Abstract
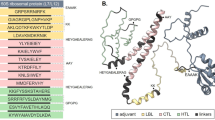
The African Swine Fever Virus (ASFV) poses a major threat to global livestock production by infecting both domestic and wild pigs, causing significant economic loss. Despite promising protective results observed with live attenuated viruses, the safety concern blocked its extensive application. In this study, we developed a novel vaccine combining two recombinant vaccinia viruses-rTTV-D-A and rTTV-K-J-that together express eight ASFV genes, including EP402R (CD2v), B646L (p72), B602L (pB602L), D117L (p17), H240R (pH240R), B438L (p49), E183L (p54), CP204L (p30), and a synthetic T antigen composed of conserved T cell epitopes from multiple ASFV proteins, aiming to induce both humoral and T-cell immune responses against different viral antigens. After demonstrating that this vaccine induced antigen-specific humoral and cellular responses in both mice and swine, its protective efficacy in swine was examined using a lethal challenge model. The vaccinated pigs showed a promising protection against the lethal challenge of a virulent genotype II ASFV strain (100 HAD50/pig), with 4 out of 6 surviving, while all control animals succumbed from 9 to 15 days post challenge. Importantly, the protection was further evidenced by the recovery to normal temperature and no ASFV infection-related clinical signs or virus shedding in surviving pigs over a 21-day observation period. Our results support the potential of rTTV-D-A and rTTV-K-J as a novel multi-immunogen vaccinia-vectored ASFV vaccine. Further studies are warranted to explore and improve its use as a standalone vaccine or in combination with other vaccine platforms to achieve broad and effective protection against ASFV.
Similar content being viewed by others
Data availability
The DNA sequence of the MTCE used in the rTTV-D-A has been deposited in the DNA DataBank of Japan under accession number LC897646.
References
Blome, S., Franzke, K. & Beer, M. African swine fever–A review of current knowledge. Virus Res. 287, 198099 (2020).
Kleiboeker, S. B. & Scoles, G. A. Pathogenesis of African swine fever virus in Ornithodoros ticks. Anim. Health Res. Rev. 2, 121–128 (2001).
Pereira De Oliveira, R. et al. Differential vector competence of Ornithodoros soft ticks for African swine fever virus: what if it involves more than just crossing organic barriers in ticks? Parasites Vectors 13, 1–15 (2020).
Li, Z. et al. African swine fever virus: a review. Life 12, 1255 (2022).
Ruedas-Torres, I., Thi to Nga, B. & Salguero, F. J. Pathogenicity and virulence of African swine fever virus. Virulence 15, 2375550 (2024).
Sánchez-Vizcaíno, J., Mur, L., Gomez-Villamandos, J. & Carrasco, L. An update on the epidemiology and pathology of African swine fever. J. Comp. Pathol. 152, 9–21 (2015).
Zhang, H. et al. Vaccines for African swine fever: an update. Front. Microbiol. 14, 1139494 (2023).
Dixon, L. K., Chapman, D. A., Netherton, C. L. & Upton, C. African swine fever virus replication and genomics. Virus Res. 173, 3–14 (2013).
Bastos, A. D. et al. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 148, 693–706 (2003).
Goatley, L. C. et al. African swine fever virus NAM P1/95 is a mixture of genotype I and genotype VIII viruses. Microbiol. Resour. Announc. 13, e00067–00024 (2024).
Njau, E. P. et al. African swine fever virus (ASFV): biology, genomics and genotypes circulating in sub-Saharan Africa. Viruses 13, 2285 (2021).
Ata, E. B. et al. African swine fever virus: a raised global upsurge and a continuous threaten to pig husbandry. Microb. Pathog. 167, 105561 (2022).
Zhao, D. et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 14, 3096 (2023).
Lee, K. et al. Molecular characterization of emerging recombinant African swine fever virus of genotype I and II in Vietnam, 2023. Emerg. Microbes Infect. 13, 2404156 (2024).
Igolkin, A. et al. Detection of the first recombinant African swine fever virus (genotypes I and II) in domestic pigs in Russia. Mol. Biol. Rep. 51, 1–9 (2024).
Vu, H. L. & McVey, D. S. Recent progress on gene-deleted live-attenuated African swine fever virus vaccines. npj Vaccines 9, 60 (2024).
van den Born, E. et al. African swine fever virus vaccine strain Asfv-G-∆ I177l reverts to virulence and negatively affects reproductive performance. npj Vaccines 10, 46 (2025).
