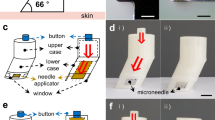
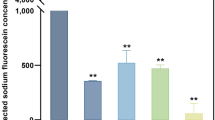
Abstract
The volume of interstitial fluid (ISF) in the human body is three times that of blood. Yet, collecting diagnostically useful ISF is more challenging than collecting blood because the extraction of dermal ISF disrupts the delicate balance of pressure between ISF, blood and lymph, and because the triggered local inflammation further skews the concentrations of many analytes in the extracted fluid. In this Perspective, we overview the most meaningful differences in the make-up of ISF and blood, and discuss why ISF cannot be viewed generally as a diagnostically useful proxy for blood. We also argue that continuous sensing of small-molecule analytes in dermal ISF via rapid assays compatible with nanolitre sample volumes or via miniaturized sensors inserted into the dermis can offer clinically advantageous utility, particularly for the monitoring of therapeutic drugs and of the status of the immune system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tobias, A., Ballard, B. D. & Mohiuddin, S. S. Physiology, Water Balance (StatPearls Publishing LLC, updated 3 October 2022); https://www.ncbi.nlm.nih.gov/books/NBK541059/
Ash, S. R. et al. Subcutaneous capillary filtrate collector for measurement of blood glucose. ASAIO J. 38, M416–M420 (1992).
Gebhart, S. et al. Glucose sensing in transdermal body fluid collected under continuous vacuum pressure via micropores in the stratum corneum. Diabetes Technol. Ther. 5, 159–166 (2003).
Miller, P. R. et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun. Biol. 1, 173 (2018).
Tran, B. Q. et al. Proteomic characterization of dermal interstitial fluid extracted using a novel microneedle-assisted technique. J. Proteome Res. 17, 479–485 (2018).
Taylor, R. M., Miller, P. R., Ebrahimi, P., Polsky, R. & Baca, J. T. Minimally-invasive, microneedle-array extraction of interstitial fluid for comprehensive biomedical applications: transcriptomics, proteomics, metabolomics, exosome research, and biomarker identification. Lab. Anim. 52, 526–530 (2018).
Aukland, K. & Reed, R. K. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol. Rev. 73, 1–78 (1993).
Vermeer, B. J., Reman, F. C. & van Gent, C. M. The determination of lipids and proteins in suction blister fluid. J. Invest. Dermatol. 73, 303–305 (1979).
Rodbard, D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol. Ther. 18 (Suppl. 2), S3–S13 (2016).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
Reed, R. K. & Rubin, K. Transcapillary exchange: role and importance of the interstitial fluid pressure and the extracellular matrix. Cardiovasc. Res. 87, 211–217 (2010).
Madden, J., O’Mahony, C., Thompson, M., O’Riordan, A. & Galvin, P. Biosensing in dermal interstitial fluid using microneedle based electrochemical devices. Sens. Biosens. Res. 29, 100348 (2020).
Kashaninejad, N. et al. Microneedle arrays for sampling and sensing skin interstitial fluid. Chemosensors 9, 83 (2021).
García-Guzmán, J. J., Pérez-Ràfols, C., Cuartero, M. & Crespo, G. A. Microneedle based electrochemical (bio)sensing: towards decentralized and continuous health status monitoring. Trends Anal. Chem. 135, 116148 (2021).
Kiang, T. K. L., Ranamukhaarachchi, S. A. & Ensom, M. H. H. Revolutionizing therapeutic drug monitoring with the use of interstitial fluid and microneedles technology. Pharmaceutics 9, 43 (2017).
Kretsos, K. & Kasting, G. B. Dermal capillary clearance: physiology and modeling. Skin Pharmacol. Physiol. 18, 55–74 (2005).
Groenendaal, W., von Basum, G., Schmidt, K. A., Hilbers, P. A. J. & van Riel, N. A. W. Quantifying the composition of human skin for glucose sensor development. J. Diabetes Sci. Technol. 4, 1032–1040 (2010).
Liao, Y.-H. et al. Quantitative analysis of intrinsic skin aging in dermal papillae by in vivo harmonic generation microscopy. Biomed. Opt. Express 5, 3266–3279 (2014).
Levick, J. R. Flow through interstitium and other fibrous matrices. Q. J. Exp. Physiol. 72, 409–437 (1987).
Wiig, H. & Swartz, M. A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol. Rev. 92, 1005–1060 (2012).
