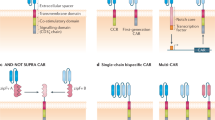
Abstract
Chimeric antigen receptor (CAR) T cell therapy has achieved remarkable success in treating haematologic malignancies. However, the rise in clinical use has highlighted substantial challenges related to T cell- and tumour-intrinsic mechanisms. Additionally, the tumour microenvironment can render these treatments dysfunctional. Extensive attempts in the field are optimizing the key elements of CAR T cell products for therapy, including antigen specificity and affinity, metabolic fitness, phenotypic stability and manufacturing. Recent efforts in transcriptomic and epigenetic profiling, as well as high-throughput functional screening methods, have identified new classes of targets, binders and mechanisms to be exploited. Advances in gene editing and delivery offer opportunities to translate those strategies into clinical trials. Here we discuss the multifaceted exploration of CAR T cell engineering approaches and emerging directions, highlighting the available strategies that can be built on to create the next generation of cellular therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sadelain, M. & Mulligan, R. C. Efficient-retroviral-mediated gene transfer into murine primary lymphocytes. In Proc. 8th International Congress of Immunology (ed. International Union of Immunological Societies) Abstract no. 34 (Springer, 1992).
Miller, A. D. Retrovirus packaging cells. Hum. Gene Ther. 1, 5–14 (1990).
Eshhar, Z., Waks, T., Gross, G. & Schindler, D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or ζ subunits of the immunoglobulin and T-cell receptors. Proc. Natl Acad. Sci. USA 90, 720–724 (1993).
Gross, G., Waks, T. & Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl Acad. Sci. USA 86, 10024–10028 (1989).
Ochi, T. et al. A single-chain antibody generation system yielding CAR-T cells with superior antitumor function. Commun. Biol. 4, 273 (2021).
Long, A. H. et al. 4-1BB costimulation ameliorates T-cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Chen, J. et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Res. 33, 341–354 (2023).
Kozani, P. S., Kozani, P. S. & O’Connor, R. S. Humanized chimeric antigen receptor (CAR) T cells. J. Cancer Immunol. 3, 183–187 (2021).
Hamers-Casterman, C. et al. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448 (1993).
Sarén, T. et al. Complementarity-determining region clustering may cause CAR-T cell dysfunction. Nat. Commun. 14, 4732 (2023).
Zhou, J. et al. The persistence and antitumor efficacy of CAR-T cells are modulated by tonic signaling within the CDR. Int. Immunopharmacol. 126, 111239 (2024).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Siegler, E., Li, S., Kim, Y. J. & Wang, P. Designed ankyrin repeat proteins as Her2 targeting domains in chimeric antigen receptor-engineered T cells. Hum. Gene Ther. 28, 726–736 (2017).
Balakrishnan, A. et al. Multispecific targeting with synthetic ankyrin repeat motif chimeric antigen receptors. Clin. Cancer Res. 25, 7506–7516 (2019).
Walsh, S. T. R., Cheng, H., Bryson, J. W., Roder, H. & Degrado, W. F. Solution structure and dynamics of a de novo designed three-helix bundle protein. Proc. Natl Acad. Sci. USA 96, 5486–5491 (1999).
Buonato, J. M. et al. Preclinical efficacy of BCMA-directed CAR T cells incorporating a novel D domain antigen recognition domain. Mol. Cancer Ther. 21, 1171–1183 (2022).
Qin, H. et al. Chimeric antigen receptors incorporating D domains targeting CD123 direct potent mono- and bi-specific antitumor activity of T cells. Mol. Ther. 27, 1262–1274 (2019).
Frigault, M. J. et al. Phase 1 study of CART-ddBCMA for the treatment of subjects with relapsed and refractory multiple myeloma. Blood Adv. 7, 768–777 (2023).
Shaffer, D. R. et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood 117, 4304–4314 (2011).
Wang, Q. J. et al. Preclinical evaluation of chimeric antigen receptors targeting CD70-expressing cancers. Clin. Cancer Res. 23, 2267–2276 (2017).
Sauer, T. et al. CD70-specific CAR T cells have potent activity against acute myeloid leukemia without HSC toxicity. Blood 138, 318–330 (2021).
Ochi, F. et al. Gene-modified human α/β-T cells expressing a chimeric CD16-CD3ζ receptor as adoptively transferable effector cells for anticancer monoclonal antibody therapy. Cancer Immunol. Res. 2, 249–262 (2014).
Kudo, K. et al. T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res. 74, 93–103 (2014).
Clémenceau, B. et al. Antibody-dependent cellular cytotoxicity (ADCC) is mediated by genetically modified antigen-specific human T lymphocytes. Blood 107, 4669–4677 (2006).
Van Cutsem, E. et al. Phase 1 studies assessing the safety and clinical activity of autologous and allogeneic NKG2D-based CAR-T therapy in metastatic colorectal cancer. Ann. Oncol. 30, iv124–iv125 (2019).
Dai, H. et al. Development of NKG2D chimeric antigen receptor-T cells as targeted therapy of liver cancer. J. Clin. Oncol. 36, e15057 (2018).
Chen, N. et al. Targeting of IL-10R on acute myeloid leukemia blasts with chimeric antigen receptor-expressing T cells. Blood Cancer J. 11, 144 (2021).
Huang, G. et al. Genetically modified T cells targeting interleukin-11 receptor α-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res. 72, 271–281 (2012).
Brown, C. E. et al. Optimization of IL13Rα2-targeted chimeric antigen receptor T cells for improved anti-tumor efficacy against glioblastoma. Mol. Ther. 26, 31–44 (2018).
Kahlon, K. S. et al. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 64, 9160–9166 (2004).
Hombach, A. et al. IL12 integrated into the CAR exodomain converts CD8+ T cells to poly-functional NK-like cells with superior killing of antigen-loss tumors. Mol. Ther. 30, 593–605 (2022).
