work. A.P. reports advisory and consulting fees from AstraZeneca, Roche, Pfizer, Novartis, Daiichi Sankyo, Ona Therapeutics, and Peptomyc, lecture fees from AstraZeneca, Roche, Novartis, and Daiichi Sankyo, institutional financial interests from AstraZeneca, Novartis, Roche, and Daiichi Sankyo; stockholder and employee of Reveal Genomics; patents filed PCT/EP2016/080056, PCT/EP2022/086493, PCT/EP2023/060810, PCT/EP2024/068197 and EP23383369; editor of NPJ Breast Cancer journal. F.B-M. reports part-time employment from Reveal Genomics and has patents filed: PCT/EP2022/086493, PCT/EP2023/060810,PCT/EP2024/068197, and EP23383369B. MBS declares Advisor o consulting fees from Pfizer, Novartis, Lilly and Astra Zeneca and travel expenses from Pfizer, Lilly, Novartis, Astra Zeneca and Gilead. R.S.-B. reports advisory/consulting/speaker fees from Roche, AstraZeneca, Novartis, Lilly, Daiichi Sankyo, Pfizer, Eisai, GlaxoSmithKline, Reveal Genomics, and Gilead; travel expenses from Pfizer, AstraZeneca, Gilead, Novartis, and Roche. E.S. reports Advisory Board and speaker honoraria of Sysmex and Astra Zenica; and consultant of Reveal Genomics INC. A.L. reports conference fees: Accord, Novartis, clinical studies: Roche, MSD, Novartis; speaker’s honorarium: Novartis; all outside the submitted work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kubeczko, M., Cobo, S., Sanchez-Bayona, R. et al. Validation of the HER2DX genomic test in first-line advanced HER2-positive breast cancer treated with trastuzumab, pertuzumab, and taxane. npj Breast Cancer (2026). https://doi.org/10.1038/s41523-026-00909-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-026-00909-0