Skobe, M. & Detmar, M. Structure, function, and molecular control of the skin lymphatic system. J. Investig. Dermatol. Symp. Proc. 5, 14–19 (2000).
Shore, A. C. Capillaroscopy and the measurement of capillary pressure. Br. J. Clin. Pharm. 50, 501–513 (2000).
Stewart, R. H. A modern view of the interstitial space in health and disease. Front. Vet. Sci. 7, 609583 (2020).
Jamalian, S. et al. Demonstration and analysis of the suction effect for pumping lymph from tissue beds at subatmospheric pressure. Sci. Rep. 7, 12080 (2017).
Levick, J. R. & Michel, C. C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 87, 198–210 (2010).
Ibrahim, R., Nitsche, J. M. & Kasting, G. B. Dermal clearance model for epidermal bioavailability calculations. J. Pharm. Sci. 101, 2094–2108 (2012).
Ono, S., Egawa, G. & Kabashima, K. Regulation of blood vascular permeability in the skin. Inflamm. Regen. 37, 11 (2017).
Pries, A. R., Secomb, T. W. & Gaehtgens, P. The endothelial surface layer. Pflügers Arch. Eur. J. Physiol. 440, 653–666 (2000).
Stan, R.-V. Structure and function of endothelial caveolae. Microsc. Res. Tech. 57, 350–364 (2002).
Davis, M. J., Rahbar, E., Gashev, A. A., Zawieja, D. C. & Moore, J. E. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am. J. Physiol. Heart Circ. Physiol. 301, H48–H60 (2011).
Mendoza, E. & Schmid-Schönbein, G. W. A model for mechanics of primary lymphatic valves. J. Biomech. Eng. 125, 407–414 (2003).
Suami, H. & Scaglioni, M. F. Anatomy of the lymphatic system and the lymphosome concept with reference to lymphedema. Semin. Plast. Surg. 32, 5–11 (2018).
Bendayan, M. Morphological and cytochemical aspects of capillary permeability. Microsc. Res. Tech. 57, 327–349 (2002).
Shen, L., Weber, C. R. & Turner, J. R. The tight junction protein complex undergoes rapid and continuous molecular remodeling at steady state. J. Cell Biol. 181, 683–695 (2008).
Anderson, J. M. & Van Itallie, C. M. Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 1, a002584 (2009).
Rippe, B. & Haraldsson, B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol. Rev. 74, 163–219 (1994).
Rutili, G. & Arfors, K. E. Protein concentration in interstitial and lymphatic fluids from the subcutaneous tissue. Acta Physiol. Scand. 99, 1–8 (1977).
Haaverstad, R., Romslo, I., Larsen, S. & Myhre, H. O. Protein concentration of subcutaneous interstitial fluid in the human leg. Int. J. Microcirc. Clin. Exp. 16, 111–117 (1996).
Michel, C. C. & Curry, F. E. Microvascular permeability. Physiol. Rev. 79, 703–761 (1999).
Vink, H. & Duling, B. R. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am. J. Physiol. Heart Circ. Physiol. 278, H285–H289 (2000).
Yuan, Y. et al. Oil-membrane protection of electrochemical sensors for fouling- and pH-insensitive detection of lipophilic analytes. ACS Appl. Mater. Interfaces 13, 53553–53563 (2021).
Norvaisas, P. & Ziemys, A. The role of payload hydrophobicity in nanotherapeutic pharmacokinetics. J. Pharm. Sci. 103, 2147–2156 (2014).
Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Bhake, R. et al. Continuous free cortisol profiles in healthy men: validation of microdialysis method. J. Clin. Endocrinol. Metab. 105, e1749–e1761 (2020).
Zhang, F., Xue, J., Shao, J. & Jia, L. Compilation of 222 drugs’ plasma protein binding data and guidance for study designs. Drug Discov. Today 17, 475–485 (2012).
Liebl, H. & Kloth, L. C. Skin cell proliferation stimulated by microneedles. J. Am. Coll. Clin. Wound Spec. 4, 2–6 (2012).
Egawa, G. et al. Intravital analysis of vascular permeability in mice using two-photon microscopy. Sci. Rep. 3, 1932 (2013).
Ripolin, A. et al. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 521, 92–101 (2017).
Nickoloff, B. J. & Naidu, Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J. Am. Acad. Dermatol. 30, 535–546 (1994).
