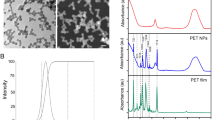
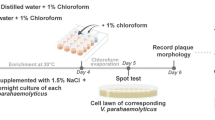
Abstract
Nanoplastics (NPs) and pathogenic bacteria are widely present in natural water, yet their interactive effects on aquatic organisms remain poorly understood. In this study, we demonstrate for the first time that Vibrio parahaemolyticus can extensively capture free NPs and facilitate their translocation through the intestinal barrier of Litopenaeus vannamei, thereby altering the distribution of NPs within shrimp and exacerbating their accumulation in the hepatopancreas. These findings provide the first evidence that bacteria act as carriers of NPs influencing their translocation. Interestingly, NPs also affect V. parahaemolyticus infection in shrimp by attenuating the virulence of pathogen, as evidenced by downregulated expression of virulence genes (Tdh and Trh), reduced bacterial loads, and improved host survival rates. Single-cell transcriptomics analysis revealed that NPs activate both energy metabolism and immune pathways, collectively enhancing the host’s antioxidative capacity and immunocompetence. These findings offer novel insights into the mechanisms of NPs-pathogen-host interactions and provide critical data for assessing the ecological risks of plastic pollution to seafood safety.

Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Sriket, P. et al. Comparative studies on the effect of the freeze-thawing process on the physicochemical properties and microstructures of black tiger shrimp (Penaeus monodon) and white shrimp (Penaeus vannamei) muscle. Food Chem. 104, 113–121 (2007).
Okasha, L. A. et al. Salinity-dependent vulnerability of whiteleg shrimp (Litopenaeus vannamei) to Vibrio parahaemolyticus: growth performance and antioxidant response. Aquacult. Int. 33, 347 (2025).
Duan, Y. et al. Toxicological effects of microplastics in Litopenaeus vannamei as indicated by an integrated microbiome, proteomic and metabolomic approach. Sci. Total Environ. 761, 143311 (2021).
Ceccarelli, D. et al. Distribution and dynamics of epidemic and pandemic Vibrio parahaemolyticus virulence factors. Front Cell Infect. Microbiol. 3, 97 (2013).
Broberg, C. A., Calder, T. J. & Orth, K. Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect. 13, 992–1001 (2011).
Jatapai, A. et al. An acute gastroenteritis outbreak of Vibrio parahaemolyticus O4:K55 in Nursing College, Thailand. Trop. Biomed. 27, 265–274 (2010).
Aagesen, A. M. et al. Persistence of Vibrio parahaemolyticus in the Pacific oyster, Crassostrea gigas, is a multifactorial process involving pili and flagella but not type III secretion systems or phase variation. Appl. Environ. Microbiol. 79(10), 3303–3305 (2013).
Khimmakthong, U. & Sukkarun, P. The spread of Vibrio parahaemolyticus in tissues of the Pacific white shrimp Litopenaeus vannamei analyzed by PCR and histopathology. Micro. Pathog. 113, 107–112 (2017).
Gigault, J. et al. Current opinion: What is a nanoplastic?. Environ. Pollut. 235, 1030–1034 (2018).
Sharma, V. K. et al. Nanoplastics are potentially more dangerous than microplastics. Environ. Chem. Lett. 21(4), 1933–1936 (2023).
Paul, M. B. et al. Complex intestinal and hepatic in vitro barrier models reveal information on uptake and impact of micro-, submicro- and nanoplastics. Environ. Int 179, 108172 (2023).
Kulkarni, S. A. & Feng, S. S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm. Res. 30(10), 2512–2522 (2013).
Mahler, G. J. et al. Oral exposure to polystyrene nanoparticles affects iron absorption. Nat. Nanotechnol. 7, 264–271 (2012).
Zhang, Y. et al. Selective bioaccumulation of polystyrene nanoplastics in fetal rat brain and damage to myelin development. Ecotoxicol. Environ. Saf. 278, 116393 (2024).
Ding, J. et al. Toxicological effects of nano- and micro-polystyrene plastics on red tilapia: Are larger plastic particles more harmless?. J. Hazard Mater. 396, 122693 (2020).
