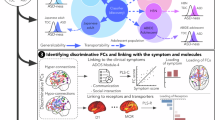
Abstract
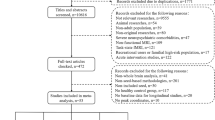
Few neuroimaging studies have examined other specified schizophrenia spectrum and other psychotic disorder (OSSO). We sought to identify features differentiating patients with OSSO from those with schizophrenia spectrum disorders (SSD) and healthy controls (HC) using auditory seed-based functional connectivity (FC) analysis. Patients with OSSO (n = 88), patients with SSD (n = 81), and HC (n = 85), matched for age, sex, and education, underwent resting-state functional magnetic resonance imaging (rs-fMRI) and clinical evaluation. To reduce heterogeneity of OSSO, individuals with specific subtypes of OSSO, i.e., pure delusion and delusion with attenuated auditory hallucinations (AHs) were only included. Using five auditory seeds, we conducted seed-to-voxel and seed-to-region of interest (ROI) analyses. We also conducted between- and within-network connectivity analyses of 13 networks, and correlations of altered FC with symptomatology were explored. The SSD group showed significantly greater connectivity between the superior temporal gyrus (STG) and precuneus, and between the temporal pole cortex (TP) and precuneus, compared to the OSSO group. Overall auditory seed-based hypoconnectivity and middle temporal gyrus-based hyperconnectivity were observed in both groups compared to HC. In OSSO, hallucination severity was positively associated with insula–putamen connectivity, whereas delusional and negative symptoms showed inverse correlations with TP–insula and STG–Heschl’s gyrus connectivity, respectively. In SSD, hallucination severity correlated positively with STG–Heschl’s gyrus and TP–insula connectivity whereas negative symptoms correlated negatively with STG–insula connectivity. These findings suggest that there are distinct differences in FC between patients with OSSO and patients with SSD, which supports the proposal that OSSO should be treated as a separate clinical syndrome with distinct neural connectomes. Future research may explore whether interventions targeting these altered connectivity patterns could help reduce the risk of progression from OSSO to SSD.
Similar content being viewed by others
Data availability
The data that support the findings of this study cannot be made publicly available for confidentiality reasons. However, data are available from the corresponding author upon reasonable request.
References
Kendler, K. S. & Walsh, D. Schizophreniform disorder, delusional disorder and psychotic disorder not otherwise specified: clinical features, outcome and familial psychopathology. Acta Psychiatr. Scand. 91, 370–378 (1995).
Ayesa-Arriola, R. et al. Diagnosis and neurocognitive profiles in first-episode non-affective psychosis patients. Eur. Arch. Psychiatry Clin. Neurosci. 266, 619–628 (2016).
Widing, L. et al. Symptom profiles in psychotic disorder not otherwise specified. Front. Psychiatry 11, 580444 (2020).
Fennig, S. et al. Gender, premorbid characteristics and negative symptoms in schizophrenia. Acta Psychiatr. Scand. 92, 173–177 (1995).
Li, L. et al. Comparison of clinical features and 1 year outcomes between patients with psychotic disorder not otherwise specified and those with schizophrenia. Early Interv. Psychiatry 16, 1309–1318 (2022).
Widing, L. et al. Long-term outcomes of people with DSM psychotic disorder NOS. Schizophr. Bull. Open 4, SGAD005 (2023).
Kim, W. S. et al. Altered functional connectivity in psychotic disorder not otherwise specified. Psychiatry Res. 317, 114871 (2022).
Crespo-Facorro, B. et al. Specific brain structural abnormalities in first-episode schizophrenia. Schizophr. Res. 115, 191–201 (2009).
Takahashi, T. et al. Diagnostic specificity of the insular cortex abnormalities in first-episode psychotic disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 651–657 (2009).
Haukvik, U. K. et al. No progressive brain changes during a 1-year follow-up of patients with first-episode psychosis. Psychol. Med. 46, 589–598 (2016).
Zuliani, R. et al. Increased gyrification in schizophrenia and non affective first episode of psychosis. Schizophr. Res. 193, 269–275 (2018).
Cho, W. R. et al. Effects of the diagnostic and statistical manual of mental disorders, fifth edition criteria changes for schizophrenia on diagnoses of first-episode schizophrenia spectrum disorders. Psychiatry Investig. 22, 212–217 (2025).
Allen, P. et al. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 32, 175–191 (2008).
Jardri, R. et al. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am. J. Psychiatry 168, 73–81 (2011).
Li, S. et al. Dysconnectivity of multiple brain networks in schizophrenia: A meta-analysis of resting-state functional connectivity. Front. Psychiatry 10, 482 (2019).
Diederen, K. M. et al. Aberrant resting-state connectivity in non-psychotic individuals with auditory hallucinations. Psychol. Med. 43, 1685–1696 (2013).
Chen, X. et al. Reduced cortical thickness in right Heschl’s gyrus associated with auditory verbal hallucinations severity in first-episode schizophrenia. BMC Psychiatry 15, 152 (2015).
Mørch-Johnsen, L. et al. Auditory cortex characteristics in schizophrenia: associations with auditory hallucinations. Schizophr. Bull. 43, 75–83 (2017).
Clos, M. et al. Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Struct. Funct. 219, 581–594 (2014).
Diederen, K. M. et al. Auditory hallucinations elicit similar brain activation in psychotic and nonpsychotic individuals. Schizophr. Bull. 38, 1074–1082 (2012).