Nguyen, T. C. et al. An African swine fever vaccine-like variant with multiple gene deletions caused reproductive failure in a Vietnamese breeding herd. Sci. Rep. 15, 14919 (2025).
Zhang, Y. et al. ASFV Subunit vaccines: strategies and prospects for future development. Microb. Pathog. 197, 107063 (2024).
Deng, Y. et al. Recombinant vaccinia vector-based vaccine (Tiantan) boosting a novel HBV subunit vaccine induced more robust and lasting immunity in rhesus macaques. Vaccine 35, 3347–3353 (2017).
Zhang, X. et al. TianTan vaccinia virus-based EBV vaccines targeting both latent and lytic antigens elicits potent immunity against lethal EBV challenge in humanized mice. Emerg. Microbes Infect. 13, 2412640 (2024).
Onisk, D. et al. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 198, 350–354 (1994).
Oura, C. A., Denyer, M., Takamatsu, H. & Parkhouse, R. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 86, 2445–2450 (2005).
Alejo, A., Matamoros, T., Guerra, M. & Andrés, G. A proteomic atlas of the African swine fever virus particle. J. Virol. 92, 01293–01218 (2018).
Reis, A. L. et al. From structure prediction to function: defining the domain on the African swine fever virus CD2v protein required for binding to erythrocytes. mBio 16, e01655–01624 (2024).
Ruiz-Gonzalvo, F., Rodríguez, F. & Escribano, J. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology 218, 285–289 (1996).
Liu, S. et al. Cryo-EM structure of the African swine fever virus. Cell Host microbe 26, 836–843.e833 (2019).
Cobbold, C., Windsor, M. & Wileman, T. A virally encoded chaperone specialized for folding of the major capsid protein of African swine fever virus. J. Virol. 75, 7221–7229 (2001).
Wang, N. et al. Architecture of African swine fever virus and implications for viral assembly. Science 366, 640–644 (2019).
Gomez-Puertas, P. et al. Neutralizing antibodies to different proteins of African swine fever virus inhibit both virus attachment and internalization. J. Virol. 70, 5689–5694 (1996).
Gómez-Puertas, P. et al. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 243, 461–471 (1998).
Barderas, M. et al. Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch. Virol. 146, 1681–1691 (2001).
Ding, M. et al. Sequential deletions of interferon inhibitors MGF110-9L and MGF505-7R result in sterile immunity against the Eurasia strain of Africa swine fever. J. Virol. 96, e01192–01122 (2022).
Tai, W. et al. An mRNA vaccine against monkeypox virus inhibits infection by co-activation of humoral and cellular immune responses. Nat. Commun. 16, 2971 (2025).
Li, Y. et al. A multivalent mRNA vaccine elicits robust immune responses and confers protection in a murine model of monkeypox virus infection. Nat. Commun. 16, 7373 (2025).
Argilaguet, J. M. et al. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antivir. Res. 98, 61–65 (2013).
Argilaguet, J. M. et al. DNA vaccination partially protects against African swine fever virus lethal challenge in the absence of antibodies. PLoS ONE 7, e40942 (2012).
Lacasta, A. et al. Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in viral pathogenesis and immune protection. Vet. Res. 46, 1–16 (2015).
Petrovan, V. et al. Role of African swine fever virus proteins EP153R and EP402R in reducing viral persistence in blood and virulence in pigs infected with BeninΔDP148R. J. Virol. 96, e01340–01321 (2022).
Goatley, L. C. et al. A pool of eight virally vectored African swine fever antigens protect pigs against fatal disease. Vaccines 8, 234 (2020).
Portugal, R. et al. Six adenoviral vectored African swine fever virus genes protect against fatal disease caused by genotype I challenge. J. Virol. 98, e00622–e00624 (2024).
Liu, W. et al. A new vaccination regimen using adenovirus-vectored vaccine confers effective protection against African swine fever virus in swine. Emerg. Microbes Infect. 12, 2233643. https://doi.org/10.1080/22221751.2023.2233643 (2023).
Sun, E. et al. Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci. China Life Sci. 64, 752–765 (2021).
Ge, S. et al. Molecular characterization of African swine fever virus, China, 2018. Emerg. Infect. Dis. 24, 2131 (2018).