Hessler, C. M. et al. The influence of capsular extracellular polymeric substances on the interaction between TiO₂ nanoparticles and planktonic bacteria. Water Res. 46, 4687–4696 (2012).
Öztürk, K., Kaplan, M. & Çalış, S. Effects of nanoparticle size, shape, and zeta potential on drug delivery. Int J. Pharm. 666, 124799 (2024).
Draviana, H. T. et al. Size and charge effects of metal nanoclusters on antibacterial mechanisms. J. Nanobiotechnol. 21, 428 (2023).
Zhu, Z. et al. Pseudomonas Stutzeri may alter the environmental fate of polystyrene nanoplastics by trapping them with increasing extracellular polymers. Sci. Total Environ. 954, 176392 (2024).
Scaria, S. S. et al. Review on impacts of micro- and nano-plastic on aquatic ecosystems and mitigation strategies. Aquat. Toxicol. 265, 106759 (2023).
Chatterjee, T. et al. The gold nanoparticle reduces Vibrio cholerae pathogenesis by inhibition of biofilm formation and disruption of the production and structure of cholera toxin. Colloids Surf. B Biointerfaces 204, 111811 (2021).
Yang, P., et al. Single-cell RNA sequencing analysis of shrimp immune cells identifies macrophage-like phagocytes. Elife 11 (2022).
Du, L. et al. Single-cell RNA sequencing reveals the heterogeneity of hepatopancreas cells and their association with gonadal development in the red swamp crayfish Procambarus clarkii. Aquaculture 601, 742311 (2025).
Zhang, B. et al. Single-cell transcriptomics uncovers potential marker genes of ochratoxin A-sensitive renal cells in an acute toxicity rat model. Cell Biol. Toxicol. 37, 7–13 (2021).
Li, Y. et al. A single-cell atlas of Drosophila trachea reveals glycosylation-mediated Notch signaling in cell fate specification. Nat. Commun. 15, 2019 (2024).
Sun, X. et al. Cell type diversity in scallop adductor muscles revealed by single-cell RNA-Seq. Genomics 113, 3582–3598 (2021).
Yu, J. et al. Heterogeneity effects of nanoplastics and lead on zebrafish intestinal cells identified by single-cell sequencing. Chemosphere 289, 133133 (2022).
Hou, L. et al. Single-cell RNA-sequencing reveals Eriocheir sinensis hemocyte subpopulations and their molecular responses to Spiroplasma eriocheiris infection. Aquaculture 594 (2025).
Cui, C. et al. Single-cell RNA-seq revealed heterogeneous responses and functional differentiation of hemocytes against white spot syndrome virus infection in Litopenaeus vannamei. J. Virol. 98, e0180523 (2024).
Sun, J. J. et al. Activation of toll pathway is different between Kuruma Shrimp and Drosophila. Front Immunol. 8, 1151 (2017).
Tassanakajon, A. et al. Shrimp humoral responses against pathogens: antimicrobial peptides and melanization. Dev. Comp. Immunol. 80, 81–93 (2018).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Dai, S. et al. Distinct lipid membrane interaction and uptake of differentially charged nanoplastics in bacteria. J. Nanobiotechnol. 20, 191 (2022).
Flemming, H. C. et al. The biofilm matrix: multitasking in a shared space. Nat. Rev. Microbiol 21, 70–86 (2023).
Bystrianský, L., Hujslová, M. & Gryndler, M. Study of the effects of mineral salts on the biofilm formation on polypropylene fibers using three quantification methods. Folia Microbiol. (Praha) 66, 133–143 (2021).
Zhu, Z. et al. Bacillus subtilis, a promising bacterial candidate for trapping nanoplastics during water treatment. J. Hazard Mater. 483, 136679 (2025).
Dogsa, I. et al. Bacillus subtilis EpsA-O: A novel exopolysaccharide structure acting as an efficient adhesive in biofilms. NPJ Biofilms Microbiomes 10, 98 (2024).
Zhao, W. et al. Contrasting effects of extracellular polymeric substances on the surface characteristics of bacterial pathogens and cell attachment to soil particles. Chem. Geol. 410, 79–88 (2015).
Davis, C. A. et al., Microbial-induced heterogeneity in the acoustic properties of porous media. Geophysical Research Letters, 2009. 36.
