Abstract
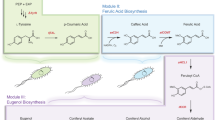
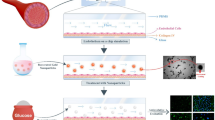
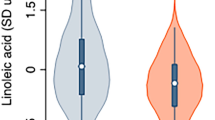
Plant-derived vesicles (PDVs) are lipid-membrane structures that enclose proteins, lipids, nucleic acids and metabolites, reflecting the phytochemical profile of their plant source. This study investigated PDVs from Rosmarinus officinalis leaves (RVs) and Coffea arabica powder (CVs), isolated using a patented method. A multidisciplinary and multi-omic approach was employed to characterize their physico-chemical properties, metabolic and lipid profiles, and in vitro biological activities using fibroblasts (BJ-T5A) and myotubes (C2C12). RVs yield showed a higher vesicles concentration, with 1.37 × 1012 nanovesicle/mL, compared to 1.74 × 1010 nanovesicles/mL for CVs. RVs were found to be rich in diterpenes, flavonoids, and free fatty acids, while CVs contained chlorogenic and phenolic acids with higher lipid diversity, mainly diacylglycerols. Both RVs and CVs exhibited a defined morphology and showed strong antioxidant activity, reducing reactive oxygen species (ROS) and malondialdehyde (MDA) production in both cell models. Additionally, they enhanced collagen production and secretion in fibroblasts and positively modulated molecular targets related to fatty acid synthesis and glucose transport in myotubes. These findings support the potential of PDVs as natural delivery systems with beneficial properties in muscle health and tissue function.
Similar content being viewed by others
Data availability
Data will be available on specific request to the authors.
References
Borrás-Linares, I. et al. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 15, 20585–20606 (2014).
Vignoli, J. A., Viegas, M. C., Bassoli, D. G. & Benassi, M. deT. Roasting process affects differently the bioactive compounds and the antioxidant activity of arabica and robusta coffees. Food Res. Int. 61, 279–285 (2014).
Saeed, M. et al. Potential nutraceutical and food additive properties and risks of coffee: a comprehensive overview. Crit. Rev. Food Sci. Nutr. 59, 3293–3319 (2019).
Alu’datt, M. H. et al. Pharmaceutical, nutraceutical and therapeutic properties of selected wild medicinal plants: thyme, spearmint, and rosemary. In Therapeutic, Probiotic, and Unconventional Foods. 275–290 (Elsevier, 2018).
Blanco-Llamero, C. et al. Bioactives in nutricosmetics: a focus on caffeine from tea to coffee. Cosmetics 11, 149 (2024).
Mena, P. et al. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 21, 1576 (2016).
Senanayake, S. P. J. N. Rosemary extract as a natural source of bioactive compounds. J. Food Bioactives 2, 51–57 (2018).
Nicoli, M. C., Anese, M., Manzocco, L. & Lerici, C. R. Antioxidant properties of coffee brews in relation to the roasting degree. LWT Food Sci. Technol. 30, 292–297 (1997).
Yashin, A., Yashin, Y., Wang, J. & Nemzer, B. Antioxidant and antiradical activity of coffee. Antioxidants 2, 230–245 (2013).
del Castillo, M. D., Ames, J. M. & Gordon, M. H. Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food Chem. 50, 3698–3703 (2002).
Farah, A., De Paulis, T., Moreira, D. P., Trugo, L. C. & Martin, P. R. Chlorogenic acids and lactones in regular and water-decaffeinated arabica coffees. J. Agric. Food Chem. 54, 374–381 (2006).
Mulinacci, N. et al. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: an HPLC/DAD/MS study. Talanta 85, 167–176 (2011).
Markom, M., Hasan, M., Daud, W. R. W., Singh, H. & Jahim, J. M. Extraction of hydrolysable tannins from Phyllanthus niruri Linn.: effects of solvents and extraction methods. Sep. Purif. Technol. 52, 487–496 (2007).
