Abstract
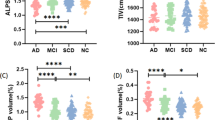
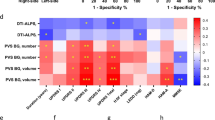
Cerebrospinal fluid (CSF) flow dynamics play a critical role in clearing pathological proteins from the brain, which may potentially influence the progression of neurodegenerative diseases. We aimed to investigate the alterations of CSF flow dynamics in patients with Parkinson’s disease (PD). We employed the multiple low b-values diffusion magnetic resonance imaging combined with CSF-based spatial statistics to evaluate changes of CSF pseudo-diffusivity within ventricles, sulci, and cisterns in PD. We assessed the relationships between CSF pseudo-diffusivity and other indirect markers of glymphatic system, including the diffusion-tensor imaging along the perivascular space (DTI-ALPS) index, the volume of the choroid plexus and perivascular spaces. We explored the association between CSF pseudo-diffusivity and the integrity of the locus coeruleus (LC). A total of 44 patients and 48 healthy controls participated in the study. PD patients showed significantly reduced CSF pseudo-diffusivity within the ventricles and sulci, with no significant changes within the cisterns. Lower CSF pseudo-diffusivity was correlated with lower DTI-ALPS index. Furthermore, decreased CSF pseudo-diffusivity was correlated with LC degeneration. These findings suggested that PD exhibit reduced CSF flow dynamics within the ventricles and sulci. Furthermore, LC-norepinephrine system dysfunction may represent a potential mechanism and target for modulating CSF flow dynamics.
Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The source code for CBSS processing is provided in https://github.com/Yutong441/CBSS.
References
Bloem, B. R., Okun, M. S. & Klein, C. Parkinson’s disease. Lancet 397, 2284–2303 (2021).
Stocchi, F., Bravi, D., Emmi, A. & Antonini, A. Parkinson disease therapy: current strategies and future research priorities. Nat. Rev. Neurol. 20, 695–707 (2024).
Yamamoto, E. A. et al. The perivascular space is a conduit for cerebrospinal fluid flow in humans: a proof-of-principle report. Proc. Natl. Acad. Sci. USA 121, e2407246121 (2024).
Iliff, J. J. et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 4, 147ra111 (2012).
Ding, X. B. et al. Impaired meningeal lymphatic drainage in patients with idiopathic Parkinson’s disease. Nat. Med. 27, 411–418 (2021).
Klostranec, J. M. et al. Current concepts in intracranial interstitial fluid transport and the glymphatic system: part I-anatomy and physiology. Radiology 301, 502–514 (2021).
Zou, W. et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl. Neurodegeneration 8, 7 (2019).
Liu, Y. et al. Non-invasive auditory and visual stimulation attenuates α-Synuclein deposition and improves motor and non-motor symptoms in PD mice. Exp. Neurol. 364, 114396 (2023).
Drieu, A. et al. Parenchymal border macrophages regulate the flow dynamics of the cerebrospinal fluid. Nature 611, 585–593 (2022).
Xue, X. et al. Aquaporin-4 deficiency reduces TGF-β1 in mouse midbrains and exacerbates pathology in experimental Parkinson’s disease. J. Cell Mol. Med. 23, 2568–2582 (2019).
Cui, H. et al. Decreased AQP4 expression aggravates ɑ-synuclein pathology in Parkinson’s disease mice, possibly via impaired glymphatic clearance. J. Mol. Neurosci. 71, 2500–2513 (2021).
Ryman, S. G. et al. Abnormal cerebrovascular activity, perfusion, and glymphatic clearance in Lewy body diseases. Mov. Disord. 39, 1258–1268 (2024).
Morawska, M. M. et al. Slow-wave sleep affects synucleinopathy and regulates proteostatic processes in mouse models of Parkinson’s disease. Sci. Transl. Med. 13, eabe7099 (2021).
