Abstract
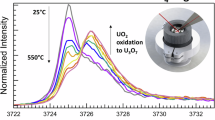
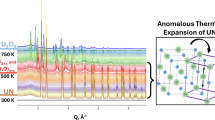
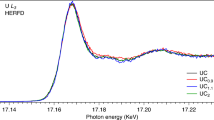
Uranium monocarbide (UC) exhibits physiochemical characteristics well-suited for nuclear fuel applications in Generation IV reactors, but its high susceptibility to oxidation remains a major barrier to deployment. A detailed understanding of the U-C-O system, including UC thermal oxidation, crystal chemistry, and thermodynamic/kinetic properties, is essential to predict its behavior under normal and off-normal reactor conditions. In this work, in situ high temperature synchrotron X-ray diffraction was conducted under sealed and open-air conditions to characterize UC thermal expansion and oxidation behaviors. From the sealed experiment, the mean coefficient of thermal expansion of UC was determined to be 9.8 × 10−6 K−1 from room temperature to 970 K. Open-air experiments conducted from room temperature to 773 K revealed the oxidation sequence UC → UO2 → U3O8. Notably, a tetragonal U(C1-xOx)2 phase, absent from current thermodynamic predictions, was observed at 840 K, lower than previously considered, suggesting potential relevance for advanced reactor fuel applications. These findings reveal ambiguities in existing knowledge of the U-C-O system, emphasizing the need for continued investigation to facilitate the use of UC-based TRISO and other carbide fuels in emerging reactor designs.
Similar content being viewed by others
Data availability
Data for this article, including 2D diffraction data are available at GitHub, https://github.com/GuoGroupWSU/UC-raw-data.
References
Meyer, M. K., Fielding, R. & Gan, J. Fuel development for gas-cooled fast reactors. J. Nucl. Mater. 371, 281–287 (2007).
Vasudevamurthy, G. & Nelson, A. T. Uranium carbide properties for advanced fuel modeling – A review. J. Nucl. Mater. 558, 153145 (2022).
Szpunar, B. & Szpunar, J. A. Thermal conductivity of uranium nitride and carbide. Int. J. Nucl. Energy 2014, 178360 (2014).
Zhou, W. & Zhou, W. Enhanced thermal conductivity accident tolerant fuels for improved reactor safety – A comprehensive review. Ann. Nucl. Energy 119, 66–86 (2018).
Hosemann, P. et al. Mechanical characteristics of SiC coating layer in TRISO fuel particles. J. Nucl. Mater. 442, 133–142 (2013).
Yun, Y. State-of-the-Art Report on Multi-scale Modelling of Nuclear Fuels. Thermal Expansion 179-186 https://inis.iaea.org/records/v6vp8-adm90 (2015).
TRISO-Coated Particle Fuel Phenomenon Identification and Ranking Tables (PIRTs) for Fission Product. NRC Web https://www.nrc.gov/reading-rm/doc-collections/nuregs/contract/cr6844/v1/index.html.
Demkowicz, P. A., Liu, B. & Hunn, J. D. Coated particle fuel: Historical perspectives and current progress. J. Nucl. Mater. 515, 434–450 (2019).
Matteo, E. et al. Advanced Reactors Spent Fuel and Waste Streams Disposition Strategies. https://doi.org/10.2172/2335764(2023).
Berthinier, C. et al. Experimental study of uranium carbide pyrophoricity. Powder Technol. 208, 312–317 (2011).
Mendez-Peñalosa, R. Thermal expansion of uranium monocarbide. Atomic Int. https://doi.org/10.2172/4629984 (1963).
Méndez-Peñalosa, R. & Taylor, R. E. Thermal expansion of uranium monocarbide. J. Am. Ceram. Soc. 47, 101–102 (1964).
Richards, H. K. Thermal expansion of uranium and tantalum monocarbides up to 2700°C. Nucl. Technol. https://doi.org/10.13182/NT71-A30947 (1971).
