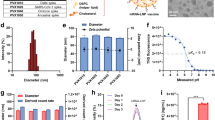
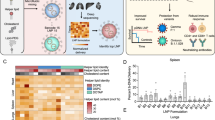
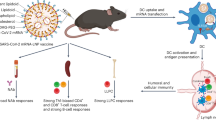
Abstract
mRNA-lipid nanoparticle (LNP) vaccines induce robust adaptive immune responses and have proven highly effective against SARS-CoV-2. However, their long-term effectiveness is limited by waning humoral responses, which decline substantially within the first six months post-boost vaccination. DNA-LNPs are being investigated as an alternative vaccine platform, offering prolonged antigen expression and robust immunity. Here, we compare DNA- and mRNA-LNP vaccines encoding CD40L-adjuvanted SARS-CoV-2 XBB.1.5 Spike (SXBB.1.5-CD40L) in a long-term in vivo challenge model. Both nucleic acid vaccines induced strong neutralizing antibody responses and conferred equivalent protection in Syrian hamsters challenged three weeks post-boost. Notably, DNA-LNP vaccination maintained high binding and neutralizing antibody titers six months post-boost, whereas mRNA-LNPs exhibited a marked decline. Correspondingly, while SXBB.1.5-CD40L DNA-LNP vaccination completely protected from weight loss, viral replication, and lung pathology at this late timepoint, SXBB.1.5-CD40L mRNA-LNP vaccination conferred minimal protection. These findings demonstrate that DNA-LNPs can sustain durable immunity, highlighting their potential as a next-generation vaccine platform that could reduce the need for frequent boosters.
Similar content being viewed by others
Data availability
All data supporting the conclusions of this study are present in the main text and supplementary materials. Additional information is available from the corresponding authors upon request.
References
Lasrado, N. et al. Waning immunity and IgG4 responses following bivalent mRNA boosting. Sci. Adv. 10, 9945 (2024).
Föhse, K. et al. The impact of BNT162b2 mRNA vaccine on adaptive and innate immune responses. Clin. Immunol. 255, 109762 (2023).
Ishii, T. et al. Waning cellular immune responses and predictive factors in maintaining cellular immunity against SARS-CoV-2 six months after BNT162b2 mRNA vaccination. Sci. Rep. 13, 1–12 (2023).
Haq, M. A. et al. Antibody longevity and waning following COVID-19 vaccination in a 1-year longitudinal cohort in Bangladesh. Sci. Rep. 14, 1–11 (2024).
Tong, X. et al. Waning and boosting of antibody Fc-effector functions upon SARS-CoV-2 vaccination. Nat. Commun. 14, 1–15 (2023). 2023 14:1.
Moore, M., Anderson, L., Schiffer, J. T., Matrajt, L. & Dimitrov, D. Durability of COVID-19 vaccine and infection induced immunity: A systematic review and meta-regression analysis. Vaccine 54, 126966 (2025).
Srivastava, K. et al. SARS-CoV-2-infection- and vaccine-induced antibody responses are long lasting with an initial waning phase followed by a stabilization phase. Immunity 57, 587–599.e4 (2024).
Widge, A. T. et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 384, 80–82 (2021).
Doria-Rose, N. et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N. Engl. J. Med. 384, 2259–2261 (2021).
Pegu, A. et al. Durability of mRNA-1273 vaccine–induced antibodies against SARS-CoV-2 variants. Science (1979) 373, 1372–1377 (2021).
Kim, W. et al. Germinal centre-driven maturation of B cell response to mRNA vaccination. Nature 604, 141–145 (2022).
Nguyen, D. C. et al. SARS-CoV-2-specific plasma cells are not durably established in the bone marrow long-lived compartment after mRNA vaccination. Nat. Med. 31, 235–244 (2025).
Florea, A. et al. Effectiveness of messenger RNA-1273 vaccine booster against coronavirus disease 2019 in immunocompetent adults. Clin. Infect. Dis. 76, 252–262 (2023).
Abu-Raddad, L. J. et al. Effect of mRNA vaccine boosters against SARS-CoV-2 omicron infection in Qatar. N. Engl. J. Med. 386, 1804–1816 (2022).
Ackerson, B. K. et al. Effectiveness and durability of mRNA-1273 BA.4/BA.5 bivalent vaccine (mRNA-1273.222) against SARS-CoV-2 BA.4/BA.5 and XBB sublineages. Hum Vaccin. Immunother. 20, 2335052 (2024).
Maslow, J. N. et al. DNA vaccines for epidemic preparedness: SARS-CoV-2 and beyond. Vaccine 11, 1016 (2023).