Skouras, S. et al. Aberrant connectivity in the hippocampus, bilateral insula and temporal poles precedes treatment resistance in first-episode psychosis: a prospective resting-state fMRI study with connectivity concordance mapping. Brain Commun. 6, FCAE094 (2024).
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn. (American Psychiatric Association, 2013).
First, M. B., Williams, J. B. & Karg, R. S. SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders Clinician Version. (American Psychiatric Association Publishing, 2016).
Oh, M. Y., Park, Y. C. & Oh, S. W. The Korean-translated Version of the SCID-5-CV: Structured Clinical Interview for DSM-5 Disorders Clinician Version: User’s Guide (Hakjisa, Seoul, 2017).
Kay, S. R., Fiszbein, A. & Opler, L. A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Yi, J. S. et al. Reliability and validity of the Korean version of the positive and negative syndrome scale. J. Korean Neuropsychiatr. Assoc. 40, 1090–1106 (2001).
Nieto-Castanon, A. & Whitfield-Gabrieli, S. CONN Functional Connectivity Toolbox (RRID) Version 19. http://www.nitrc.org/projects/conn (2019).
Heleven, E. & Van Overwalle, F. The person within: memory codes for persons and traits using fMRI repetition suppression. Soc. Cogn. Affect. Neurosci. 11, 159–171 (2015).
Behzadi, Y. et al. A component-based noise correction method (CompCor) for BOLD and perfusion-based fMRI. Neuroimage 37, 90–101 (2007).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
McNabb, C. B. et al. Functional network dysconnectivity as a biomarker of treatment resistance in schizophrenia. Schizophrenia Res. 195, 160–167 (2018).
Gordon, E. M. et al. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303 (2016).
Di Pellegrino, G. Superior temporal gyrus: a neglected cortical area?. Trends Cogn. Sci. 5, 330 (2001).
Price, C. J. The anatomy of language: Contributions from functional neuroimaging. J. Anat. 197, 335–359 (2000).
Hoekert, M. et al. Time course of the involvement of the right anterior superior temporal gyrus and the right fronto-parietal operculum in emotional prosody perception. PLoS One 3, e2244 (2008).
Cavanna, A. E. & Trimble, M. R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006).
Molnar-Szakacs, I. & Uddin, L. Q. Self-processing and the default mode network: Interactions with the mirror neuron system. Front. Hum. Neurosci. 7, 571 (2013).
Herlin, B., Navarro, V. & Dupont, S. The temporal pole: From anatomy to function—a literature appraisal. J. Chem. Neuroanat. 113, 101925 (2021).
Davey, J. et al. Exploring the role of the posterior middle temporal gyrus in semantic cognition: Integration of anterior temporal lobe with executive processes. Neuroimage 137, 165–177 (2016).
Morawetz, C. et al. Neural representation of emotion regulation goals. Hum. Brain Mapp. 37, 600–620 (2016).
Craig, A. D. How do you feel-now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Gogolla, N. The insular cortex. Curr. Biol. 27, R573–R591 (2017).
Cromwell, H. C., Hassani, O. K. & Schultz, W. Relative reward processing in primate striatum. Exp. Brain Res. 162, 520–525 (2005).
Lau, B. & Glimcher, P. W. Action and outcome encoding in the primate caudate nucleus. J. Neurosci. 27, 14502–14514 (2007).
Spironelli, C. et al. fMRI fluctuations within the language network are correlated with severity of hallucinatory symptoms in schizophrenia. Schizophrenia (Heidelberg). 9, 75 (2023).
Ferri, J. et al. Resting-state thalamic dysconnectivity in schizophrenia and relationships with symptoms. Psychol. Med. 48, 2492–2499 (2018).
Dierks, T. et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron 22, 615–621 (1999).
Lennox, B. R. et al. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 100, 13–20 (2000).
Bohlken, M. M., Hugdahl, K. & Sommer, I. E. Auditory verbal hallucinations: neuroimaging and treatment. Psychol. Med. 47, 199–208 (2017).
Slotema, C. W. et al. Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: Update and effects after one month. Schizophr. Res. 142, 40–45 (2012).
Orlov, N. D. et al. Real-time fMRI neurofeedback to down-regulate superior temporal gyrus activity in patients with schizophrenia and auditory hallucinations: a proof-of-concept study. Transl. Psychiatry 8, 46 (2018).
Molina, J. L. et al. Central auditory processing deficits in schizophrenia: Effects of auditory-based cognitive training. Schizophr. Res. 236, 135–141 (2021).
Acknowledgements
The corresponding author would like to thank all participants in the study and father for the guidance and support (SDG). This study was supported by a grant of the Korean Mental Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HL19C0015), a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (RS-2018-KH049511) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00335201).
Author information
Authors and Affiliations
Contributions
Y.-C.C. conceptualized the study. W.-S.K., S.O., and Y.-C.C. performed the study and acquired data. W.-S.K. conducted experiment and statistical analysis. W.-S.K. drafted the manuscript. W.-S.K., S.O., K.-H.L., N.-I.K., E.-J.J., A.S., L.L., F.Z.R., S.N. and Y.-C.C. critically reviewed the manuscript and Y.-C.C. finalized it. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, WS., Odkhuu, S., Jeon, EJ. et al. Altered auditory seed-based functional connectivity in other specified schizophrenia spectrum and other psychotic disorder compared to schizophrenia spectrum disorders. Schizophr (2026). https://doi.org/10.1038/s41537-025-00708-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-025-00708-9