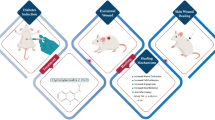
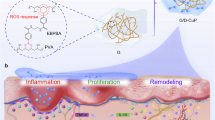
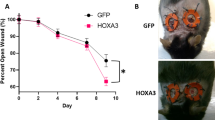
Abstract
Chronic diabetic wounds represent a major clinical challenge, compounded by persistent inflammation, microbial invasion, and deficient angiogenesis. To address these intertwined pathophysiological features, we developed a copper-ion coordinated andrographolide-loaded hydrogel (ASFH), significantly enhancing andrographolide solubility and promoting wound healing dynamics. In vitro assessments demonstrated superior antimicrobial activity, optimal mechanical strength, self-healing ability, and cytocompatibility. In diabetic mice, ASFH notably accelerated wound closure, stimulated collagen maturation and re-epithelialization, dynamically shifted macrophages toward an anti-inflammatory phenotype, and markedly enhanced angiogenesis. Mechanistic studies integrating network pharmacology, molecular docking, dynamics simulations, and SPR validation pinpointed the Rac1/JNK1/Jun/Fos signaling cascade as a primary mediator of these regenerative effects. This work presents ASFH as a translationally relevant dressing system, simultaneously addressing critical limitations in diabetic wound management through targeted molecular therapeutic intervention.

Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials. The raw datasets used and/or analyzed during the current study are available from the corresponding author.
References
Yin, G. N. et al. Latrophilin-2 is a novel receptor of LRG1 that rescues vascular and neurological abnormalities and restores diabetic erectile function. Exp. Mol. Med. 54, 626–638 (2022).
Fabio, F. et al. Prospective study on microangiopathy in type 2 diabetic foot ulcer. Diabetologia 59, 1542–1548 (2016).
Yang, L., Rong, G. C. & Wu, Q. N. Diabetic foot ulcer: challenges and future. World J. Diab. 13, 1014–1034 (2022).
He, W. et al. The cGAS-STING pathway: a therapeutic target in diabetes and its complications. Burns Trauma 12, tkad050 (2024).
Wu, X. et al. Elucidating the dual roles of apoptosis and necroptosis in diabetic wound healing: implications for therapeutic intervention. Burns Trauma 13, tkae061 (2025).
Deng, J.-Y. et al. Targeting DNA methylation and demethylation in diabetic foot ulcers. J. Adv. Res. 54, 119–131 (2023).
Cui, T. et al. Micro-gel ensembles for accelerated healing of chronic wound via pH regulation. Adv. Sci. 9, e2201254 (2022).
Ma, W. J. et al. Polydopamine decorated microneedles with Fe-MSC-derived nanovesicles encapsulation for wound healing. Adv. Sci. 9, e2103317 (2022).
Xu, Z. et al. Nanofiber-mediated sequential photothermal antibacteria and macrophage polarization for healing MRSA-infected diabetic wounds. J. Nanobiotechnol. 19, 404 (2021).
Raghavan, J. V. et al. Immunomodulatory bandage for accelerated healing of diabetic wounds. ACS Bio. Med. Chem. Au 2, 409–418 (2022).
Wu, X. Q. et al. Macrophage polarization in diabetic wound healing. Burns Trauma 10, tkac051 (2022).
Wang, C. et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics 9, 65–76 (2019).
Zou, F. et al. A novel bioactive polyurethane with controlled degradation and L-Arg release used as strong adhesive tissue patch for hemostasis and promoting wound healing. Bioact. Mater. 17, 471–487 (2022).
Zhong, G. F. et al. A photo-induced cross-linking enhanced A and B combined multi-functional spray hydrogel instantly protects and promotes of irregular dynamic wound healing. Small 20, e2309568 (2024).
Han, K. et al. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int J. Biol. Macromol. 183, 2142–2151 (2021).
Zhang, W. et al. Antibacterial coaxial hydro-membranes accelerate diabetic wound healing by tuning surface immunomodulatory functions. Mater. Today Bio. 16, 100395 (2022).
Sharmeen, S. et al. Polyethylene glycol functionalized carbon nanotubes/gelatin-chitosan nanocomposite: an approach for significant drug release. Bioact. Mater. 3, 236–244 (2018).
Chen, X. et al. Efficient drug delivery and anticancer effect of micelles based on vitamin E succinate and chitosan derivatives. Bioact. Mater. 6, 3025–3035 (2021).
Geng, Y. T. et al. Recent advances in carboxymethyl chitosan-based materials for biomedical applications. Carbohydr. Polym. 305, 120555 (2023).
Tran, Q. T. N., Tan, W. S. D., Wong, W. S. F. & Chai, C. L. L. Polypharmacology of andrographolide: beyond one molecule one target. Nat. Prod. Rep. 38, 682–692 (2021).
Dai, Y. et al. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Crit. Rev. Food Sci. 59, S17–S29 (2019).