Clough, G. F., Jackson, C. L., Lee, J. J. P., Jamal, S. C. & Church, M. K. What can microdialysis tell us about the temporal and spatial generation of cytokines in allergen-induced responses in human skin in vivo? J. Invest. Dermatol. 127, 2799–2806 (2007).
Kolluru, C. et al. Monitoring drug pharmacokinetics and immunologic biomarkers in dermal interstitial fluid using a microneedle patch. Biomed. Microdevices 21, 14 (2019).
Blicharz, T. M. et al. Microneedle-based device for the one-step painless collection of capillary blood samples. Nat. Biomed. Eng. 2, 151–157 (2018).
Svedman, C., Yu, B. B., Ryan, T. J. & Svensson, H. Plasma proteins in a standardised skin mini-erosion (I): permeability changes as a function of time. BMC Dermatol. 2, 3 (2002).
Svedman, C., Yu, B. B., Ryan, T. J. & Svensson, H. Plasma proteins in a standardised skin mini-erosion (II): effects of extraction pressure. BMC Dermatol. 2, 4 (2002).
Stout, P. et al. Site-to-site variation of glucose in interstitial fluid samples and correlation to venous plasma glucose. Clin. Chem. 45, 1674–1675 (1999).
Kramer, G. C., Sibley, L., Aukland, K. & Renkin, E. M. Wick sampling of interstitial fluid in rat skin: further analysis and modifications of the method. Microvasc. Res. 32, 39–49 (1986).
Noddeland, H. Colloid osmotic pressure of human subcutaneous interstitial fluid sampled by nylon wicks: evaluation of the method. Scand. J. Clin. Lab. Invest. 42, 123–130 (1982).
Wiig, H., Heir, S. & Aukland, K. Colloid osmotic pressure of interstitial fluid in rat subcutis and skeletal muscle: comparison of various wick sampling techniques. Acta Physiol. Scand. 133, 167–175 (1988).
Samant, P. P. & Prausnitz, M. R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl Acad. Sci. USA 115, 4583–4588 (2018).
Laszlo, E., De Crescenzo, G., Nieto-Argüello, A., Banquy, X. & Brambilla, D. Superswelling microneedle arrays for dermal interstitial fluid (prote)omics. Adv. Funct. Mater. 31, 2106061 (2021).
Woodley, D. et al. Localization of basement membrane components after dermal-epidermal junction separation. J. Invest. Dermatol. 81, 149–153 (1983).
Samant, P. P. et al. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl Med. 12, eaaw0285 (2020).
Korf, J., Huinink, K. D. & Posthuma-Trumpie, G. A. Ultraslow microdialysis and microfiltration for in-line, on-line and off-line monitoring. Trends Biotechnol. 28, 150–158 (2010).
Chaurasia, C. S. et al. AAPS-FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm. Res. 24, 1014–1025 (2007).
Nightingale, A. M. et al. Monitoring biomolecule concentrations in tissue using a wearable droplet microfluidic-based sensor. Nat. Commun. 10, 2741 (2019).
Collison, M. E. et al. Analytical characterization of electrochemical biosensor test strips for measurement of glucose in low-volume interstitial fluid samples. Clin. Chem. 45, 1665–1673 (1999).
Wang, Z. et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat. Biomed. Eng. 5, 64–76 (2021).
Zheng, H. et al. Hydrogel microneedle-assisted assay integrating aptamer probes and fluorescence detection for reagentless biomarker quantification. ACS Sens. 7, 2387–2399 (2022).
Bouissou, C. C., Sylvestre, J.-P., Guy, R. H. & Delgado-Charro, M. B. Reverse iontophoresis of amino acids: identification and separation of stratum corneum and subdermal sources in vitro. Pharm. Res. 26, 2630–2638 (2009).
Tierney, M. J. et al. Design of a biosensor for continual, transdermal glucose monitoring. Clin. Chem. 45, 1681–1683 (1999).
Cengiz, E. & Tamborlane, W. V. A tale of two compartments: interstitial versus blood glucose monitoring. Diabetes Technol. Ther. 11, S11–S16 (2009).
Chen, C. et al. Recent advances in electrochemical glucose biosensors: a review. RSC Adv. 3, 4473–4491 (2013).
Hirsch, I. B. & Wright, E. E. Using flash continuous glucose monitoring in primary practice. Clin. Diabetes 37, 150–161 (2019).