Han, X. et al. Adnectin-based design of chimeric antigen receptor for T cell engineering. Mol. Ther. 25, 2466–2476 (2017).
Salzer, B. et al. Engineering AvidCARs for combinatorial antigen recognition and reversible control of CAR function. Nat. Commun. 11, 4166 (2020).
Zajc, C. U. et al. A conformation-specific ON-switch for controlling CAR T cells with an orally available drug. Proc. Natl Acad. Sci. USA 117, 14926–14935 (2020).
Kuwana, Y. et al. Expression of chimeric receptor composed of immunoglobulin-derived V resions and T-cell receptor-derived C regions. Biochem. Biophys. Res. Commun. 149, 960–968 (1987).
Irving, B. A. & Weiss, A. The cytoplasmic domain of the T cell receptor ζ chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 64, 891–901 (1991).
Feucht, J. et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat. Med. 25, 82–88 (2019).
Schoutrop, E. et al. Tuned activation of MSLN-CAR T cells induces superior antitumor responses in ovarian cancer models. J. Immunother. Cancer 11, e005691 (2023).
Park, J. H. et al. A phase I study of CD19-targeted 19(T2)28z1xx CAR T cells in adult patients with relapsed or refractory diffuse large B-cell lymphoma. Blood 140, 403–404 (2022).
Krause, A. et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J. Exp. Med. 188, 619–626 (1998).
Xu, T. et al. A novel TREM1/DAP12-based multiple chain CAR-T cell targets PTK7 in ovarian cancer therapy. Med. Oncol. 40, 226 (2023).
Velasco Cárdenas, R. M. H. et al. Harnessing CD3 diversity to optimize CAR T cells. Nat. Immunol. 24, 2135–2149 (2023).
Gudipati, V. et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 21, 848–856 (2020).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022).
Zhong, X. S., Matsushita, M., Plotkin, J., Riviere, I. & Sadelain, M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI 3 kinase/AKT/Bcl-X L activation and CD8 T cell-mediated tumor eradication. Mol. Ther. 18, 413–420 (2010).
Textor, A. et al. Cd28 co-stimulus achieves superior CAR T cell effector function against solid tumors than 4-1BB co-stimulus. Cancers (Basel) 13, 1050 (2021).
Porter, D. L. et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 7, 303ra139 (2015).
Davila, M. L. et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 6, 224ra25 (2014).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Zhao, Z. et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 28, 415–428 (2015).
van der Stegen, S. J. C., Hamieh, M. & Sadelain, M. The pharmacology of second-generation chimeric antigen receptors. Nat. Rev. Drug Discov. 14, 499–509 (2015).
Guedan, S. et al. Single residue in CD28-costimulated CAR-T cells limits long-term persistence and antitumor durability. J. Clin. Invest. 130, 3087–3097 (2020).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022).
Boroughs, A. C. et al. A distinct transcriptional program in human CAR T cells bearing the 4-1BB signaling domain revealed by scRNA-Seq. Mol. Ther. 28, 2577–2592 (2020).
Kawalekar, O. U. et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44, 380–390 (2016).
Carpenito, C. et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA 106, 3360–3365 (2009).
Del Bufalo, F. et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med. 388, 1284–1295 (2023).
Kagoya, Y. et al. A novel chimeric antigen receptor containing a JAK-STAT signaling domain mediates superior antitumor effects. Nat. Med. 24, 352–359 (2018).
Guedan, S. et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood 124, 1070–1080 (2014).
Guedan, S. et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 3, e96976 (2018).
Moreno-Cortes, E. et al. ICOS and OX40 tandem co-stimulation enhances CAR T-cell cytotoxicity and promotes T-cell persistence phenotype. Front. Oncol. 13, 1200914 (2023).
Song, D. G. et al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 119, 696–706 (2012).
Prinzing, B. et al. MyD88/CD40 signaling retains CAR T cells in a less differentiated state. JCI Insight 5, e136093 (2020).
Collinson-Pautz, M. R. et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia 33, 2195–2207 (2019).
Hudecek, M. et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin. Cancer Res. 19, 3153–3164 (2013).
Levin-Piaeda, O. et al. The intracellular domain of CD40 is a potent costimulatory element in chimeric antigen receptors. J. Immunother. 44, 209–213 (2021).
Jonnalagadda, M. et al. Chimeric antigen receptors with mutated IgG4 Fc spacer avoid fc receptor binding and improve T cell persistence and antitumor efficacy. Mol. Ther. 23, 757–768 (2015).
Alabanza, L. et al. Function of novel anti-CD19 chimeric antigen receptors with human variable regions is affected by hinge and transmembrane domains. Mol. Ther. 25, 2452–2465 (2017).
Li, S. et al. DAP10 integration in CAR-T cells enhances the killing of heterogeneous tumors by harnessing endogenous NKG2D. Mol. Ther. Oncolytics 26, 15–26 (2022).
Singh, N. et al. Antigen-independent activation enhances the efficacy of 4-1BB-costimulated CD22 CAR T cells. Nat. Med. 27, 842–850 (2021).
Rodriguez-Marquez, P. et al. CAR density influences antitumoral efficacy of BCMA CAR T cells and correlates with clinical outcome. Sci. Adv. 8, eabo0514 (2022).
Fraietta, J. A. et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature 558, 307–312 (2018).
Glaser, V. et al. Combining different CRISPR nucleases for simultaneous knock-in and base editing prevents translocations in multiplex-edited CAR T cells. Genome Biol. 24, 89 (2023).
Liao, C. et al. CD38-specific CAR integrated into CD38 locus driven by different promoters causes distinct antitumor activities of T and NK cells. Adv. Sci. 10, e2207394 (2023).