Zajac, M. D. et al. Immunization of pigs with replication-incompetent adenovirus-vectored African swine fever virus multi-antigens induced humoral immune responses but no protection following contact challenge. Front. Vet. Sci. 10, 1208275 (2023).
Qin, L., Liang, M. & Evans, D. H. Genomic analysis of vaccinia virus strain TianTan provides new insights into the evolution and evolutionary relationships between Orthopoxviruses. Virology 442, 59–66 (2013).
Li, M. et al. Long-lasting humoral and cellular memory immunity to vaccinia virus Tiantan provides pre-existing immunity against mpox virus in Chinese population. Cell Rep. 43, 113609 (2024).
Drew, D. R., Lightowlers, M. & Strugnell, R. A. Humoral immune responses to DNA vaccines expressing secreted, membrane bound and non-secreted forms of the: Taenia ovis 45W antigen. Vaccine 18, 2522–2532 (2000).
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 19, 329–336 (2013).
Gaudreault, N. N., Madden, D. W., Wilson, W. C., Trujillo, J. D. & Richt, J. A. African swine fever virus: an emerging DNA arbovirus. Front. Vet. Sci. 7, 215 (2020).
Borca, M. V. et al. ASFV-G-∆I177L as an effective oral nasal vaccine against the Eurasia strain of Africa swine fever. Viruses 13, https://doi.org/10.3390/v13050765 (2021).
Bourry, O. et al. Oronasal or intramuscular immunization with a thermo-attenuated ASFV strain provides full clinical protection against Georgia 2007/1 challenge. Viruses 14, 2777 (2022).
Vlasov, M. et al. Administration routes and doses of the attenuated African swine fever virus strain PSA-1NH influence cross-protection of pigs against heterologous challenge. Animals 14, 1277 (2024).
Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol. 40, 840–854 (2022).
Yuan, F. et al. Selection, Design, and Immunogenicity Studies of ASFV Antigens for Subunit mRNA Cocktail Vaccines with Specific Immune Response Profiles. ACS Infectious Diseases 11, 1907–1921 (2025).
Hu, X. et al. Development and efficacy of a novel mRNA cocktail for the delivery of African swine fever virus antigens and induction of immune responses. Microbiol. Spectr. 13, e02909–e02924 (2025).
Tian, C. et al. Immunogenicity and efficacy of an LNP-mRNA prepared from African swine fever virus K205R1. J. Integr. Agric 24, 4026–40333 (2024).
Diep, N. V. et al. Genotype II live-attenuated ASFV vaccine strains unable to completely protect pigs against the emerging recombinant ASFV genotype I/II strain in Vietnam. Vaccines 12, 1114 (2024).
Xie, X. et al. Influenza vaccine with consensus internal antigens as immunogens provides cross-group protection against influenza A viruses. Front. Microbiol. 10, 1630 (2019).
King, D. P. et al. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 107, 53–61 (2003).
Zhao, D. et al. Replication and virulence in pigs of the first African swine fever virus isolated in China. Emerg. Microbes Infect. 8, 438–447 (2019).
Cardiff, R. D., Miller, C. H. & Munn, R. J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, prot073411 (2014).
Acknowledgements
This work was funded by National Key Research and Development Program of China (2024YFC2311100) and Prevention and Control of Emerging and Major Infectious Diseases-National Science and Technology Major Project (2025ZD01904400). The authors also acknowledge a donation from ShangFuShaFei (Shanghai) Biotechnology Co., Ltd. that supported the challenge experiments.
Author information
Authors and Affiliations
Contributions
J.X. conceptualized and supervised the research; J.X., X.Z., C.Z., D.Z., K.C., and L.D. designed the study and reviewed all data; L.D., N.G., R.L., A.X., T.Y., H.P., C.Z., and Z.Z. performed experiments; L.D., N.G., and R.L. collected and analyzed the data; L.D. drafted the manuscript; J.X., X.Z., and C.Z. revised the manuscript. L.D., N.G., and R.L. are co-first authors of this manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
Patent applications related to this work have been filed in China (application number: 202411253959.6) and internationally under the Patent Cooperation Treaty (application number: PCT/CN2024/117421), with J.X., X.Z., and L.D. listed as inventors. All other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dong, L., Gao, N., Liu, R. et al. Recombinant vaccinia vectored ASFV vaccine enhances swine survival against genotype II challenge. npj Vaccines (2026). https://doi.org/10.1038/s41541-026-01377-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-026-01377-0