McClatchey, P. M. et al. Fibrotic encapsulation is the dominant source of continuous glucose monitor delays. Diabetes 68, 1892–1901 (2019).
Hoath, S. B. & Leahy, D. G. The organization of human epidermis: functional epidermal units and phi proportionality. J. Invest. Dermatol. 121, 1440–1446 (2003).
Garg, S. K. et al. Evaluation of accuracy and safety of the next-generation up to 180-day long-term implantable eversense continuous glucose monitoring system: the PROMISE study. Diabetes Technol. Ther. https://doi.org/10.1089/dia.2021.0182 (2021).
Boscari, F. et al. Implantable and transcutaneous continuous glucose monitoring system: a randomized cross over trial comparing accuracy, efficacy and acceptance. J. Endocrinol. Invest. https://doi.org/10.1007/s40618-021-01624-2 (2021).
Christiansen, M. P. et al. A prospective multicenter evaluation of the accuracy and safety of an implanted continuous glucose sensor: the PRECISION study. Diabetes Technol. Ther. 21, 231–237 (2019).
Wan, W. et al. Cost-effectiveness of continuous glucose monitoring for adults with type 1 diabetes compared with self-monitoring of blood glucose: the DIAMOND randomized trial. Diabetes Care 41, 1227–1234 (2018).
Hoskins, M. & Tenderich, A. Senseonics stops sales of eversense implantable CGM in wake of COVID-19 crisis. Healthline (31 March 2020); https://www.healthline.com/diabetesmine/senseonics-suspends-eversense-implantable-cgm
Joseph, J. I. Review of the long-term implantable senseonics continuous glucose monitoring system and other continuous glucose monitoring systems. J. Diabetes Sci. Technol. 15, 167–173 (2021).
Kanick, S. C., Schneider, P. A., Klitzman, B., Wisniewski, N. A. & Rebrin, K. Continuous monitoring of interstitial tissue oxygen using subcutaneous oxygen microsensors: in vivo characterization in healthy volunteers. Microvasc. Res. 124, 6–18 (2019).
Gill, H. S., Denson, D. D., Burris, B. A. & Prausnitz, M. R. Effect of microneedle design on pain in human subjects. Clin. J. Pain 24, 585–594 (2008).
Levin, Y., Kochba, E., Hung, I. & Kenney, R. Intradermal vaccination using the novel microneedle device MicronJet600: past, present, and future. Hum. Vaccin. Immunother. 11, 991–997 (2015).
Leone, M. et al. Universal applicator for digitally-controlled pressing force and impact velocity insertion of microneedles into skin. Pharmaceutics 10, 211 (2018).
Rawson, T. M. et al. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: a first-in-human evaluation in healthy volunteers. Lancet Digit. Health 1, e335–e343 (2019).
Jina, A. et al. Design, development, and evaluation of a novel microneedle array-based continuous glucose monitor. J. Diabetes Sci. Technol. 8, 483–487 (2014).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00887-1 (2022).
Wu, Y. et al. Microneedle aptamer-based sensors for continuous, real-time therapeutic drug monitoring. Anal. Chem. https://doi.org/10.1021/acs.analchem.2c00829 (2022).
Lin, S. et al. Wearable microneedle-based electrochemical aptamer biosensing for precision dosing of drugs with narrow therapeutic windows. Sci. Adv. 8, eabq4539 (2022).
Spehar-Délèze, A.-M., Anastasova, S. & Vadgama, P. Monitoring of lactate in interstitial fluid, saliva and sweat by electrochemical biosensor: the uncertainties of biological interpretation. Chemosensors 9, 195 (2021).
Kretsos, K., Miller, M. A., Zamora-Estrada, G. & Kasting, G. B. Partitioning, diffusivity and clearance of skin permeants in mammalian dermis. Int. J. Pharm. 346, 64–79 (2008).
Heikenfeld, J. et al. Wearable sensors: modalities, challenges, and prospects. Lab Chip 18, 217–248 (2018).
Rocchitta, G. et al. Enzyme biosensors for biomedical applications: strategies for safeguarding analytical performances in biological fluids. Sensors 16, 780 (2016).
Maahs, D. M. et al. Effect of acetaminophen on CGM glucose in an outpatient setting. Diabetes Care 38, e158–e159 (2015).
