Abstract
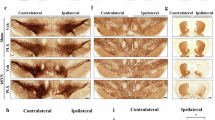
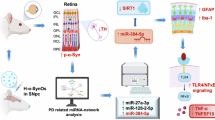
Parkinson’s disease (PD) affects motor and non-motor systems; however, retinal changes and their molecular basis are not well understood. Using a transgenic mouse model overexpressing A53T-mutant human α-synuclein, we examined retinal function, structure, and proteomics at 6- and 16 months. Early retinal dysfunction was detected by a reduction in scotopic oscillatory potential amplitudes on electroretinography. Optical coherence tomography showed early thinning of the retinal nerve fiber layer/ganglion cell layer, and photoreceptor layer, accompanied by thickening of the inner plexiform layer. Phosphorylated α-synuclein accumulation, increased glial fibrillary acidic protein, and loss of the ribbon synapse protein CtBP2 were observed. Proteomic profiling revealed stage-dependent alterations involving α-synuclein, oxidative stress markers, and crystallins. Network analysis showed progression from α-synuclein-associated disruption to inflammation and metabolic remodeling. These results highlight retinal alterations as early indicators of PD neurodegeneration and provide mechanistic insights into the molecular events that precede neuronal loss.
Similar content being viewed by others
Data availability
Mass spectrometry proteomic data were deposited in the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD066087.
References
Bartels, A. L. & Leenders, K. L. Parkinson’s disease: the syndrome, the pathogenesis and pathophysiology. Cortex 45, 915–921 (2009).
Ekker, M. S. et al. Ocular and visual disorders in Parkinson’s disease: common but frequently overlooked. Parkinsonism Relat. Disord. 40, 1–10 (2017).
Nowacka, B., Lubinski, W., Honczarenko, K., Potemkowski, A. & Safranow, K. Bioelectrical function and structural assessment of the retina in patients with early stages of Parkinson’s disease (PD). Doc. Ophthalmol. 131, 95–104 (2015).
Mello, L. G. M. et al. Electroretinography reveals retinal dysfunction in Parkinson’s disease despite normal high-resolution optical coherence tomography findings. Parkinsonism Relat. Disord. 101, 90–95 (2022).
Lee, J. Y. et al. Multimodal brain and retinal imaging of dopaminergic degeneration in Parkinson disease. Nat. Rev. Neurol. 18, 203–220 (2022).
Zhou, W. C., Tao, J. X. & Li, J. Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: a systematic review and meta-analysis. Eur. J. Neurol. 28, 763–774 (2021).
Chorostecki, J. et al. Characterization of retinal architecture in Parkinson’s disease. J. Neurol. Sci. 355, 44–48 (2015).
Rascuna, C. et al. Retinal thickness and microvascular pattern in early Parkinson’s disease. Front. Neurol. 11, 533375 (2020).
Unlu, M., Gulmez Sevim, D., Gultekin, M. & Karaca, C. Correlations among multifocal electroretinography and optical coherence tomography findings in patients with Parkinson’s disease. Neurol. Sci. 39, 533–541 (2018).
Campagnolo, M. et al. Optical coherence tomography reveals retinal structural abnormalities in alpha-synucleinopathies: insights from the Padua-CESNE cohort. J. Neural Transm. 132, 1013–1022 (2025).
Sung, M. S. et al. Inner retinal thinning as a biomarker for cognitive impairment in de novo Parkinson’s disease. Sci. Rep. 9, 11832 (2019).
Hasanov, S. et al. Functional and morphological assessment of ocular structures and follow-up of patients with early-stage Parkinson’s disease. Int. Ophthalmol. 39, 1255–1262 (2019).
Ahn, J. et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 91, e1003–e1012 (2018).
Ma, L. J. et al. Progressive changes in the retinal structure of patients with Parkinson’s disease. J. Parkinsons Dis. 8, 85–92 (2018).
Soto Linan, V. et al. Early detection of Parkinson’s disease: retinal functional impairments as potential biomarkers. Neurobiol. Dis. 208, 106872 (2025).
Djamgoz, M. B., Hankins, M. W., Hirano, J. & Archer, S. N. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vis. Res. 37, 3509–3529 (1997).
Tran, K. K. N. et al. Levodopa rescues retinal function in the transgenic A53T alpha-synuclein model of Parkinson’s disease. Biomedicines 12 https://doi.org/10.3390/biomedicines12010130 (2024).
Sanchez-Saez, X., Ortuno-Lizaran, I., Sanchez-Castillo, C., Lax, P. & Cuenca, N. Starburst amacrine cells, involved in visual motion perception, loose their synaptic input from dopaminergic amacrine cells and degenerate in Parkinson’s disease patients. Transl. Neurodegener. 12, 17 (2023).
