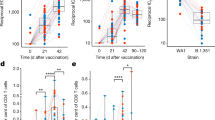
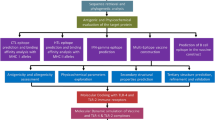
Abstract
Type I interferons (IFN) are key mediators of innate immune activation, promoting upregulation of costimulatory molecules and Major Histocompatibility Complex (MHC) I/II on antigen-presenting cells (APCs). However, IFN also suppress endogenous translation to restrict viral replication. Critically, IFN-stimulated APCs lose the capacity to acquire new antigens, making the timing of IFN signaling a crucial determinant of vaccine efficacy. Here, we show that both DC-specific loss of IFNα/β receptor (IFNαR) and transient blockade of IFNαR before vaccination enhances vaccine uptake and expression within DCs, improves CD8⁺ T cell priming, and leads to superior tumor control. We also demonstrate that IFN signaling before vaccination, triggered by prior infection or administration of a different vaccine, impairs dendritic cell uptake of mRNA-LNP vaccines and reduces the magnitude of vaccine-specific CD8⁺ T cell responses. These findings highlight the dual-edged nature of IFN signaling and offer a potential strategy for enhancing vaccine-induced immunity.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Arevalo, C. P. et al. A multivalent nucleoside-modified mRNA vaccine against all known influenza virus subtypes. Science 378, 899–904 (2022).
Xie, Z. et al. mRNA-LNP HIV-1 trimer boosters elicit precursors to broad neutralizing antibodies. Science 384, eadk0582 (2024).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Rojas, L. A. et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 618, 144–150 (2023).
Liu, C. et al. mRNA-based cancer therapeutics. Nat. Rev. Cancer 23, 526–543 (2023).
Kranz, L. M. et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401 (2016).
Sethna, Z. et al. RNA neoantigen vaccines prime long-lived CD8+ T cells in pancreatic cancer. Nature 639, 1042–1051 (2025).
Sittplangkoon, C. et al. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 13, 983000 (2022).
Arunachalam, P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature 596, 410–416 (2021).
Li, C. et al. Mechanisms of innate and adaptive immunity to the Pfizer-BioNTech BNT162b2 vaccine. Nat. Immunol. 23, 543–555 (2022).
Kim, S. et al. Innate immune responses against mRNA vaccine promote cellular immunity through IFN-β at the injection site. Nat. Commun. 15, 7226 (2024).
McNab, F., Mayer-Barber, K., Sher, A., Wack, A. & O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 15, 87–103 (2015).
Kolumam, G. A., Thomas, S., Thompson, L. J., Sprent, J. & Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 202, 637–650 (2005).
Sikora, A. G. et al. IFN-α enhances peptide vaccine-induced CD8+ T cell numbers, effector function, and antitumor activity. J. Immunol. 182, 7398–7407 (2009).
Ngoi, S. M., Tovey, M. G. & Vella, A. T. Targeting Poly I:C to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFNα/β. J. Immunol. 181, 7670–7680 (2008).
Hall, J. C. & Rosen, A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat. Rev. Rheumatol. 6, 40–49 (2010).
Su, A. I. et al. Genomic analysis of the host response to hepatitis C virus infection. Proc. Natl. Acad. Sci. USA 99, 15669–15674 (2002).
O’Garra, A. et al. The immune response in tuberculosis. Annu. Rev. Immunol. 31, 475–527 (2013).
McNab, F. W. et al. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J. Immunol. 191, 1732–1743 (2013).
Berry, M. P. R. et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466, 973–977 (2010).
Sallusto, F., Cella, M., Danieli, C. & Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 (1995).
Wilson, N. S. et al. Systemic activation of dendritic cells by Toll-like receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat. Immunol. 7, 165–172 (2006).
Theisen, D. J. et al. Batf3-dependent genes control tumor rejection induced by dendritic cells independently of cross-presentation. Cancer Immunol. Res. 7, 29–39 (2019).
Sheehan, K. C. F. et al. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J. Interferon Cytokine Res. 26, 804–819 (2006).
Palacio, N. et al. Early type I IFN blockade improves the efficacy of viral vaccines. J. Exp. Med. 217, e20191220 (2020).
Bullock, C. B. et al. Type I interferon signaling in dendritic cells limits direct antigen presentation and CD8+ T cell responses against an arthritogenic alphavirus. mBio. 15, e02930-24 (2024).
Broomfield, B. J. et al. Transient inhibition of type I interferon enhances CD8+ T cell stemness and vaccine protection. J. Exp. Med 222, e20241148 (2025).