Tipanee, J., Samara-Kuko, E., Gevaert, T., Chuah, M. K. & VandenDriessche, T. Universal allogeneic CAR T cells engineered with Sleeping Beauty transposons and CRISPR-CAS9 for cancer immunotherapy. Mol. Ther. 30, 3155–3175 (2022).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Deep, G. & Panigrahi, G. K. Hypoxia-induced signaling promotes prostate cancer progression: exosomes role as messenger of hypoxic response in tumor microenvironmen. Crit. Rev. Oncog. 20, 419–434 (2015).
Kosti, P. et al. Hypoxia-sensing CAR T cells provide safety and efficacy in treating solid tumors. Cell Rep. Med. 2, 100227 (2021).
Gu, X., He, D., Li, C., Wang, H. & Yang, G. Development of inducible CD19-CAR T cells with a Tet-on system for controlled activity and enhanced clinical safety. Int. J. Mol. Sci. 19, 3455 (2018).
Agarwal, S. et al. In vivo generation of CAR T cells selectively in human CD4+ lymphocytes. Mol. Ther. 28, 1783–1794 (2020).
Da Vià, M. C. et al. Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma. Nat. Med. 27, 616–619 (2021).
Rasche, L. et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 8, 268 (2017).
Liu, Y. et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 13, eabb5191 (2021).
Simon, S. et al. Design of sensitive monospecific and bispecific synthetic chimeric T cell receptors for cancer therapy. Nat. Cancer 6, 647–665 (2025).
Larrayoz, M. et al. Preclinical models for prediction of immunotherapy outcomes and immune evasion mechanisms in genetically heterogeneous multiple myeloma. Nat. Med. 29, 632–645 (2023).
Denk, D. et al. Expansion of T memory stem cells with superior anti-tumor immunity by Urolithin A-induced mitophagy. Immunity 55, 2059–2073.e8 (2022).
Vogt, M. et al. Targeting MYC effector functions in pancreatic cancer by inhibiting the ATPase RUVBL1/2. Gut 73, 1509–1528 (2024).
Van Der Windt, G. J. W. et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl Acad. Sci. USA 110, 14336–14341 (2013).
Panetti, S. et al. Engineering amino acid uptake or catabolism promotes CAR T-cell adaption to the tumor environment. Blood Adv. 7, 1754–1761 (2023).
Quintana, F. J. et al. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 (2008).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014).
Navarro, F. et al. Overcoming T cell dysfunction in acidic pH to enhance adoptive T cell transfer immunotherapy. Oncoimmunology 11, 2070337 (2022).
Fultang, L. et al. Metabolic engineering against the arginine microenvironment enhances CAR-T cell proliferation and therapeutic activity. Blood 136, 1155–1160 (2020).
Hickman, T. L. et al. BOXR1030, an anti-GPC3 CAR with exogenous GOT2 expression, shows enhanced T cell metabolism and improved anti-cell line derived tumor xenograft activity. PLoS ONE 17, e0266980 (2022).
Si, X. et al. Mitochondrial isocitrate dehydrogenase impedes CAR T cell function by restraining antioxidant metabolism and histone acetylation. Cell Metab. 36, 176–192.e10 (2024).
Xu, W. et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19, 17–30 (2011).
Schmidt, N. M. et al. Targeting human Acyl-CoA:cholesterol acyltransferase as a dual viral and T cell metabolic checkpoint. Nat. Commun. 12, 2814 (2021).
Jung, I. Y. et al. CRISPR/Cas9-mediated knockout of DGK improves antitumor activities of human T cells. Cancer Res. 78, 4692–4703 (2018).
Zhao, L. et al. Inhibition of cholesterol esterification enzyme enhances the potency of human chimeric antigen receptor T cells against pancreatic carcinoma. Mol. Ther. Oncolytics 16, 262–271 (2020).
Lontos, K. et al. Metabolic reprogramming via an engineered PGC-1α improves human chimeric antigen receptor T-cell therapy against solid tumors. J. Immunother. Cancer 11, e006522 (2023).
Laletin, V. et al. Negative intracellular regulators of T-cell receptor (TCR) signaling as potential antitumor immunotherapy targets. J. Immunother. Cancer 11, e005845 (2023).
Shui, J. W. et al. Hematopoietic progenitor kinase 1 negatively regulates T cell receptor signaling and T cell-mediated immune responses. Nat. Immunol. 8, 84–91 (2007).
Wang, X. et al. Attenuation of T cell receptor signaling by serine phosphorylation-mediated lysine 30 ubiquitination of SLP-76 protein. J. Biol. Chem. 287, 34091–34100 (2012).
Liu, M. et al. CRISPR/Cas9-mediated knockout of intracellular molecule SHP-1 enhances tumor-killing ability of CD133-targeted CAR T cells in vitro. Exp. Hematol. Oncol. 12, 88 (2023).
Du, X., Wiede, F., Darcy, P. K. & Tiganis, T. Abstract PR04: CRISPR-mediated PTPN2 deletion in CAR T cells enhances anti-tumor efficacy. Cancer Immunol. Res. 10, PR04 (2022).
Naramura, M. et al. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 3, 1192–1199 (2002).
Wiede, F. et al. PTPN 2 phosphatase deletion in T cells promotes anti‐tumour immunity and CAR T‐cell efficacy in solid tumours. EMBO J. 39, e103637 (2020).
Wu, L. et al. CD28-CAR-T cell activation through FYN kinase signaling rather than LCK enhances therapeutic performance. Cell Rep. Med. 4, 100917 (2023).
Kalinin, R. S. et al. Engineered removal of PD-1 from the surface of CD19 CAR-T cells results in increased activation and diminished survival. Front. Mol. Biosci 8, 745286 (2021).
Mestermann, K. et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci. Transl. Med. 11, eaau5907 (2019).