Krikorian, O. H. Thermal expansion of high temperature materials. UCRL--6132, 4137098 http://www.osti.gov/servlets/purl/4137098/, https://doi.org/10.2172/4137098 (1960).
Meerson, G. A., Kotel’nikov, R. B. & Bashlykov, S. N. Uranium monocarbide. Sov. J. Energy 9, 927–931 (1961).
Le Guyadec, F. et al. Thermodynamic and experimental study of UC powders ignition. J. Nucl. Mater. 393, 333–342 (2009).
Gasparrini, C. et al. Oxidation of UC: an in situ high temperature environmental scanning electron microscopy study. J. Nucl. Mater. 494, 127–137 (2017).
Gasparrini, C. et al. Uranium carbide oxidation from 873 K to 1173. K. Corros. Sci. 151, 44–56 (2019).
Dell, R. M., Wheeler, V. J. & McIver, E. J. Oxidation of uranium mononitride and uranium monocarbide. Trans. Faraday Soc. 62, 3591–3606 (1966).
Berthinier, C. et al. Experimental kinetic study of oxidation of uranium monocarbide powders under controlled oxygen partial pressures below 230. C. J. Nucl. Mater. 432, 505–519 (2013).
Goncharov, V. G. et al. Energetics of oxidation and formation of uranium monocarbide. J. Nucl. Mater. 581, 154446 (2023).
Goncharov, V. G. et al. Energetics of oxidation and formation of uranium mononitride. J. Nucl. Mater. 569, 153904 (2022).
Van Tets, A. Reaction of uranium monocarbide powder in oxidizing atmospheres. Thermochim. Acta 6, 195–203 (1973).
Guéneau, C. et al. A thermodynamic approach for advanced fuels of gas-cooled reactors. J. Nucl. Mater. 344, 191–197 (2005).
Chevalier, P. Y. & Fischer, E. Thermodynamic modelling of the C–U and B–U binary systems. J. Nucl. Mater. 288, 100–129 (2001).
Guéneau, C. et al. Thermodynamic modelling of advanced oxide and carbide nuclear fuels: description of the U–Pu–O–C systems. J. Nucl. Mater. 419, 145–167 (2011).
Heiss, A. Etude thermodynamique du systeme U-O-C par mesure des pressions d’oxyde de carbone a l’equilibre. J. Nucl. Mater. 55, 207–223 (1975).
Henry, J. L., Paulson, D. L., Blickensderfer, H. J. & Kelly, H. J. Phase Relations in the Uranium Monocarbide Region of the System Uranium-Carbon-Oxygen at 1,7000 C. (U.S. Department of the Interior, Bureau of Mines, Washington, D.C., 1967).
Stoops, R. F. & Hamme, J. V. Phase relations in the system uranium—Carbon—Oxygen. J. Am. Ceram. Soc. 47, 59–62 (1964).
Reiche, H. M., Vogel, S. C. & Tang, M. In situ synthesis and characterization of uranium carbide using high temperature neutron diffraction. J. Nucl. Mater. 471, 308–316 (2016).
Gronvold, F. High-temperature X-ray study of uranium oxides in the UO2-U3O8 region. J. Inorg. Nucl. Chem. 1, 357–370 (1955).
Leinders, G., Cardinaels, T., Binnemans, K. & Verwerft, M. Accurate lattice parameter measurements of stoichiometric uranium dioxide. J. Nucl. Mater. 459, 135–142 (2015).
Bruneval, F., Freyss, M. & Crocombette, J.-P. Lattice constant in nonstoichiometric uranium dioxide from first principles. Phys. Rev. Mater. 2, 023801 (2018).
De Bona, E. et al. Oxidation of micro- and nanograined UO2 pellets by in situ synchrotron X-ray diffraction. Inorg. Chem. 61, 1843–1850 (2022).
Teixeira, S. R. & Imakuma, K. High temperature X-ray diffraction study of the U4O9 formation on UO2 sintered plates. J. Nucl. Mater. 178, 33–39 (1991).