Cortazar, E. et al. Optimisation of microwave-assisted extraction for the determination of nonylphenols and phthalate esters in sediment samples and comparison with pressurised solvent extraction. Anal. Chim. Acta 534, 247–254 (2005).
Ivanović, M., Islamčević Razboršek, M. & Kolar, M. Innovative extraction techniques using deep eutectic solvents and analytical methods for the isolation and characterization of natural bioactive compounds from plant material. Plants 9, 1428 (2020).
Bhadange, Y. A., Carpenter, J. & Saharan, V. K. A comprehensive review on advanced extraction techniques for retrieving bioactive components from natural sources. ACS Omega 9, 31274–31297 (2024).
Armendáriz-Barragán, B. et al. Plant extracts: from encapsulation to application. Expert Opin. Drug Deliv. 13, 1165–1175 (2016).
Wang, B. et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol. Therapy 22, 522–534 (2014).
Raimondo, S. et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget 6, 19514-27 (2015).
Yang, M., Liu, X., Luo, Q., Xu, L. & Chen, F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J. Nanobiotechnol. 18, 100 (2020).
Cai, Y., Zhang, L., Zhang, Y. & Lu, R. Plant-derived exosomes as a drug-delivery approach for the treatment of inflammatory bowel disease and colitis-associated cancer. Pharmaceutics 14, 822 (2022).
Raimondo, S. et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK NF-κB signalling pathways. J. Cell Mol. Med. 26, 4195–4209 (2022).
Yazdanpanah, S. et al. Plant-derived exosomes: carriers and cargo of natural bioactive compounds: emerging functions and applications in human health. Nanomaterials 15, 1005 (2025).
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 23, 7 (2018).
Böttcher, N., Suhr, F., Pufe, T., Wruck, C. J. & Fragoulis, A. Luteolin induces Nrf2 activity in C2C12 cells: implications for muscle health. Int J. Mol. Sci. 26, 4092 (2025).
Mendias, C. L. Fibroblasts take the centre stage in human skeletal muscle regeneration. J. Physiol. 595, 5005 (2017).
Sharma, K. D., Heberle, F. A. & Waxham, M. N. Visualizing lipid membrane structure with cryo-EM: past, present, and future. Emerg. Top. Life Sci. 7, 55 (2023).
Shi, D. & Huang, R. Analysis and comparison of electron radiation damage assessments in Cryo-EM by single particle analysis and micro-crystal electron diffraction. Front. Mol. Biosci. 9, 988928 (2022).
Garcia, D. & Shaw, R. J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell 66, 789–800 (2017).
Mackey, A. L., Magnan, M., Chazaud, B. & Kjaer, M. Human skeletal muscle fibroblasts stimulate in vitro myogenesis and in vivo muscle regeneration. J. Physiol. 595, 5115–5127 (2017).
Sephel, G. C. & Davidson, J. M. Elastin production in human skin fibroblast cultures and its decline with age. J. Invest. Dermatol. 86, 279–285 (1986).
Baldini, N. et al. Exosome-like nanovesicles isolated from citrus limon L. exert antioxidative effect. Curr. Pharm. Biotechnol. 19, 877–885 (2018).
Berger, E. et al. Use of nanovesicles from orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Mol. Ther. Methods Clin. Dev. 18, 880–892 (2020).
Zhuang, X. et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J. Extracell Vesicles 4, 28713 (2015).
Sánchez-López, C. M., Manzaneque-López, M. C., Pérez-Bermúdez, P., Soler, C. & Marcilla, A. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 13, 12870–12882 (2022).
Liu, H. et al. Construction of curcumin-fortified juices using their self-derived extracellular vesicles as natural delivery systems: grape, tomato, and orange juices. Food Funct. 14, 9364–9376 (2023).
Kilasoniya, A. et al. Potential of plant exosome vesicles from grapefruit (citrus × paradisi) and tomato (Solanum lycopersicum) juices as functional ingredients and targeted drug delivery vehicles. Antioxidants 12, 943 (2023).