Zhang, Y. et al. CRISPR-Cas9 mediated LAG-3 disruption in CAR-T cells. Front. Med. 11, 554–562 (2017).
Hu, B. et al. Nucleofection with plasmid DNA for CRISPR/Cas9-mediated inactivation of programmed cell death protein 1 in CD133-specific CAR T cells. Hum. Gene Ther. 30, 446–458 (2019).
Cui, Q. et al. CD38-directed CAR-T cell therapy: a novel immunotherapy strategy for relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J. Hematol. Oncol. 14, 82 (2021).
Agarwal, S. et al. Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Immunity 56, 2388–2407.e9 (2023).
Masoumi, E. et al. Genetic and pharmacological targeting of A2a receptor improves function of anti-mesothelin CAR T cells. J. Exp. Clin. Cancer Res. 39, 49 (2020).
Tang, N. et al. TGF-β inhibition via CRISPR promotes the long-term efficacy of CAR T cells against solid tumors. JCI Insight 5, e133977 (2020).
Chen, X. et al. Secretion of bispecific protein of anti-PD-1 fused with TGF-β trap enhances antitumor efficacy of CAR-T cell therapy. Mol. Ther. Oncolytics 21, 144–157 (2021).
Kloss, C. C. et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol. Ther. 26, 1855–1866 (2018).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Liu, G. et al. Disruption of adenosine 2A receptor improves the anti-tumor function of anti-mesothelin CAR T cells both in vitro and in vivo. Exp. Cell. Res. 409, 112886 (2021).
Li, N. et al. Improving the anti-solid tumor efficacy of CAR-T cells by inhibiting adenosine signaling pathway. Oncoimmunology 9, 1824643 (2020).
Künkele, A. et al. Functional tuning of CARs reveals signaling threshold above which CD8+ CTL antitumor potency is attenuated due to cell fas-FasL-dependent AICD. Cancer Immunol. Res. 3, 368–379 (2015).
Tschumi, B. O. et al. CART cells are prone to Fas- and DR5-mediated cell death. J. Immunother. Cancer 6, 92 (2018).
Ren, J. et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 8, 17002–17011 (2017).
Prinzing, B. et al. Deleting DNMT3A in CAR T cells prevents exhaustion and enhances antitumor activity. Sci. Transl. Med. 13, eabh0272 (2021).
Lopez-Cobo, S. et al. SUV39H1 ablation enhances long-term CAR T function in solid tumors. Cancer Discov 14, 120–141 (2023).
Someya, K. et al. Improvement of Foxp3 stability through CNS2 demethylation by TET enzyme induction and activation. Int. Immunol. 29, 365–375 (2017).
Yue, X. et al. Control of Foxp3 stability through modulation of TET activity. J. Exp. Med. 213, 377–397 (2016).
Jain, N. et al. Disruption of SUV39H1-mediated H3K9 methylation sustains CAR T-cell function. Cancer Discov. 14, 142–157 (2024).
Seo, H. et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8+ T cell exhaustion. Proc. Natl Acad. Sci. USA 116, 12410–12415 (2019).
Chen, J. et al. NR4A transcription factors limit CAR T cell function in solid tumours. Nature 567, 530–534 (2019).
Jung, I. Y. et al. BLIMP1 and NR4A3 transcription factors reciprocally regulate antitumor CAR T cell stemness and exhaustion. Sci. Transl. Med. 14, eabn7336 (2022).
Wei, J. et al. Targeting REGNASE-1 programs long-lived effector T cells for cancer therapy. Nature 576, 471–476 (2019).
Zhao, H. et al. Genome-wide fitness gene identification reveals Roquin as a potent suppressor of CD8 T cell expansion and anti-tumor immunity. Cell Rep. 37, 110083 (2021).
Zheng, W. et al. Regnase-1 suppresses TCF-1+ precursor exhausted T-cell formation to limit CAR–T-cell responses against ALL. Blood 138, 122–135 (2021).
Behrens, G. et al. Disrupting Roquin-1 interaction with Regnase-1 induces autoimmunity and enhances antitumor responses. Nat. Immunol. 22, 1563–1576 (2021).
Wang, D. et al. CRISPR screening of CAR T cells and cancer stem cells reveals critical dependencies for cell-based therapies. Cancer Discov. 11, 1192–1211 (2021).
Bandyopadhyay, S., Valdor, R. & Macian, F. Tle4 regulates epigenetic silencing of gamma interferon expression during effector T helper cell tolerance. Mol. Cell. Biol. 34, 233–245 (2014).
Gogishvili, T. et al. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood 130, 2838–2847 (2017).
Prommersberger, S. et al. CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma. Gene Ther. 28, 560–571 (2021).
Georgiadis, C. et al. Base-edited CAR T cells for combinational therapy against T cell malignancies. Leukemia 35, 3466–3481 (2021).
Srinivasan, S. et al. Investigation of ALLO-316: a fratricide-resistant allogeneic CAR T targeting CD70 as a potential therapy for the treatment of AML. Blood 136, 23 (2020).
Breman, E. et al. Overcoming target driven fratricide for T cell therapy. Front. Immunol. 9, 2940 (2018).
Wellhausen, N. et al. Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy. Sci. Transl. Med. 15, eadi1145 (2023).
Moon, E. K. et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin. Cancer Res. 17, 4719–4730 (2011).
Di Stasi, A. et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113, 6392–6402 (2009).
Jin, L. et al. CXCR1- or CXCR2-modified CAR T cells co-opt IL-8 for maximal antitumor efficacy in solid tumors. Nat. Commun. 10, 4016 (2019).
Li, G. et al. CXCR5 guides migration and tumor eradication of anti-EGFR chimeric antigen receptor T cells. Mol. Ther. Oncolytics 22, 507–517 (2021).
Grover, N. S. et al. CD30-directed CAR-T cells co-expressing CCR4 in relapsed/refractory Hodgkin Lymphoma and CD30+ cutaneous T cell lymphoma. Blood 138, 742 (2021).