Arroyo-Currás, N., Dauphin-Ducharme, P., Scida, K. & Chávez, J. L. From the beaker to the body: translational challenges for electrochemical, aptamer-based sensors. Anal. Methods 12, 1288–1310 (2020).
Leung, K. K., Downs, A. M., Ortega, G., Kurnik, M. & Plaxco, K. W. Elucidating the mechanisms underlying the signal drift of electrochemical aptamer-based sensors in whole blood. ACS Sens. 6, 3340–3347 (2021).
Shaver, A. & Arroyo-Currás, N. The challenge of long-term stability for nucleic acid-based electrochemical sensors. Curr. Opin. Electrochem. 32, 100902 (2022).
Watkins, Z., Karajić, A., Young, T., White, R. & Heikenfeld, J. Week-long operation of electrochemical aptamer sensors: new insights into self-assembled monolayer degradation mechanisms and solutions for stability in biofluid at body temperature. Preprint at ChemRxiv https://doi.org/10.26434/chemrxiv-2022-s1qj9 (2022).
Kurnik, M., Pang, E. Z. & Plaxco, K. W. An electrochemical biosensor architecture based on protein folding supports direct real-time measurements in whole blood. Angew. Chem. Int. Ed. 59, 18442–18445 (2020).
Dauphin-Ducharme, P. et al. Electrochemical aptamer-based sensors for improved therapeutic drug monitoring and high-precision, feedback-controlled drug delivery. ACS Sens. 4, 2832–2837 (2019).
Chien, J.-C., Baker, S. W., Soh, H. T. & Arbabian, A. Design and analysis of a sample-and-hold CMOS electrochemical sensor for aptamer-based therapeutic drug monitoring. IEEE J. Solid State Circuits 55, 2914–2929 (2020).
Mage, P. L. et al. Closed-loop control of circulating drug levels in live animals. Nat. Biomed. Eng. 1, 0070 (2017).
Ye, Z.-K., Tang, H.-L. & Zhai, S.-D. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS ONE 8, e77169 (2013).
Roberts, J. A., Norris, R., Paterson, D. L. & Martin, J. H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 73, 27–36 (2012).
Sime, F. B., Roberts, M. S., Peake, S. L., Lipman, J. & Roberts, J. A. Does beta-lactam pharmacokinetic variability in critically ill patients justify therapeutic drug monitoring? A systematic review. Ann. Intensive Care 2, 35 (2012).
Ates, H. C. et al. On-site therapeutic drug monitoring. Trends Biotechnol. 38, 1262–1277 (2020).
Kiang, T. K. L., Häfeli, U. O. & Ensom, M. H. H. A comprehensive review on the pharmacokinetics of antibiotics in interstitial fluid spaces in humans: implications on dosing and clinical pharmacokinetic monitoring. Clin. Pharmacokinet. 53, 695–730 (2014).
Harbarth, S., Samore, M. H., Lichtenberg, D. & Carmeli, Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 101, 2916–2921 (2000).
Skhirtladze, K. et al. Impaired target site penetration of vancomycin in diabetic patients following cardiac surgery. Antimicrob. Agents Chemother. 50, 1372–1375 (2006).
Hamada, Y., Kuti, J. L. & Nicolau, D. P. Vancomycin serum concentrations do not adequately predict tissue exposure in diabetic patients with mild to moderate limb infections. J. Antimicrob. Chemother. 70, 2064–2067 (2015).
Paneni, F., Beckman, J. A., Creager, M. A. & Cosentino, F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur. Heart J. 34, 2436–2443 (2013).
Kennedy, J. M. & Van Riji, A. M. Effects of surgery on the pharmacokinetic parameters of drugs. Clin. Pharmacokinet. 35, 293–312 (1998).
De Paepe, P., Belpaire, F. M. & Buylaert, W. A. Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clin. Pharmacokinet. 41, 1135–1151 (2002).
Blot, S. I., Pea, F. & Lipman, J. The effect of pathophysiology on pharmacokinetics in the critically ill patient — concepts appraised by the example of antimicrobial agents. Adv. Drug Deliv. Rev. 77, 3–11 (2014).
LaCount, T. D. et al. Modeling temperature-dependent dermal absorption and clearance for transdermal and topical drug applications. AAPS J. 22, 70 (2020).
Riviere, J. E. & Williams, P. L. Pharmacokinetic implications of changing blood flow in skin. J. Pharm. Sci. 81, 601–602 (1992).