Arslan, D. & Musa Özcan, M. Evaluation of drying methods with respect to drying kinetics, mineral content and colour characteristics of rosemary leaves. Energy Convers. Manag. 49, 1258–1264 (2008).
Fisher, G. J. et al. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 174, 101–114 (2009).
Martins, S. G., Zilhão, R., Thorsteinsdóttir, S. & Carlos, A. R. Linking oxidative stress and DNA damage to changes in the expression of extracellular matrix components. Front. Genet. 29, 12 (2021).
Lian, D., Chen, M. M., Wu, H., Deng, S. & Hu, X. The role of oxidative stress in skeletal muscle myogenesis and muscle disease. Antioxidants 11, 755 (2022).
Habtemariam, S. Anti-inflammatory therapeutic mechanisms of natural products: insight from rosemary diterpenes, carnosic acid and carnosol. Biomedicines 11, 545 (2023).
Satoh, T., Trudler, D., Oh, C. K. & Lipton, S. A. Potential therapeutic use of the rosemary diterpene carnosic acid for Alzheimer’s disease, Parkinson’s disease, and long-COVID through NRF2 activation to counteract the NLRP3 inflammasome. Antioxidants 11, 124 (2022).
Qiao, S. et al. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 39, 995–1003 (2005).
Ai, X. Y. et al. Apigenin inhibits colonic inflammation and tumorigenesis by suppressing STAT3-NF-κB signaling. Oncotarget 8, 100216–100226 (2017).
Duarte, S. et al. Apigenin protects endothelial cells from lipopolysaccharide (LPS)-induced inflammation by decreasing caspase-3 activation and modulating mitochondrial function. Int J. Mol. Sci. 14, 17664–17679 (2013).
Han, X., Wu, X., Liu, F., Chen, H. & Hou, H. Inhibition of LPS-induced inflammatory response in RAW264.7 cells by natural Chlorogenic acid isomers involved with AKR1B1 inhibition. Bioorg. Med. Chem. 114, 117942 (2024).
Kim, S. H. et al. Chlorogenic acid suppresses lipopolysaccharide-induced nitric oxide and interleukin-1β expression by inhibiting JAK2/STAT3 activation in RAW264.7 cells. Mol. Med. Rep. 16, 9224–9232 (2017).
Huang, Q., Shan, Q., Ma, F., Li, S. & Sun, P. Chlorogenic acid mitigates heat stress-induced oxidative damage in bovine mammary epithelial cells by inhibiting NF-κB-mediated NLRP3 inflammasome activation via upregulating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 301, 140133 (2025).
Petersen, M. et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 70, 1663–1679 (2009).
Lee, J. & Kim, G. Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 22, 75 (2010).
Sugahara, Y. et al. Anti-skin-aging effects of human ceramides via collagen and fibrillin expression in dermal fibroblasts. Biosci. Biotechnol. Biochem. 86, 1240–1246 (2022).
Casati, S. et al. Lipidomics of cell secretome combined with the study of selected bioactive lipids in an in vitro model of osteoarthritis. Stem Cells Transl. Med. 11, 959–970 (2022).
Li, Y. et al. Age-associated increase in skin fibroblast–derived prostaglandin E2 contributes to reduced collagen levels in elderly human skin. J. Invest. Dermatol. 135, 2181–2188 (2015). Sep.
Subbaramaiah, K., Chung, W. J. & Dannenberg, A. J. Ceramide regulates the transcription of cyclooxygenase-2. J. Biol. Chem. 273, 32943–32949 (1998).
Merrill, G. F., Kurth, E. J., Hardie, D. G. & Winder, W. W. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am. J. Physiol. Endocrinol. Metab. 273, E1107-12 (1997).
Holmes, B. F., Kurth-Kraczek, E. J. & Winder, W. W. Chronic activation of 5’-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle. J. Appl. Physiol. 87, 1990-5 (1999).