Ren, D. et al. High prevalence of biofilm synergy among bacterial soil isolates in cocultures indicates bacterial interspecific cooperation. Isme j. 9, 81–89 (2015).
Aquino, S. F. & Stuckey, D. C. Soluble microbial products formation in anaerobic chemostats in the presence of toxic compounds. Water Res 38, 255–266 (2004).
Hu, X. et al. Extracellular Polymeric Substances Acting as a Permeable Barrier Hinder the Lateral Transfer of Antibiotic Resistance Genes. Front Microbiol 10, 736 (2019).
Wu, S. et al. An invisible workforce in soil: The neglected role of soil biofilms in conjugative transfer of antibiotic resistance genes. Crit. Rev. Environ. Sci. Technol. 52, 2720–2748 (2022).
Costa, O. Y. A., Raaijmakers, J. M. & Kuramae, E. E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front Microbiol 9, 1636 (2018).
Gao, X. et al. Comparing the effects and mechanisms of exposure to polystyrene nanoplastics with different functional groups on the male reproductive system. Sci. Total Environ. 922, 171299 (2024).
Malinowska, K. et al. Polystyrene nanoparticles: the mechanism of their genotoxicity in human peripheral blood mononuclear cells. Nanotoxicology 16, 791–811 (2022).
Wang, Y. et al. Comparative transcriptome analysis reveals the different roles between hepatopancreas and intestine of Litopenaeus vannamei in immune response to aflatoxin B1 (AFB1) challenge. Comp. Biochem Physiol. C. Toxicol. Pharm. 222, 1–10 (2019).
Lu, Y. et al. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 50, 4054–4060 (2016).
Vagner, M. et al. Experimental evidence that polystyrene nanoplastics cross the intestinal barrier of European seabass. Environ. Int 166, 107340 (2022).
Claus, S. P., Guillou, H. & Ellero-Simatos, S. The gut microbiota: a major player in the toxicity of environmental pollutants?. NPJ Biofilms Microbiomes 2, 16003 (2016).
Meng, X. et al. Systemic effects of nanoplastics on multi-organ at the environmentally relevant dose: The insights in physiological, histological, and oxidative damages. Sci. Total Environ. 892, 164687 (2023).
Garcia-Garcia, E., Galindo-Villegas, J. & Mulero, V. Mucosal immunity in the gut: the non-vertebrate perspective. Dev. Comp. Immunol. 40, 278–288 (2013).
Soonthornchai, W. et al. Expression of immune-related genes in the digestive organ of shrimp, Penaeus monodon, after an oral infection by Vibrio harveyi. Dev. Comp. Immunol. 34, 19–28 (2010).
Zhang, J. et al. Differentially Charged Nanoplastics Induce Distinct Effects on the Growth and Gut of Benthic Insects (Chironomus kiinensis) via Charge-Specific Accumulation and Perturbation of the Gut Microbiota. Environ. Sci. Technol. 57, 11218–11230 (2023).
She, Q. et al. Impacts of circadian rhythm and melatonin on the specific activities of immune and antioxidant enzymes of the Chinese mitten crab (Eriocheir sinensis). Fish. Shellfish Immunol. 89, 345–353 (2019).
Amparyup, P., Charoensapsri, W. & Tassanakajon, A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish. Shellfish Immunol. 34, 990–1001 (2013).
Sang, W. et al. Transcriptome analysis of hepatopancreas of Chinese grass shrimp, Palaemonetes sinensis, infected by Enterocytospora artemiae. Fish. Shellfish Immunol. 133, 108557 (2023).
Xu, Y. et al. Toxicity of the microcystin-producing cyanobacteria Microcystis aeruginosa to shrimp Litopenaeus vannamei. Ecotoxicology 31, 1403–1412 (2022).
Lin, Z. et al. Micro/Nanoplastics in plantation agricultural products: behavior process, phytotoxicity under biotic and abiotic stresses, and controlling strategies. J. Nanobiotechnology 23, 231 (2025).