Barre, J., Didey, F., Delion, F. & Tillement, J.-P. Problems in therapeutic drug monitoring: free drug level monitoring. Ther. Drug Monit. 10, 133–143 (1988).
Zeitlinger, M. A. et al. Protein binding: do we ever learn? Antimicrob. Agents Chemother. 55, 3067–3074 (2011).
Butterfield, J. M. et al. Refining vancomycin protein binding estimates: identification of clinical factors that influence protein binding. Antimicrob. Agents Chemother. 55, 4277–4282 (2011).
Tisoncik, J. R. et al. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 76, 16–32 (2012).
Guo, Y.-R. et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil. Med. Res. 7, 11 (2020).
Schulert, G. S. & Grom, A. A. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu. Rev. Med. 66, 145–159 (2015).
Nestle, F. O., Di Meglio, P., Qin, J.-Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 9, 679–691 (2009).
Clark, R. A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Nedrebø, T., Reed, R. K., Jonsson, R., Berg, A. & Wiig, H. Differential cytokine response in interstitial fluid in skin and serum during experimental inflammation in rats. J. Physiol. 556, 193–202 (2004).
Zaleska, M., Olszewski, W. L., Durlik, M. & Miller, N. E. Signaling proteins are represented in tissue fluid/lymph from soft tissues of normal human legs at concentrations different from serum. Lymphat. Res. Biol. 11, 203–210 (2013).
Kumar, A. et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit. Care Med. 34, 1589–1596 (2006).
Panelli, M. C. et al. Forecasting the cytokine storm following systemic interleukin (IL)-2 administration. J. Transl Med. 2, 17 (2004).
Acknowledgements
The authors at the University of Cincinnati acknowledge support from the US Air Force Office of Scientific Research (USAF Contract No. FA9550-20-1-0117), a National Science Foundation CBET Award (No. 2125056), a National Science Foundation ECCS Award (No. 2025720) and a US Office of Naval Research Award (No. N00014-20-1-2764). The authors at Stanford University thank M. Eisenstein for his editorial contributions, and appreciate financial support from W. L Gore and Associates, the Helmsley Trust and the National Institutes of Health (NIH, OT2OD025342). I.A.P.T. acknowledges support from the Medtronic Foundation Stanford Graduate Fellowship and from the Natural Sciences and Engineering Research Council of Canada (NSERC, 416353855). Sandia National Laboratories is a multimission laboratory managed and operated by National Technology and Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc., for the US Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525. This Perspective describes objective technical results and analysis. Any subjective views or opinions that might be expressed here do not necessarily represent the views of the US Department of Energy or the United States Government.
Author information
Authors and Affiliations
Contributions
M.F. and I.A.P.T. contributed equally to writing and revising all sections. G.K. contributed to the writing of the sections ‘Structure and composition of the dermis’ and ‘Partitioning of analytes in dermal ISF’. R.P. contributed to the sections ‘Partitioning of analytes in dermal ISF’ and ‘Applications of ISF in diagnostics’. D.C. contributed to the section ‘Challenges in obtaining true ISF via extraction’. J.H. and H.T.S led the project and contributed to the writing and revision of all sections.
Corresponding authors
Ethics declarations
Competing interests
J.H. is a co-founder of Kilele Health Inc., which is pursuing the commercialization of wearables for the continuous monitoring of analytes in ISF. H.T.S. is a co-founder of Eigen Biosciences, which seeks to commercialize technologies for measuring biomarkers in ISF. The other authors declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks Srikanth Singamaneni and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Friedel, M., Thompson, I.A.P., Kasting, G. et al. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng 7, 1541–1555 (2023). https://doi.org/10.1038/s41551-022-00998-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-022-00998-9
This article is cited by
-
Dual-responsive zwitterionic hydrogel microneedle patch for the on-site detection of two alzheimer’s disease - related miRNAs
Journal of Nanobiotechnology (2026)
-
Challenges and opportunities of wearable molecular sensors in endocrinology and metabolism
Nature Reviews Endocrinology (2026)
-
Noninvasive On-Skin Biosensors for Monitoring Diabetes Mellitus
Nano-Micro Letters (2026)
-
Microneedle-aided nanotherapeutics delivery and nanosensor intervention in advanced tissue regeneration
Journal of Nanobiotechnology (2025)
-
A nanoMIP sensor for real-time in vivo monitoring of levodopa pharmacokinetics in precision Parkinson’s therapy
Nature Communications (2025)