Crane, J., Kalish, H. S. & Litton, F. B. The development o. uranium carbide as a nuclear fuel. Third Annual Report, September 1, 1961 to October 31, 1962. UNC-5048, 4721743 http://www.osti.gov/servlets/purl/4721743-tY9hb6/, https://doi.org/10.2172/4721743(1963).
Freyss, M. First-principles study of uranium carbide: accommodation of point defects and of helium, xenon, and oxygen impurities. Phys. Rev. B 81, 014101 (2010).
Quémard, L. et al. On the origin of the sigmoid shape in the UO2 oxidation weight gain curves. J. Eur. Ceram. Soc. 29, 2791–2798 (2009).
McEachern, R. J. & Taylor, P. A review of the oxidation of uranium dioxide at temperatures below 400. C. J. Nucl. Mater. 254, 87–121 (1998).
Austin, A. E. Carbon positions in uranium carbides. Acta Crystallogr 12, 159–161 (1959).
Shi, H., Zhang, P., Li, S.-S., Wang, B. & Sun, B. First-principles study of UC2 and U2C3. J. Nucl. Mater. 396, 218–222 (2010).
Belbeoch, B., Piekarski, C. & Perio, P. Structure d’U4O9. https://doi.org/10.3406/bulmi.1960.5408 (1960).
Lauriat, J. P., Chevrier, G. & Boucherle, J. X. Space group of U4O9 in the beta phase. J. Solid State Chem. 80, 80–93 (1989).
Bevan, D. J. M., Grey, I. E. & Willis, B. T. M. The crystal structure of β-U4O9−y. J. Solid State Chem. 61, 1–7 (1986).
Masaki, N. & Doi, K. Analysis of the superstructure of U4O9 by neutron diffraction. Acta Crystallogr. B 28, 785–791 (1972).
Lipson, H. S., Stokes, A. R. & Bragg, W. L. The structure of graphite. Proc. R. Soc. Lond. Ser. Math. Phys. Sci. 181, 101–105 (1997).
Wyckoff, R. W. G. Crystal Structures (Interscience Publishers, 1963).
Atoji, M. & Medrud, R. C. Structures of calcium dicarbide and uranium dicarbide by neutron diffraction. J. Chem. Phys. 31, 332–337 (1959).
Nunez, U. et al. Structure of UC2 and U2C3: XRD, 13C NMR and Exafs Study. J. Alloys Compd. 589, 234–239 (2013).
Guéneau, C., Baichi, M., Labroche, D., Chatillon, C. & Sundman, B. Thermodynamic assessment of the uranium–oxygen system. J. Nucl. Mater. 304, 161–175 (2002).
Guéneau, C., Chatain, S. & Dumas. J.S. FUELBASE: a thermodynamic database for advanced nuclear fuels. In Proc. HTR2006 (HTR-2006, 2006).
Farr, J. D., Huber, E. J., Head, E. L. & Holley, C. E. The preparation of uranium monocarbide and its heat of formation. J. Phys. Chem. 63, 1455–1456 (1959).
Huber, E. J., Head, E. L. & Holley, C. E. The heat of formation of uranium dicarbide1,2. J. Phys. Chem. 67, 1730–1731 (1963).
Grenthe, I. et al. Chemical Thermodynamics of Uranium (North-Holland, 1992).
Macleod, A. C. High-temperature thermodynamic properties of uranium dicarbide. J. Inorg. Nucl. Chem. 31, 715–725 (1969).
Leitnaker, J. M. & Godfrey, T. G. Thermodynamic properties of uranium carbides. J. Nucl. Mater. 21, 175–189 (1967).
Levinson, L. S. Heat content of uranium dicarbide from 1484° to 2581°K. J. Chem. Phys. 38, 2105–2106 (1963).
Marcial, J. et al. Thermodynamic non-ideality and disorder heterogeneity in actinide silicate solid solutions. Npj Mater. Degrad. 5, 1–14 (2021).
Wen, X.-D., Martin, R. L., Henderson, T. M. & Scuseria, G. E. Density functional theory studies of the electronic structure of solid state actinide oxides. Chem. Rev. 113, 1063–1096 (2013).