McKnight, C. D., Rouleau, R. M., Donahue, M. J. & Claassen, D. O. The regulation of cerebral spinal fluid flow and its relevance to the glymphatic system. Curr. Neurol. Neurosci. Rep. 20, 58 (2020).
Miyazaki, M. et al. Physical exercise alters egress pathways for intrinsic CSF outflow: an investigation performed with spin-labeling MR imaging. Magn. Reson. Med. Sci. 23, 171–183 (2024).
Hauglund, N. L. et al. Norepinephrine-mediated slow vasomotion drives glymphatic clearance during sleep. Cell https://doi.org/10.1016/j.cell.2024.11.027 (2025).
Sun, X. et al. 40 Hz light flickering facilitates the glymphatic flow via adenosine signaling in mice. Cell Discov. 10, 81 (2024).
Ringstad, G. et al. Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3, https://doi.org/10.1172/jci.insight.121537 (2018).
Taoka, T. et al. Diffusion analysis of fluid dynamics with incremental strength of motion proving gradient (DANDYISM) to evaluate cerebrospinal fluid dynamics. Jpn. J. Radiol. 39, 315–323 (2021).
Bito, Y., Harada, K., Ochi, H. & Kudo, K. Low b-value diffusion tensor imaging for measuring pseudorandom flow of cerebrospinal fluid. Magn. Reson. Med. 86, 1369–1382 (2021).
Chen, Y., Hong, H., Nazeri, A., Markus, H. S. & Luo, X. Cerebrospinal fluid-based spatial statistics: towards quantitative analysis of cerebrospinal fluid pseudodiffusivity. Fluids Barriers CNS 21, 59 (2024).
Pierobon Mays, G. et al. Reduced cerebrospinal fluid motion in patients with Parkinson’s disease revealed by magnetic resonance imaging with low b-value diffusion weighted imaging. Fluids Barriers CNS 21, 40 (2024).
Atchley, T. J., Vukic, B., Vukic, M. & Walters, B. C. Review of cerebrospinal fluid physiology and dynamics: a call for medical education reform. Neurosurgery 91, 1–7 (2022).
Wang, Y. et al. Cerebrovascular activity is a major factor in the cerebrospinal fluid flow dynamics. NeuroImage 258, 119362 (2022).
Rasmussen, M. K., Mestre, H. & Nedergaard, M. The glymphatic pathway in neurological disorders. Lancet Neurol. 17, 1016–1024 (2018).
Tarasoff-Conway, J. M. et al. Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 (2015).
Ghaderi, S., Mohammadi, S., Jouzdani, A. F., Ahmadzadeh, A. M. & Fatehi, F. A systematic review and meta-analysis on glymphatic flow dysfunction in Parkinson’s disease and Parkinsonism spectrum. NPJ Parkinson’s. Dis. 11, 306 (2025).
Banta, A. R. et al. Quantification of perivascular flow dynamics of human cerebrospinal fluid along major arteries using phase-contrast MRI. Radiology 316, e243521 (2025).
Williamson, N. H., Komlosh, M. E., Benjamini, D. & Basser, P. J. Limits to flow detection in phase contrast MRI. J. Magn. Reson. Open 2–3, https://doi.org/10.1016/j.jmro.2020.100004 (2020).
Zhang, W. et al. Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage 238, 118257 (2021).
Taoka, T. et al. Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn. J. Radiol. 35, 172–178 (2017).
Zhou, C. et al. Glymphatic system dysfunction and risk of clinical milestones in patients with Parkinson disease. Eur. J. Neurol. 31, e16521 (2024).
Schilling, K. G. et al. White matter geometry confounds diffusion tensor imaging along perivascular space (DTI-ALPS) measures. Hum. Brain Mapp. 46, e70282 (2025).
Betts, M. J. et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142, 2558–2571 (2019).
Knudsen, K. et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 17, 618–628 (2018).
Postuma, R. B. et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601 (2015).
Jost, S. T. et al. Levodopa dose equivalency in Parkinson’s disease: updated systematic review and proposals. Mov. Disord. 38, 1236–1252 (2023).