Abe, D., Saito, T. & Nogata, Y. Rosmarinic acid regulates fatty acid and glucose utilization by activating the CaMKK/AMPK pathway in C2C12 myotubes. Food Sci. Technol. Res. 22, 779–785 (2016).
Naimi, M., Vlavcheski, F., Murphy, B., Hudlicky, T. & Tsiani, E. Carnosic acid as a component of rosemary extract stimulates skeletal muscle cell glucose uptake via AMPK activation. Clin. Exp. Pharm. Physiol. 44, 94–102 (2017).
Eid, H. M., Thong, F., Nachar, A. & Haddad, P. S. Caffeic acid methyl and ethyl esters exert potential antidiabetic effects on glucose and lipid metabolism in cultured murine insulin-sensitive cells through mechanisms implicating activation of AMPK. Pharm. Biol. 55, 2026–2034 (2017).
Piazzon, A. et al. Antioxidant activity of phenolic acids and their metabolites: synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 60, 12312–12323 (2012).
Prabhakar, P. K. & Doble, M. Synergistic effect of phytochemicals in combination with hypoglycemic drugs on glucose uptake in myotubes. Phytomedicine 16, 1119–1126 (2009).
Singdam, P. et al. The mechanisms of neochlorogenic acid (3-caffeoylquinic acid) in improving glucose and lipid metabolism in rats with insulin resistance induced by a high fat-high fructose diet. Trends Sci. 20, 6455 (2023).
Foukas, L. C. et al. Direct effects of caffeine and theophylline on p110δ and other phosphoinositide 3-kinases. J. Biol. Chem. 277, 37124–37130 (2002).
Bollati, C., Fanzaga, M., d’Adduzio, L. & Lammi, C. Molecular and human in vivo study of an innovative plant-derived multifunctional peptide signaling the collagen and elastin pathways and melanin production. Cosmetics 12, 100 (2025).
Bi, J. et al. Regulation of skeletal myogenesis in C2C12 cells through modulation of Pax7, MyoD, and myogenin via different low-frequency electromagnetic field energies. Technol. Health Care 30, 371–382 (2022).
Zanoni, C., Aiello, G., Arnoldi, A. & Lammi, C. Investigations on the hypocholesterolaemic activity of LILPKHSDAD and LTFPGSAED, two peptides from lupin β-conglutin: focus on LDLR and PCSK9 pathways. J. Funct. Foods 32, 1–8 (2017).
Acknowledgements
We acknowledge UNITECH OMICs, mass spectrometry platform of Università degli Studi di Milano, for running mass spectrometry analyses. We are indebted to Carlo Sirtori Foundation (Milan, Italy) for having provided part of equipment used in this experimentation. The method described in this paper, which includes RVs and CVs isolation, purification and stabilization is referenced in the patent WO 2024/223549 A1 (Method for the production of plant-derived nanovesicles and their applications) and licensed to Plantech srl (Italy).
Author information
Authors and Affiliations
Contributions
Conceptualization C.L.; methodology L.d’A., G.A., U.M., G.F., G.A., M.F., C.B.; formal analysis L.d’A., G.A., M.F., C.B., M.S.M; resources C.L.; data curation: L.d’A., G.A., M.F., C.B.; supervision: C.L. writing L.d’A., G.A., C.L.—original draft preparation and review. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
Patent WO 2024/223549 A1 (‘Method for the production of plant-derived nanovesicles and their applications’), which is directly related to the content of this publication, is owned by Università degli Studi di Milano. The patent has been licensed to Plantech s.r.l., a start-up of which C.L. is a scientific director and co-founder.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
d’Adduzio, L., Aiello, G., Musazzi, U. et al. From rosemary and coffee to bioactive nanovesicles: exploring new frontiers in food functional ingredients. npj Sci Food (2026). https://doi.org/10.1038/s41538-026-00723-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41538-026-00723-9