Nicolas-Boluda, A. et al. Tumor stiffening reversion through collagen crosslinking inhibition improves T cell migration and anti-PD-1 treatment. eLife 10, e58688 (2021).
Caruana, I. et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat. Med. 21, 524–529 (2015).
Liu, X. et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 76, 1578–1590 (2016).
Hoogi, S. et al. A TIGIT-based chimeric co-stimulatory switch receptor improves T-cell anti-tumor function. J. Immunother. Cancer 7, 243 (2019).
Ma, Q. et al. A PD-L1-targeting chimeric switch receptor enhances efficacy of CAR-T cell for pleural and peritoneal metastasis. Signal Transduct. Target. Ther. 7, 380 (2022).
Liu, H. et al. CD19-specific CAR T cells that express a PD-1/CD28 chimeric switch-receptor are effective in patients with PD-L1⇓positive B-cell lymphoma. Clin. Cancer Res. 27, 473–484 (2021).
Brog, R. A. et al. Superkine IL-2 and IL-33 armored CAR T cells reshape the tumor microenvironment and reduce growth of multiple solid tumors. Cancer Immunol. Res. 10, 962–977 (2022).
Hou, A. J. et al. IL-13Rα2/TGF-β bispecific CAR-T cells counter TGF-β-mediated immune suppression and potentiate anti-tumor responses in glioblastoma. Neuro Oncol. 26, 1850–1866 (2024).
Liu, X. et al. A novel dominant-negative PD-1 armored anti-CD19 CAR T cell is safe and effective against refractory/relapsed B cell lymphoma. Transl. Oncol. 14, 101085 (2021).
Yamamoto, T. N. et al. T cells genetically engineered to overcome death signaling enhance adoptive cancer immunotherapy. J. Clin. Invest. 129, 1551–1565 (2019).
Yang, F. et al. Self-delivery of TIGIT-blocking scFv enhances CAR-T immunotherapy in solid tumors.Front. Immunol. 14, 1175920 (2023).
Rafiq, S. et al. Targeted delivery of a PD-1-blocking scFV by CAR-T cells enhances anti-tumor efficacy in vivo. Nat. Biotechnol. 36, 847–856 (2018).
Huang, S. W. et al. BiTE-secreting CAR-γδT as a dual targeting strategy for the treatment of solid tumors. Adv. Sci 10, e2206856 (2023).
Choi, B. D. et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 37, 1049–1058 (2019).
Porter, C. E. et al. Oncolytic adenovirus armed with BiTE, cytokine, and checkpoint inhibitor enables CAR T cells to control the growth of heterogeneous tumors. Mol. Ther. 28, 1251–1262 (2020).
Yin, Y. et al. Locally secreted BiTEs complement CAR T cells by enhancing killing of antigen heterogeneous solid tumors. Mol. Ther. 30, 2537–2553 (2022).
Li, G. et al. Fn14-targeted BiTE and CAR-T cells demonstrate potent preclinical activity against glioblastoma. Oncoimmunology 10, 1983306 (2021).
Choi, B. D. et al. Intraventricular CARv3-TEAM-E T cells in recurrent glioblastoma. N. Engl. J. Med. 390, 1290–1298 (2024).
Adachi, K. et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat. Biotechnol. 36, 346–351 (2018).
Liu, L. et al. Enhanced CAR-T activity against established tumors by polarizing human T cells to secrete interleukin-9. Nat. Commun. 11, 5902 (2020).
Jaspers, J. E. et al. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J. Clin. Invest. 133, e166028 (2023).
Ma, X. et al. Interleukin-23 engineering improves CAR T cell function in solid tumors. Nat. Biotechnol. 38, 448–459 (2020).
Krenciute, G. et al. Transgenic expression of IL15 improves antiglioma activity of IL13Rα2-CAR T cells but results in antigen loss variants. Cancer Immunol. Res. 5, 571–581 (2017).
Kueberuwa, G., Kalaitsidou, M., Cheadle, E., Hawkins, R. E. & Gilham, D. E. CD19 CAR T cells expressing IL-12 eradicate lymphoma in fully lymphoreplete mice through induction of host immunity. Mol. Ther. Oncolytics 8, 41–51 (2018).
Lynn, R. C. et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature 576, 293–300 (2019).
Blaeschke, F. et al. Modular pooled discovery of synthetic knockin sequences to program durable cell therapies. Cell 186, 4216–4234.e33 (2023).
Zhang, X. et al. Depletion of BATF in CAR-T cells enhances antitumor activity by inducing resistance against exhaustion and formation of central memory cells. Cancer Cell 40, 1407–1422.e7 (2022).
Ohno, M. et al. Expression of miR-17-92 enhances anti-tumor activity of T-cells transduced with the anti-EGFRvIII chimeric antigen receptor in mice bearing human GBM xenografts. J. Immunother. Cancer 1, 21 (2013).
Johnson, L. R. et al. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell 184, 4981–4995.e14 (2021).
Zhang, J. et al. Co-expression of miR155 or LSD1 shRNA increases the anti-tumor functions of CD19 CAR-T cells. Front. Immunol 12, 811364 (2022).
Stepanov, A. V. et al. Control of the antitumour activity and specificity of CAR T cells via organic adapters covalently tethering the CAR to tumour cells. Nat. Biomed. Eng. 8, 529–543 (2024).
Micklethwaite, K. P. et al. Investigation of product-derived lymphoma following infusion of piggyBac-modified CD19 chimeric antigen receptor T cells. Blood 138, 1391–1405 (2021).
Elsallab, M. et al. Second primary malignancies after commercial CAR T-cell therapy: analysis of the FDA Adverse Events Reporting System. Blood 143, 2099–2105 (2024).