Ou, F. et al. Enhanced in vitro type 1 conventional dendritic cell generation via the recruitment of hematopoietic stem cells and early progenitors by Kit ligand. Eur. J. Immunol. 53, e2250201 (2023).
Ryman, K. D. et al. Sindbis virus translation is inhibited by a PKR/RNase L-independent effector induced by alpha/beta interferon priming of dendritic cells. J. Virol. 79, 1487–1499 (2005).
Ahn, E. et al. Role of PD-1 during effector CD8 T cell differentiation. Proc. Natl. Acad. Sci. USA 115, 4749–4754 (2018).
Zahm, C. D., Colluru, V. T. & McNeel, D. G. Vaccination with high-affinity epitopes impairs antitumor efficacy by increasing PD-1 expression on CD8+ T cells. Cancer Immunol. Res. 5, 630–641 (2017).
Zheng, L., Bandara, S. R., Tan, Z. & Leal, C. Lipid nanoparticle topology regulates endosomal escape and delivery of RNA to the cytoplasm. Proc. Natl. Acad. Sci. USA 120, e2301067120 (2023).
Caton, M. L., Smith-Raska, M. R. & Reizis, B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J. Exp. Med. 204, 1653–1664 (2007).
Prigge, J. R. et al. Type-I-IFNs act upon hematopoietic progenitors to protect and maintain hematopoiesis during Pneumocystis lung infection in mice. J. Immunol. 195, 5347–5357 (2015).
Durai, V. et al. Cryptic activation of an Irf8 enhancer governs cDC1 fate specification. Nat. Immunol. 20, 1161–1173 (2019).
Liu, T.-T. et al. Ablation of cDC2 development by triple mutations within the Zeb2 enhancer. Nature https://doi.org/10.1038/s41586-022-04866-z (2022).
Shresta, S., Sharar, K. L., Prigozhin, D. M., Beatty, P. R. & Harris, E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 80, 10208–10217 (2006).
Chen, H.-W. et al. The roles of IRF-3 and IRF-7 in innate antiviral immunity against dengue virus. J. Immunol. 191, 4194–4201 (2013).
Chen, H.-C., Hofman, F. M., Kung, J. T., Lin, Y.-D. & Wu-Hsieh, B. A. Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J. Virol. 81, 5518–5526 (2007).
Ferris, S. T. et al. cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature https://doi.org/10.1038/s41586-020-2611-3 (2020).
Yan, N. & Chen, Z. J. Intrinsic antiviral immunity. Nat. Immunol. 13, 214–222 (2012).
Busselaar, J., Sijbranda, M. & Borst, J. The importance of type I interferon in orchestrating the cytotoxic T-cell response to cancer. Immunol. Lett. 270, 106938 (2024).
Orozco, S. et al. Characterization of a model of lethal dengue virus 2 infection in C57BL/6 mice deficient in the alpha/beta interferon receptor. J. Gen. Virol. 93, 2152–2157 (2012).
Acknowledgements
Stephen T. Ferris is supported by the President’s Research Fund of Saint Louis University School of Medicine (S.F.). Elise Alspach, PhD, is supported by a Research Scholar Grant, RSG-24-1251974-01-IBCD, from the American Cancer Society (https://doi.org/10.53354/ACS.RSG-24-1251974-01-IBCD.pc.gr.193734), and is the recipient of a Cancer Research Institute CLIP Grant (CRI5509). We thank the NIH Tetramer Core Facility (NIH Contract 75N93020D00005 and RRID:SCR_026557) for providing H2-Kb chicken ova 257-264 SIINFEKL Monomer.
Author information
Authors and Affiliations
Contributions
Conceptualization: S.T.F., T.A.L., and A.D.; Methodology: S.T.F., T.A.L., E.A., and L.V.T.; Investigation: T.A.L., S.T.F., S.B., J.A.C., Y.D., and W.G.; Visualization: T.A.L. and S.T.F.; Funding acquisition: S.T.F.; Project administration: S.T.F. and R.J.D.; Supervision: S.T.F.; Writing—original draft: T.A.L. and S.T.F.; Writing—review & editing: T.A.L., S.T.F., E.A., L.V.T., and W.G.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lobb, T.A., Dickson, A., Guo, W. et al. Type I interferon restricts mRNA vaccine efficacy through suppression of antigen uptake in cDCs. npj Vaccines (2026). https://doi.org/10.1038/s41541-025-01362-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-025-01362-z