Petit, L., Svane, A., Szotek, Z., Temmerman, W. M. & Stocks, G. M. Electronic structure and ionicity of actinide oxides from first principles. Phys. Rev. B 81, 045108 (2010).
Zinkle, S. J. & Was, G. S. Materials challenges in nuclear energy. Acta Mater. 61, 735–758 (2013).
Sengupta, A. K., Agarwal, R. & Kamath, H. S. 3.03 - Carbide Fuel. in Comprehensive Nuclear Materials (ed. Konings, R. J. M.) 55–86 (Elsevier, Oxford). https://doi.org/10.1016/B978-0-08-056033-5.00051-3 (2012).
Stecura, S. & Campbell, W. J. Thermal expansion and phase inversion of rare-earth oxides. BM-RI-5847, 4840970 http://www.osti.gov/servlets/purl/4840970-djI5pv/, https://doi.org/10.2172/4840970 (1960).
Thermophysical Properties of Matter - the TPRC Data Series. Volume 13. Thermal Expansion - Nonmetallic Solids. https://apps.dtic.mil/sti/citations/ADA129116.
Rahemtulla, A. et al. The high energy diffraction beamline at the Canadian light source. J. Synchrotron Radiat. 32, 750-756 (2025).
Toby, B. H. & Von Dreele, R. B. GSAS-II: the genesis of a modern open-source all purpose crystallography software package. J. Appl. Crystallogr. 46, 544–549 (2013).
Fei, Y. Thermal expansion. in Mineral Physics & Crystallography 29–44. https://doi.org/10.1029/RF002p0029 (American Geophysical Union (AGU), 1995).
Andersson, J.-O. et al. Computational tools for materials science. Calphad 26, 273–312 (2002).
Acknowledgements
This work was supported by the United States Nuclear Regulatory Commission, Office of Nuclear Regulatory Research (RES) under award No. 31310023M0011. Support of E.C.K. and N.S.Y. is through the support of a Department of Energy, Office of Nuclear Energy, University Nuclear Leadership Program Graduate Fellowship. Research presented in this article was also supported by the Laboratory Directed Research and Development program of Los Alamos National Laboratory under project number 20220053DR. Los Alamos National Laboratory is operated by Triad National Security, LLC, for the National Nuclear Security Administration of U.S. Department of Energy (Contract No. 89233218CNA000001). Portions of this research were also supported by Alexandra Navrotsky Institute for Experimental Thermodynamics. This research used beamline 28-ID-2 of the National Synchrotron Light Source II, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract No. DE-SC0012704. Part of the research described in this paper was also performed at the Canadian Light Source, a national research facility of the University of Saskatchewan, which is supported by the Canada Foundation for Innovation (CFI), the Natural Sciences and Engineering Research Council (NSERC), the Canadian Institutes of Health Research (CIHR), the Government of Saskatchewan, and the University of Saskatchewan.
Author information
Authors and Affiliations
Contributions
Emma C. Kindall (formal analysis, writing—original draft); Natalie S. Yaw (investigation, writing—review and editing); Malin C. Dixon Wilkins (investigation, writing—review and editing); Juejing Liu (investigation, writing—review and editing); Sam Karcher (investigation); Bryn Merrill (investigation); Rushi Gong (investigation, writing—review and editing); Shun-Li Shang (writing—review and editing); Zi-Kui Liu (writing—review and editing); John McCloy (writing—review and editing, supervision); Hongwu Xu (writing—review and editing); Adrien J. Terricabras (investigation, writing—review and editing); Scarlett Widgeon Paisner (writing—review and editing); Arjen van Veelen (writing—review and editing); Joshua T. White (writing—review and editing, supervision); Xiaofeng Guo (conceptualization, methodology, writing—review & editing, funding acquisition, supervision).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kindall, E.C., Yaw, N.S., Wilkins, M.C.D. et al. Thermal oxidation and high temperature structural behavior of uranium carbide. npj Mater Degrad (2026). https://doi.org/10.1038/s41529-025-00732-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41529-025-00732-1