Ghilardi, G. et al. T cell lymphoma and secondary primary malignancy risk after commercial CAR T cell therapy. Nat. Med. 30, 984–989 (2024).
Tsai, S. Q. et al. GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat. Biotechnol. 33, 187–197 (2015).
Głów, D. et al. LATE–a novel sensitive cell-based assay for the study of CRISPR/Cas9-related long-term adverse treatment effects. Mol. Ther. Methods Clin. Dev. 22, 249–262 (2021).
Nobles, C. L. et al. IGUIDE: an improved pipeline for analyzing CRISPR cleavage specificity Jin-Soo Kim. Genome Biol. 20, 14 (2019).
Donnadieu, E. et al. Time to evolve: predicting engineered T cell-associated toxicity with next-generation models. J. Immunother. Cancer 10, e003486 (2022).
Guedan, S. et al. Time 2EVOLVE: predicting efficacy of engineered T-cells—how far is the bench from the bedside?. J. Immunother. Cancer 10, e003487 (2022).
O'Rourke, D. M. et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci. Transl. Med. 9, eaaa0984 (2017).
Wadman, M. FDA no longer needs to require animal tests before human drug trials. Science 379, 127–128 (2023).
Qasim, W. et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 9, eaaj2013 (2017).
Stadtmauer, E. A. et al. CRISPR-engineered T cells in patients with refractory cancer. Science 367, eaba7365 (2020).
Diorio, C. et al. Cytosine base editing enables quadruple-edited allogeneic CART cells for T-ALL. Blood 140, 619–629 (2022).
Bengsch, B. et al. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity 45, 358–373 (2016).
Wang, X. et al. Engineering tolerance toward allogeneic CAR-T cells by regulation of MHC surface expression with Human Herpes Virus-8 proteins. Mol. Ther. 29, 718–733 (2021).
Bishop, D. C. et al. Development of CAR T-cell lymphoma in 2 of 10 patients effectively treated with piggyBac-modified CD19 CAR T cells. Blood 138, 1504–1509 (2021).
Wang, B. et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 5, 429–440 (2021).
Kagoya, Y. et al. Genetic ablation of HLA class I, class II, and the T-cell receptor enables allogeneic T cells to be used for adoptive T-cell therapy. Cancer Immunol. Res. 8, 926–936 (2020).
Perica, K. et al. HIV immune evasin Nef enhances allogeneic CAR T cell potency. Nature 640, 793–801 (2025).
Grosskopf, A. et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 8, eabn8264 (2022).
Frank, A. M. et al. Combining T-cell-specific activation and in vivo gene delivery through CD3-targeted lentiviral vectors. Blood Adv. 4, 5702–5715 (2020).
Nicolai, C. J. et al. In vivo CAR T-cell generation in nonhuman primates using lentiviral vectors displaying a multidomain fusion ligand. Blood 144, 977–987 (2024).
Hamilton, J. R. et al. In vivo human T cell engineering with enveloped delivery vehicles. Nat. Biotechnol. 42, 1684–1692 (2024).
Haghikia, A. et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 15, 2542 (2024).
Müller, F. et al. CD19 CAR T-cell therapy in autoimmune disease—a case series with follow-up. N. Engl. J. Med. 390, 687–700 (2024).
Staudt, S. et al. Microbial metabolite-guided CAR T cell engineering enhances anti-tumor immunity via epigenetic-metabolic crosstalk. Preprint at https://www.biorxiv.org/content/10.1101/2024.08.19.608538v1 (2024).
Gottschlich, A. et al. Single-cell transcriptomic atlas-guided development of CAR-T cells for the treatment of acute myeloid leukemia. Nat. Biotechnol. 41, 1618–1632 (2023).
Hort, S. et al. Deciphering and advancing CAR T-cell therapy with single-cell sequencing technologies. Mol. Cancer 9, 1–25 (2022).
Qiu, S. et al. CAR-Toner: an AI-driven approach for CAR tonic signaling prediction and optimization. Cell Res. 34, 386–388 (2023).
Hort, S. et al. Toward rapid, widely available autologous CAR-T cell therapy—artificial intelligence and automation enabling the smart manufacturing hospital. Front. Med. 9, 913287 (2022).
Tousley, A. M. et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 615, 507–516 (2023).
Sharifzadeh, Z. et al. Genetically engineered T cells bearing chimeric nanoconstructed receptors harboring TAG-72-specific camelid single domain antibodies as targeting agents. Cancer Lett. 334, 237–244 (2013).
Li, N., Fu, H., Hewitt, S. M., Dimitrov, D. S. & Ho, M. Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proc. Natl Acad. Sci. USA 114, E6623–E6631 (2017).
An, N. et al. Anti-multiple myeloma activity of nanobody-based anti-CD38 chimeric antigen receptor T cells. Mol. Pharm. 15, 4577–4588 (2018).
De Munter, S. et al. Nanobody based dual specific CARs. Int. J. Mol. Sci. 19, 403 (2018).
Shadman, M. et al. CD20 targeted CAR-T for high-risk B-cell non-Hodgkin lymphomas. Blood 134, 3235 (2019).
Xie, Y. J. et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl Acad. Sci. USA 116, 7624–7631 (2019).
McComb, S. et al. Programmable attenuation of antigenic sensitivity for a nanobody-based EGFR chimeric antigen receptor through hinge domain truncation. Front. Immunol. 13, 864868 (2022).
Zhang, M. et al. A single-arm, open-label, pilot trial of autologous CD7-CAR-T cells for CD7 positive relapsed and refractory T-lymphoblastic leukemia/lymphoma. Blood 138, 3829 (2021).
Pan, J. et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J. Clin. Oncol. 39, 3340–3351 (2021).
Mo, F. et al. Nanobody-based chimeric antigen receptor T cells designed by CRISPR/Cas9 technology for solid tumor immunotherapy. Signal Transduct. Target. Ther. 6, 80 (2021).