Blakney, A. K. & Bekker, L. G. DNA vaccines join the fight against COVID-19. Lancet 399, 1281–1282 (2022).
Feliciano, L. - et al. Improved DNA vaccine delivery with needle-free injection systems. Vaccines 11, 280 (2023).
Sabnis, S. et al. A novel amino lipid series for mRNA delivery: Improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 26, 1509–1519 (2018).
Hassett, K. J. et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids 15, 1–11 (2019).
Connors, J. et al. Lipid nanoparticles (LNP) induce activation and maturation of antigen presenting cells in young and aged individuals. Commun. Biol. 6, 1–13 (2023).
Alameh, M. G. et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 54, 2877–2892.e7 (2021).
Pfeifle, A. et al. DNA lipid nanoparticle vaccine targeting outer surface protein C affords protection against homologous Borrelia burgdorferi needle challenge in mice. Front Immunol. 14, 1020134 (2023).
Guimaraes, L. C. et al. Nanoparticle-based DNA vaccine protects against SARS-CoV-2 variants in female preclinical models. Nat. Commun. 15, 1–19 (2024). 2024 15:1.
Liao, H. C. et al. Lipid nanoparticle-encapsulated DNA vaccine robustly induce superior immune responses to the mRNA vaccine in Syrian hamsters. Mol. Ther. Methods Clin. Dev. 32, 101169 (2024).
Tursi, N. J. et al. Modulation of lipid nanoparticle-formulated plasmid DNA drives innate immune activation promoting adaptive immunity. Cell Rep. Med 6, 102035 (2025).
Li, M. et al. Lipid nanoparticles outperform electroporation in delivering therapeutic HPV DNA vaccines. Vaccines (Basel) 12, 666 (2024).
Zhang, W. et al. The expression kinetics and immunogenicity of lipid nanoparticles delivering plasmid DNA and mRNA in mice. Vaccines (Basel) 11, 1580 (2023).
Patel, M. N. et al. Safer non-viral DNA delivery using lipid nanoparticles loaded with endogenous anti-inflammatory lipids. Nat. Biotechnol. 2025 1–11 https://doi.org/10.1038/s41587-025-02556-5 (2025).
F. D. A. Takes Action on Updated mRNA COVID-19 Vaccines to Better Protect Against Currently Circulating Variants | FDA. https://www.fda.gov/news-events/press-announcements/fda-takes-action-updated-mrna-covid-19-vaccines-better-protect-against-currently-circulating.
Tamming, L. A. et al. DNA based vaccine expressing SARS-CoV-2 Spike-CD40L fusion protein confers protection against challenge in a Syrian Hamster Model. Front Immunol 12, 785349 (2022).
Tamming, L. et al. Lipid nanoparticle encapsulation of a Delta spike-CD40L DNA vaccine improves effectiveness against Omicron challenge in Syrian hamsters. Mol. Ther. Methods Clin. Dev. 32, 101325 (2024).
Kaku, Y., Uriu, K., Okumura, K., Ito, J. & Sato, K. Virological characteristics of the SARS-CoV-2 KP.3.1.1 variant. Lancet Infect. Dis. 24, e609 (2024).
Nunes da Silva, W., Dias Moura Prazeres, P. H. & Goulart Guimarães, P. P. PLA-PEG as an alternative to PEGylated lipids for nanoparticle-based DNA vaccination against SARS-CoV-2. Mol. Ther. Nucleic Acids 35, 102293 (2024).
Lai, D. C., Nguyen, T. N., Trinh, G. P., Steffen, D. & Vu, H. L. X. Lipid nanoparticle-encapsulated DNA vaccine induces balanced antibody and T-cell responses in pigs with maternally derived antibodies. J. Virol. https://doi.org/10.1128/JVI.01123-25 (2025).
Lai, D. C., Nguyen, T. N., Poonsuk, K., McVey, D. S. & Vu, H. L. X. Lipid nanoparticle-encapsulated DNA vaccine encoding African swine fever virus p54 antigen elicits robust immune responses in pigs. Vet. Microbiol 305, 110508 (2025).
Mucker, E. M. et al. Lipid nanoparticle formulation increases efficiency of DNA-vectored vaccines/immunoprophylaxis in animals including transchromosomic bovines. Sci. Rep. 10, 8764 (2020).
AM, H. et al. CD40 ligand preferentially modulates immune response and enhances protection against influenza virus. J. Immunol. 193, 722–734 (2014).
Kometani, K. & Kurosaki, T. Differentiation and maintenance of long-lived plasma cells. Curr. Opin. Immunol. 33, 64–69 (2015).