Tran, K. K. N. et al. Retinal alpha-synuclein accumulation correlates with retinal dysfunction and structural thinning in the A53T mouse model of Parkinson’s disease. Front. Neurosci. 17, 1146979 (2023).
Xu, T. et al. Abnormal alpha-synuclein aggregates cause synaptic- and microcircuit-specific deficits in the retinal rod pathway. Am. J. Pathol. 194, 796–809 (2024).
Ortuno-Lizaran, I. et al. Dopaminergic retinal cell loss and visual dysfunction in Parkinson disease. Ann. Neurol. 88, 893–906 (2020).
Inzelberg, R., Ramirez, J. A., Nisipeanu, P. & Ophir, A. Retinal nerve fiber layer thinning in Parkinson disease. Vis. Res. 44, 2793–2797 (2004).
Hajee, M. E. et al. Inner retinal layer thinning in Parkinson disease. Arch. Ophthalmol. 127, 737–741 (2009).
Elanwar, R. et al. Retinal functional and structural changes in patients with Parkinson’s disease. BMC Neurol. 23, 330 (2023).
Wagner, S. K. et al. Retinal optical coherence tomography features associated with incident and prevalent Parkinson disease. Neurology 101, e1581–e1593 (2023).
Lim, J. K. H. et al. Retinal functional and structural changes in the 5xFAD mouse model of Alzheimer’s disease. Front. Neurosci. 14, 862 (2020).
Ortuno-Lizaran, I. et al. Phosphorylated alpha-synuclein in the retina is a biomarker of Parkinson’s disease pathology severity. Mov. Disord. 33, 1315–1324 (2018).
Fu, C. et al. Mutant mice with rod-specific VPS35 deletion exhibit retinal alpha-synuclein pathology-associated degeneration. Nat. Commun. 15, 5970 (2024).
Pfeiffer, R. L., Marc, R. E. & Jones, B. W. Persistent remodeling and neurodegeneration in late-stage retinal degeneration. Prog. Retin Eye Res. 74, 100771 (2020).
Caramiello, A. M. & Pirota, V. Novel therapeutic horizons: SNCA targeting in Parkinson’s disease. Biomolecules 14 https://doi.org/10.3390/biom14080949 (2024).
He, Q. et al. Early synaptic dysfunction of striatal parvalbumin interneurons in a mouse model of Parkinson’s disease. iScience 27, 111253 (2024).
Lanoue, A. C., Blatt, G. J. & Soghomonian, J. J. Decreased parvalbumin mRNA expression in dorsolateral prefrontal cortex in Parkinson’s disease. Brain Res. 1531, 37–47 (2013).
Fan, Y. & Xiao, S. Progression rate associated peripheral blood biomarkers of Parkinson’s disease. J. Mol. Neurosci. 65, 312–318 (2018).
Dai, H., Wang, L., Li, L., Huang, Z. & Ye, L. Metallothionein 1: a new spotlight on inflammatory diseases. Front. Immunol. 12, 739918 (2021).
Templeton, J. P. et al. A crystallin gene network in the mouse retina. Exp. Eye Res. 116, 129–140 (2013).
Wang, F. et al. alphaB-crystallin alleviates endotoxin-induced retinal inflammation and inhibits microglial activation and autophagy. Front. Immunol. 12, 641999 (2021).
Fort, P. E. & Lampi, K. J. New focus on alpha-crystallins in retinal neurodegenerative diseases. Exp. Eye Res. 92, 98–103 (2011).
Zhao, N., Quicksall, Z., Asmann, Y. W. & Ren, Y. Network approaches for omics studies of neurodegenerative diseases. Front. Genet. 13, 984338 (2022).
Acknowledgements
This study was supported by the Nano-Material Technology Development Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (NRF-2022M3H4A4085936); the Korea Mouse Phenotyping Project (RS-2024-00400118) from the Ministry of Science and ICT through the National Research Foundation. The funding bodies had no role in the design of the study, collection, analysis, and interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
C.-E.M. and S.J.L. contributed equally to this study. C.-E.M. contributed to the conceptualization, methodology, formal analysis, investigation, data curation, original draft writing, and visualization. S.J.L. contributed to original draft writing, review, and editing. H.S., H.K., J.-K.L., and H.J.K. were involved in the investigation. H.S.K. and I.H.M. were involved in writing. S.S.K., H.K.L., K.Y.S., and S.-R.C. contributed to supervision and project administration. Y.W.J. contributed to conceptualization, writing, supervision, and project administration. All the authors have read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Moon, CE., Lee, S.J., Shin, H. et al. Early retinal synaptic dysfunction and proteomic remodeling precede neurodegeneration in a Parkinson’s disease model. npj Parkinsons Dis. (2026). https://doi.org/10.1038/s41531-026-01261-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-026-01261-7