Li, D. et al. Camel nanobody-based B7-H3 CAR-T cells show high efficacy against large solid tumours. Nat. Commun. 14, 5920 (2023).
Hammill, J. A. et al. Designed ankyrin repeat proteins are effective targeting elements for chimeric antigen receptors. J. Immunother. Cancer 3, 55 (2015).
Stepanov, A. V. et al. Switchable targeting of solid tumors by BsCAR T cells. Proc. Natl Acad. Sci. USA 119, e2210562119 (2022).
Wang, Y. et al. Targeting FLT3 in acute myeloid leukemia using ligand-based chimeric antigen receptor-engineered T cells. J. Hematol. Oncol. 11, 60 (2018).
Kubo, H. et al. Development of non-viral, ligand-dependent, EPHB4-specific chimeric antigen receptor T cells for treatment of rhabdomyosarcoma. Mol. Ther. Oncolytics 20, 646–658 (2021).
Wang, D. et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci. Transl. Med. 12, eaaw2672 (2020).
Davies, D. M. et al. Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol. Med. 18, 565–576 (2012).
Schmidts, A. et al. Rational design of a trimeric April-based CAR-binding domain enables efficient targeting of multiple myeloma. Blood Adv. 3, 3248–3260 (2019).
Wong, D. P. et al. A BAFF ligand-based CAR-T cell targeting three receptors and multiple B cell cancers. Nat. Commun. 13, 217 (2022).
Vedvyas, Y. et al. Manufacturing and preclinical validation of CAR T cells targeting ICAM-1 for advanced thyroid cancer therapy. Sci. Rep. 9, 10634 (2019).
Urbanska, K., Stashwick, C., Poussin, M. & Powell, D. J. Follicle-stimulating hormone receptor as a target in the redirected T-cell therapy for cancer. Cancer Immunol. Res. 3, 1130–1137 (2015).
Zoine, J. T. et al. Thrombopoietin-based CAR-T cells demonstrate in vitro and in vivo cytotoxicity to MPL positive acute myelogenous leukemia and hematopoietic stem cells. Gene Ther. 29, 1–12 (2022).
Hasegawa, A. et al. Mutated GM-CSF-based CAR-T cells targeting CD116/CD131 complexes exhibit enhanced anti-tumor effects against acute myeloid leukaemia. Clin. Transl. Immunol. 10, e1282 (2021).
Chinsuwan, T. et al. Ligand-based, piggyBac-engineered CAR-T cells targeting EGFR are safe and effective against non-small cell lung cancers. Mol. Ther. Oncolytics 31, 100728 (2023).
D’aloia, M. M. et al. T lymphocytes engineered to express a CD16-chimeric antigen receptor redirect T-cell immune responses against immunoglobulin G-opsonized target cells. Cytotherapy 18, 278–290 (2016).
Wu, M. R., Zhang, T., DeMars, L. R. & Sentman, C. L. B7H6-specific chimeric antigen receptors lead to tumor elimination and host antitumor immunity. Gene Ther. 22, 675–684 (2015).
Zhang, T., Wu, M.-R. & Sentman, C. L. An NKp30-based chimeric antigen receptor promotes T cell effector functions and antitumor efficacy in vivo. J. Immunol. 189, 2290–2299 (2012).
Butler, S. E. et al. Engineering a natural ligand-based CAR: directed evolution of the stress-receptor NKp30. Cancer Immunol. Immunother. 71, 165–176 (2022).
Quintarelli, C. et al. Choice of costimulatory domains and of cytokines determines CAR T-cell activity in neuroblastoma. Oncoimmunology 7, e1433518 (2018).
Hombach, A. A., Heiders, J., Foppe, M., Chmielewski, M. & Abken, H. OX40 costimulation by a chimeric antigen receptor abrogates CD28 and IL-2 induced IL-10 secretion by redirected CD4+ T cells. Oncoimmunology 1, 458–466 (2012).
Pulè, M. A. et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 12, 933–941 (2005).
M, G. et al. CD28.OX40 co-stimulatory combination is associated with long in vivo persistence and high activity of CAR.CD30 T-cells. Haematologica 106, 987–999 (2021).
Zhang, H. et al. A chimeric antigen receptor with antigen-independent OX40 signaling mediates potent antitumor activity. Sci. Transl. Med. 13, eaba7308 (2021).
Duong, C. P. M. et al. Engineering T cell function using chimeric antigen receptors identified using a DNA library approach. PLoS One 8, e63037 (2013).
Han, Y., Xie, W., Song, D. G. & Powell, D. J. Control of triple-negative breast cancer using ex vivo self-enriched, costimulated NKG2D CAR T cells. J. Hematol. Oncol. 11, 92 (2018).
Yu, L. et al. GD2-specific chimeric antigen receptor-modified T cells for the treatment of refractory and/or recurrent neuroblastoma in pediatric patients. J. Cancer Res. Clin. Oncol. 148, 2643–2652 (2021).
Posey, A. D. & June, C. H. 211. CD2, the first identified T cell co-stimulator, demonstrates more effective chimeric antigen receptor activity over CD28 and 4-1BB. Mol. Ther. 10.1016/S1525-0016(16)33816-3 (2015).
Weng, J. et al. A novel generation 1928zT2 CAR T cells induce remission in extramedullary relapse of acute lymphoblastic leukemia. J. Hematol. Oncol. 11, 25 (2018).
Lai, Y. et al. Toll-like receptor 2 costimulation potentiates the antitumor efficacy of CAR T Cells. Leukemia 32, 801–808 (2018).
Nair, S. et al. Functional improvement of chimeric antigen receptor through intrinsic interleukin-15Rα signaling. Curr. Gene Ther. 19, 40–53 (2018).