Brook, B. et al. Adjuvantation of a SARS-CoV-2 mRNA vaccine with controlled tissue-specific expression of an mRNA encoding IL-12p70. Sci. Transl. Med. 16, eadm8451 (2024).
Wang, L. et al. IL-7 promotes mRNA vaccine-induced long-term immunity. Immun. J. Nanobiotechnology 22, 716 (2024).
Pardi, N. et al. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Controlled Release 217, 345–351 (2015).
Swingle, K. L. et al. Circular RNA lipid nanoparticle vaccine against SARS-CoV-2. Proc. Natl. Acad. Sci. 122, e2505718122 (2025).
Qu, L. et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185, 1728–1744.e16 (2022).
Wan, J. et al. Circular RNA vaccines with long-term lymph node-targeting delivery stability after lyophilization induce potent and persistent immune responses. mBio 15, e0177523 (2024).
Krawczyk, P. S. et al. Re-adenylation by TENT5A enhances efficacy of SARS-CoV-2 mRNA vaccines. Nature 641, 984–992 (2025).
Robinson, M. J. et al. Long-lived plasma cells accumulate in the bone marrow at a constant rate from early in an immune response. Sci Immunol 7, eabm8389 (2022).
Muecksch, F. et al. Affinity maturation of SARS-CoV-2 neutralizing antibodies confers potency, breadth, and resilience to viral escape mutations. Immunity 54, 1853–1868.e7 (2021).
Colwill, K. et al. A scalable serology solution for profiling humoral immune responses to SARS-CoV-2 infection and vaccination. Clin. Transl. Immunol. 11, e1380 (2022).
Nie, J. et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 15, 3699–3715 (2020).
Reed, L. J. & Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27, 493–497 (1938).
Wölfel, R. et al. Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469 (2020).
Zivcec, M., Safronetz, D., Haddock, E., Feldmann, H. & Ebihara, H. Validation of assays to monitor immune responses in the Syrian golden hamster (Mesocricetus auratus). J. Immunol. Methods 368, 24–35 (2011).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 − ΔΔCT method. Methods 25, 402–408 (2001).
Harris, G., Holbein, B. E., Zhou, H., Howard Xu, H. & Chen, W. Potential mechanisms of mucin-enhanced acinetobacter baumannii virulence in the mouse model of intraperitoneal infection. Infect Immun 87, e00591–19 (2019).
Lien, C. E. et al. CpG-adjuvanted stable prefusion SARS-CoV-2 spike protein protected hamsters from SARS-CoV-2 challenge. Sci. Rep. 2021 11, 1–7 (2021).
Acknowledgements
We gratefully acknowledge the histology and staining services provided by the Louise Pelletier HCF at the University of Ottawa. We gratefully acknowledge the technical contribution of Simon Lord-Dufour, Brian Cass, and Louis Bisson at the NRC-HHT for recombinant spike production. We also would like to acknowledge the assistance provided by the Animal Care Facility staff at Health Canada and the National Research Council of Canada. We thank Dr. Lu Huixin and Dr. Roger Tam for commenting on the manuscript and Greg Beaudoin for preparing the photomicrographs. Schematic representations were created in BioRender. This work is supported by the Government of Canada (intramural funding from Health Canada).
Author information
Authors and Affiliations
Contributions
Conceptualization, L.T., A.T., M.J.W.J., X.L.; Methodology, L.T., C.L., W.Z., G.F., M.S., A.T.; Formal Analysis and Visualization, L.T., A.S.; Investigation, L.T., C.L., W.Z., D.D., J.B., G.F., A.P., S.N.R. J.W., C.G., A.S., W.C.; Resources, C.L., S.N.R., C.G., M.S., Y.D., A.T.; Writing—Original Draft, L.T.; Writing—Review & Editing, L.T., C.L., W.Z., D.D., J.B., G.F., A.P., S.N.R. J.W., C.G., A.S., M.S., Y.D., W.C., L.W., S.S., A.T., M.J.W.J., X.L.; Supervision, Y.D., L.W., S.S., A.T., M.J.W.J., X.L.; Project Administration L.T., A.T., M.J.W.J., X.L.; Funding Acquisition, Y.D., L.W., S.S., A.T., M.J.W.J., X.L.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no Competing Financial or Non-Financial Interests to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tamming, L., Lansdell, C., Zhang, W. et al. Durability of DNA-LNP and mRNA-LNP vaccine-induced immunity against sars-cov-2 xbb.1.5. npj Vaccines (2026). https://doi.org/10.1038/s41541-026-01382-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-026-01382-3