Islam, M. T. Andrographolide, a new hope in the prevention and treatment of metabolic syndrome. Front. Pharm. 8, 571 (2017).
Veeresham, C., Swetha, E., Rao, A. R. & Asres, K. In vitro and in vivo aldose reductase inhibitory activity of standardized extracts and the major constituent of andrographis paniculata. Phytother. Res. 27, 412–416 (2012).
Zhang, Z. et al. Hypoglycemic and beta cell protective effects of andrographolide analogue for diabetes treatment. J. Transl. Med. 7, 62 (2009).
Messire, G., Serreau, R. & Berteina-Raboin, S. Antioxidant effects of catechins (EGCG), andrographolide, and curcuminoids compounds for skin protection, cosmetics, and dermatological uses: an update. Antioxidants 12, 1317 (2023).
Mussard, E. et al. Andrographis paniculata and its bioactive diterpenoids against inflammation and oxidative stress in keratinocytes. Antioxidants 9, 530 (2020).
Mussard, E. et al. Andrographis paniculata and its bioactive diterpenoids protect dermal fibroblasts against inflammation and oxidative stress. Antioxidants 9, 432 (2020).
Li, C. X. et al. Andrographolide suppresses thymic stromal lymphopoietin in phorbol myristate acetate/calcium ionophore A23187-activated mast cells and 2,4-dinitrofluorobenzene-induced atopic dermatitis-like mice model. Drug Des. Dev. Ther. 10, 781–791 (2016).
Shao, F. L. et al. Andrographolide alleviates imiquimod-induced psoriasis in mice via inducing autophagic proteolysis of MyD88. Biochem. Pharm. 115, 94–103 (2016).
Zhan, J. Y. X. et al. Andrographolide sodium bisulfate prevents UV-induced skin photoaging through inhibiting oxidative stress and inflammation. Mediat. Inflamm. 2016, 3271451 (2016).
Wahid, F., Wang, H.-S., Zhong, C. & Chu, L.-Q. Facile fabrication of moldable antibacterial carboxymethyl chitosan supramolecular hydrogels cross-linked by metal ions complexation. Carbohydr. Polym. 165, 455–461 (2017).
Haas, K. L. & Franz, K. J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 109, 4921–4960 (2009).
Lee, Y., Choi, K., Kim, J. E., Cha, S. & Nam, J. M. Integrating, validating, and expanding information space in single-molecule surface-enhanced Raman spectroscopy for biomolecules. ACS Nano 18, 25359–25371 (2024).
Raza, A. & Wu, W. Metal-organic frameworks in oral drug delivery. Asian J. Pharm. Sci. 19, 100951 (2024).
Septiani, D. A., Hakim, A., Patech, L. R., Zulhalifah, Z. & Siswadi, S. Isolation and identification of andrographolide compounds from the leaves of sambiloto plant (Andrographis paniculata ness). Acta Chim. Asian. 4, 108–113 (2021).
Clarke, D. E., Pashuck, E. T., Bertazzo, S., Weaver, J. V. M. & Stevens, M. M. Self-healing, self-assembled β-sheet peptide-poly(γ-glutamic acid) hybrid hydrogels. J. Am. Chem. Soc. 139, 7250–7255 (2017).
Liu, Y. et al. Asiaticoside-nitric oxide promoting diabetic wound healing through the miRNA-21-5p/TGF-β1/SMAD7/TIMP3 signaling pathway. J. Ethnopharmacol. 319, 117266 (2024).
Li, S., Ding, X., Yan, X., Qian, J. & Tan, Q. ceAF ameliorates diabetic wound healing by alleviating inflammation and oxidative stress via TLR4/NF- κ B and Nrf2 pathways. J. Diab. Res. 2023, 2422303 (2023).
Lan, C. C., Wu, C. S., Huang, S. M., Wu, I. H. & Chen, G. S. High-glucose environment enhanced oxidative stress and increased interleukin-8 secretion from keratinocytes: new insights into impaired diabetic wound healing. Diabetes 62, 2530–2538 (2013).
Suzuki, K. et al. Hydrogen sulfide replacement therapy protects the vascular endothelium in hyperglycemia by preserving mitochondrial function. Proc. Natl. Acad. Sci. USA 108, 13829–13834 (2011).
Ming, T. et al. Mono-phosphorylation at Ser4 of barrier-to-autointegration factor (Banf1) significantly reduces its DNA binding capability by inducing critical changes in its local conformation and DNA binding surface. Phys. Chem. Chem. Phys. 25, 24657–24677 (2023).
Tang, M. et al. Targeting the COMMD4–H2B protein complex in lung cancer. Br. J. Cancer 129, 2014–2024 (2023).
Young, M. J. et al. Nicotine binds to the transthyretin-thyroxine complex and reduces its uptake by placental trophoblasts. Mol. Cell Endocrinol. 549, 111642 (2022).