Sökmen, T. et al. Polystyrene nanoplastics (20 nm) are able to bioaccumulate and cause oxidative DNA damages in the brain tissue of zebrafish embryo (Danio rerio). Neurotoxicology 77, 51–59 (2020).
van Rensburg, G. J. et al. Oxidative stress in the freshwater shrimp Caridina africana following exposure to atrazine. Bull. Environ. Contamination Toxicol. 109, 443–449 (2022).
Park, K. S. et al. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 72, 6659–6665 (2004).
Goodman, K. E. et al. Exposure of Human Lung Cells to Polystyrene Microplastics Significantly Retards Cell Proliferation and Triggers Morphological Changes. Chem. Res Toxicol. 34, 1069–1081 (2021).
Wang, Y. L. et al. The Kidney-Related Effects of Polystyrene Microplastics on Human Kidney Proximal Tubular Epithelial Cells HK-2 and Male C57BL/6 Mice. Environ. Health Perspect. 129, 57003 (2021).
Cui, H. et al. Single-cell RNA sequencing analysis to evaluate antimony exposure effects on cell-lineage communications within the Drosophila testicular niche. Ecotoxicol. Environ. Saf. 270, 115948 (2024).
Vogt, G. Cytology, function and dynamics of stem and progenitor cells in decapod crustaceans. Biol. Rev. Camb. Philos. Soc. 97, 817–850 (2022).
Sonakowska, L. et al. Structure and Ultrastructure of the Endodermal Region of the Alimentary Tract in the Freshwater Shrimp Neocaridina heteropoda (Crustacea, Malacostraca). PLoS One 10, e0126900 (2015).
Vogt, G. Functional cytology of the hepatopancreas of decapod crustaceans. J. Morphol. 280, 1405–1444 (2019).
Ramzan, R., et al., Cytochrome c Oxidase Inhibition by ATP Decreases Mitochondrial ROS Production. Cells, 2022. 11.
Wu, Q. et al. Polystyrene nanoplastics-induced lung apoptosis and ferroptosis via ROS-dependent endoplasmic reticulum stress. Sci. Total Environ. 912, 169260 (2024).
Li, Y. et al. ROS and DRP1 interactions accelerate the mitochondrial injury induced by polystyrene nanoplastics in human liver HepG2 cells. Chem. Biol. Interact. 379, 110502 (2023).
Bu, W. et al. Unmasking the Invisible Threat: Biological Impacts and Mechanisms of Polystyrene Nanoplastics on Cells. Toxics, 2024. 12.
Winiarska, E., Jutel, M. & Zemelka-Wiacek, M. The potential impact of nano- and microplastics on human health: Understanding human health risks. Environ. Res. 251, 118535 (2024).
Wang, J. et al. White spot syndrome virus (WSSV) infection impacts intestinal microbiota composition and function in Litopenaeus vannamei. Fish. Shellfish Immunol. 84, 130–137 (2019).
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grants 42377363 and 42177253 to Yan Muting]; the Guangdong Basic and Applied Basic Research Foundation [Grants 2024A1515011401 and 2024A1515030201 to Yan Muting and Han Gong, respectively]; the Guangdong Provincial Special Project for Promoting Urban-Rural and Regional Coordinated Development through Sci-Tech Achievements into Counties and Towns [Grant 2025B0202010035 to Yan Muting]; the Young Talent Support Project of Guangzhou Association for Science and Technology [Grant QT-2025-014 to Yan Muting]; and the Guangzhou Science and Technology Project, Basic and Applied Basic Research project [Grant 2025A04J5419 to Yan Muting]. Additionally, we acknowledge BioRender.com for assistance in creating graphical elements and Shanghai Majorbio Bio-pharm Technology Co., Ltd. (Meiji, China) for providing technical support in single-cell RNA sequencing.
Author information
Authors and Affiliations
Contributions
R.Z. and X.F. conducted the experiments and analyzed the data. C.L. prepared Figures 1-4 and performed statistical analysis. B.Z. and G.Z. contributed to data collection and technical support. H.G. and M.Y. conceived and supervised the project, provided funding, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhong, R., Fang, X., Li, C. et al. Combined Vibrio and nanoplastics stress promotes nanoplastic accumulation while reducing bacterial lethality in shrimp. npj Sci Food (2026). https://doi.org/10.1038/s41538-025-00697-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-025-00697-0