Han, G. et al. Age- and time-of-day dependence of glymphatic function in the human brain measured via two diffusion MRI methods. Front. Aging Neurosci. 15, 1173221 (2023).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125, 1063–1078 (2016).
Tournier, J. D. et al. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Avants, B. B., Tustison, N. J., Wu, J., Cook, P. A. & Gee, J. C. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics 9, 381–400 (2011).
Vogt, N. M. et al. Cortical microstructural alterations in mild cognitive impairment and Alzheimer’s disease dementia. Cereb. Cortex 30, 2948–2960 (2020).
Billot, B. et al. Robust machine learning segmentation for large-scale analysis of heterogeneous clinical brain MRI datasets. Proc. Natl. Acad. Sci. USA 120, e2216399120 (2023).
Liu, X. et al. Cross-Vendor test-retest validation of diffusion tensor image analysis along the perivascular space (DTI-ALPS) for evaluating glymphatic system function. Aging Dis. 15, 1885–1898 (2024).
Huang, S. Y. et al. Glymphatic system dysfunction predicts amyloid deposition, neurodegeneration, and clinical progression in Alzheimer’s disease. Alzheimer’s. Dement. 20, 3251–3269 (2024).
He, P. et al. The association of the glymphatic function with Parkinson’s disease symptoms: neuroimaging evidence from longitudinal and cross-sectional studies. Ann. Neurol. 94, 672–683 (2023).
Li, Y. et al. Choroid plexus enlargement exacerbates white matter hyperintensity growth through glymphatic impairment. Ann. Neurol. 94, 182–195 (2023).
Jeong, S. H. et al. Association of choroid plexus volume with motor symptoms and dopaminergic degeneration in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 94, 1047–1055 (2023).
Bown, C. W., Carare, R. O., Schrag, M. S. & Jefferson, A. L. Physiology and clinical relevance of enlarged perivascular spaces in the aging brain. Neurology 98, 107–117 (2022).
Boutinaud, P. et al. 3D segmentation of perivascular spaces on T1-weighted 3 Tesla MR images with a convolutional autoencoder and a U-shaped neural network. Front. Neuroinform. 15, 641600 (2021).
Zhou, C. et al. Locus coeruleus degeneration is associated with disorganized functional topology in Parkinson’s disease. NeuroImage Clin. 32, 102873 (2021).
Acknowledgements
We thank Shanghai Tengyun Biotechnology Co., Ltd for developing Hiplot Pro platform (https://hiplot.com.cn/) and providing valuable tools for data analysis and visualization. Hiplot is a free tool and none of the authors have any financial interests or relationships to disclose that could inappropriately influence, or be perceived to influence, the work presented in this manuscript. This work was supported by the National Natural Science Foundation of China (Grant Nos. 82302132, 82271935, 82171888, 82202091, 82001767, 91630314, 82071997, 82302136, 82202089, 82371906, 82302135, and 81971577), the Natural Science Foundation of Zhejiang Province (Grant No. LY22H180002, LQ21H180008, and Z24H180002), the 13th Five-year Plan for National Key Research and Development Program of China (Grant No. 2016YFC1306600), and the China Postdoctoral Science Foundation (2023M733085).
Author information
Authors and Affiliations
Contributions
M.Z. has full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: C.Z., H.H., and M.Z. Acquisition, analysis, or interpretation of data: C.Z., X.G., T.G., and X.X. Drafting of the manuscript: C.Z. Critical review of the manuscript for important intellectual content: All authors. Statistical analysis: C.Z., H.H., and Y.C. Obtained funding: M.Z., C.Z., X.G., T.G., and X.X. Administrative, technical, or material support: M.Z. Supervision: M.Z.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, C., Hong, H., Chen, Y. et al. Alterations of cerebrospinal fluid flow dynamics in Parkinson’s disease. npj Parkinsons Dis. (2026). https://doi.org/10.1038/s41531-025-01257-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-025-01257-9