Ye, P. et al. SOX family transcription factors as therapeutic targets in wound healing: a comprehensive review. Int J. Biol. Macromol. 253, 127243 (2023).
Busik, J. V., Mohr, S. & Grant, M. B. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57, 1952–1965 (2008).
Wang, R. et al. Targeting oxidative damage in diabetic foot ulcers: integrative strategies involving antioxidant drugs and nanotechnologies. Burns Trauma 13, tkaf020 (2025).
Uberoi, A., McCready-Vangi, A. & Grice, E. A. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol 22, 507–521 (2024).
Brandt, B., Abou-Eladab, E. F., Tiedge, M. & Walzel, H. Role of the JNK/c-Jun/AP-1 signaling pathway in galectin-1-induced T-cell death. Cell Death Dis. 1, e23 (2010).
Xu, H. et al. Integrative single-cell RNA-Seq and ATAC-Seq analysis of peripheral mononuclear cells in patients with ankylosing spondylitis. Front. Immunol. 12, 760381 (2021).
Yang, L. et al. Initial IL-10 production dominates the therapy of mesenchymal stem cell scaffold in spinal cord injury. Theranostics 14, 879–891 (2024).
Li, Z. et al. Self-healing hydrogel bioelectronics. Adv. Mater. 36, e2306350 (2024).
Rocasalbas, G. et al. Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohydr. Polym. 92, 989–996 (2013).
Osi, A. R. et al. Three-dimensional-printable thermo/photo-cross-linked methacrylated chitosan-gelatin hydrogel composites for tissue engineering. ACS Appl. Mater. Inter. 13, 22902–22913 (2021).
Ressler, A. et al. Injectable chitosan-hydroxyapatite hydrogels promote the osteogenic differentiation of mesenchymal stem cells. Carbohydr. Polym. 197, 469–477 (2018).
Liu, T. et al. PSMC2 promotes the progression of gastric cancer via induction of RPS15A/mTOR pathway. Oncogenesis 11, 12 (2022).
Jonkman, J. E. et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adh. Migr. 8, 440–451 (2014).
Liang, C. C., Park, A. Y. & Guan, J. L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2, 329–333 (2007).
Jin, E. et al. Lemon-derived nanoparticle-functionalized hydrogels regulate macrophage reprogramming to promote diabetic wound healing. J. Nanobiotechnol. 23, 68 (2025).
Gurtner, M. R. N. K. C. A. B. G. C. Wound healing: a cellular perspective. Physiol. Rev. 99, 665–706 (2019).
Wang, X. et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45, W356–W360 (2017).
Tang, M., Gandhi, N. S., Burrage, K. & Gu, Y. Adsorption Of Collagen-like Peptides Onto Gold Nanosurfaces. Langmuir 35, 4435–4444 (2019).
Wu, X. et al. The Gut Microbiota-xanthurenic Acid-aromatic Hydrocarbon Receptor Axis Mediates The Anticolitic Effects Of Trilobatin. Adv. Sci. 12, e2412234 (2025).
Wei, J. et al. Targeting FDX1 by trilobatin to inhibit cuproptosis in doxorubicin-induced cardiotoxicity. Br. J. Pharm. 182, 2409–2425 (2025).
Mu, X. et al. Asiaticoside–nitric oxide synergistically accelerate diabetic wound healing by regulating key metabolites and SRC/STAT3 signaling. Burns Trauma 13, tkaf009 (2025).
Ye, P. et al. ACNO hydrogel enhances diabetic wound healing by modulating the Bcl-2/Bax/Caspase-3/PARP pathway. Int Immunopharmacol. 147, 113997 (2025).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (82460792, 82160770), the Zunyi Science and Technology Talent Platform Carrier Construction Project (ZSKRPT2023-1), the Department of Science and Technology of Guizhou Province (QKHPTRC-CXTD [2023] 024), the Guizhou Key Laboratory of Modern Traditional Chinese Medicine Creation (Qian Ke He Platform ZSYS [2025] 019), and the Zunyi City Municipal and University Joint Science and Technology Funding Project (Zunshi Kehe HZ [2025]251). The funder played no role in study design, data collection, analysis and interpretation of data, or the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
Penghui Ye—original draft, methodology, investigation, formal analysis, and data curation. Yuhe Dai and Qianbo Zhang—investigation and data curation. Junqi Yang, Lele Liu, and Xiuying Guo—software, Formal analysis, and data curation. Rifang Gu, Ming Tang, Min Tan, Huan Zhu, Jitao Chen, and Felicity Han—Writing—review and editing. Xuqiang Nie—Writing—review and editing, supervision, conceptualization, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ye, P., Dai, Y., Zhang, Q. et al. Novel copper-ion coordinated andrographolide-loaded hydrogel activates Rac1/JNK1 axis for enhancing diabetic wound healing. npj Regen Med (2026). https://doi.org/10.1038/s41536-026-00457-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41536-026-00457-y