Ma, Y.-J. et al. Successful application of PD-1 knockdown CLL-1 CAR-T therapy in two AML patients with post-transplant relapse and failure of anti-CD38 CAR-T cell treatment. Am. J. Cancer Res 12, 615–621 (2022).
Zhou, J. E. et al. ShRNA-mediated silencing of PD-1 augments the efficacy of chimeric antigen receptor T cells on subcutaneous prostate and leukemia xenograft. Biomed. Pharmacother. 137, 111339 (2021).
Liu, G., Rui, W., Zhao, X. & Lin, X. Enhancing CAR-T cell efficacy in solid tumors by targeting the tumor microenvironment. Cell. Mol. Immunol. 18, 1085–1095 (2021).
Simon, B. et al. Enhancing lentiviral transduction to generate melanoma-specific human T cells for cancer immunotherapy. J. Immunol. Methods 472, 55–64 (2019).
Lee, Y. H. et al. PD-1 and TIGIT downregulation distinctly affect the effector and early memory phenotypes of CD19-targeting CAR T cells. Mol. Ther. 30, 579–592 (2022).
Jafarzadeh, L. et al. Targeted knockdown of Tim3 by short hairpin RNAs improves the function of anti-mesothelin CAR T cells. Mol. Immunol. 139, 1–9 (2021).
Beavis, P. A. et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J. Clin. Invest. 127, 929–941 (2017).
Jain, N. et al. TET2 guards against unchecked BATF3-induced CAR T cell expansion. Nature 615, 315–322 (2023).
López-Cobo, S. et al. SUV39H1 ablation enhances long-term CAR T function in solid tumors. Cancer Discov. 14, 120–141 (2024).
Mai, D. et al. Combined disruption of T cell inflammatory regulators regnase-1 and roquin-1 enhances antitumor activity of engineered human T cells. Proc. Natl Acad. Sci. USA 120, e2218632120 (2023).
Craddock, J. A. et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 33, 780–788 (2010).
Kuhn, N. F. et al. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell 35, 473–488.e6 (2019).
Hu, W. et al. Chimeric antigen receptor modified T cell (CAR-T) co-expressed with ICOSL-41BB promote CAR-T proliferation and tumor rejection. Biomed. Pharmacother. 118, 109333 (2019).
Kalbasi, A. et al. Publisher Correction: Potentiating adoptive cell therapy using synthetic IL-9 receptors. Nature 612, E10 (2022).
Chen, C. et al. Construction of PD1/CD28 chimeric-switch receptor enhances anti-tumor ability of c-Met CAR-T in gastric cancer. Oncoimmunology 10, 1901434 (2021).
Supimon, K. et al. Cytotoxic activity of anti-mucin 1 chimeric antigen receptor T cells expressing PD-1-CD28 switch receptor against cholangiocarcinoma cells. Cytotherapy 25, 148–161 (2023).
Zhao, S. et al. Switch receptor T3/28 improves long-term persistence and antitumor efficacy of CAR-T cells. J. Immunother. Cancer 9, e003176 (2021).
Beck, C. et al. Development of a TGFβ–IL-2/15 switch receptor for use in adoptive cell therapy. Biomedicines 11, 459 (2023).
Leen, A. M. et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol. Ther. 22, 1211–1220 (2014).
Lange, S. et al. A chimeric GM-CSF/IL18 receptor to sustain CAR T-cell function. Cancer Discov. 11, 1661–1671 (2021).
Durgin, J. S. et al. Enhancing CAR T function with the engineered secretion of C. perfringens neuraminidase. Mol. Ther. 30, 1201–1214 (2022).
Qu, Y. et al. Adenosine deaminase 1 overexpression enhances the antitumor efficacy of chimeric antigen receptor-engineered T cells. Hum. Gene Ther. 33, 223–236 (2022).
Bai, Y. et al. Enhancement of the in vivo persistence and antitumor efficacy of CD19 chimeric antigen receptor T cells through the delivery of modified TERT mRNA. Cell Discov. 1, 15040 (2015).
Xie, Y. J. et al. Improved antitumor efficacy of chimeric antigen receptor T cells that secrete single-domain antibody fragments. Cancer Immunol. Res. 8, 518–529 (2020).
Suarez, E. R. et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget 7, 34341–34355 (2016).
Zhang, Y. et al. Chimeric antigen receptor T cells engineered to secrete CD40 agonist antibodies enhance antitumor efficacy. J. Transl. Med. 19, 82 (2021).
Yi, Y. et al. CRISPR-edited CART with GM-CSF knockout and auto secretion of IL6 and IL1 blockers in patients with hematologic malignancy. Cell Discov. 7, 27 (2021).
Cao, G. et al. GPC3-targeted CAR-T cells secreting B7H3-targeted BiTE exhibit potent cytotoxicity activity against hepatocellular carcinoma cell in the in vitro assay. Biochem. Biophys. Rep. 31, 101324 (2022).
Wehrli, M. et al. Abstract 569: Mesothelin CAR T cells secreting FAP specific T cell engaging molecule (TEAM) target pancreatic cancer and its tumor microenvironment (TME). Cancer Res. 82, 569 (2022).
Luo, H. et al. Coexpression of IL7 and CCL21 increases efficacy of CAR-T cells in solid tumors without requiring preconditioned lymphodepletion. Clin. Cancer Res. 26, 5494–5505 (2020).
Lei, W. et al. Safety and feasibility of anti-CD19 CAR T cells expressing inducible IL-7 and CCL19 in patients with relapsed or refractory large B-cell lymphoma. Cell Discov. 10, 5 (2024).
Swan, S. L. et al. IL7 and IL7 Flt3L co-expressing CAR T cells improve therapeutic efficacy in mouse EGFRvIII heterogeneous glioblastoma. Front. Immunol 14, 1085547 (2023).
Liu, Y. et al. Armored inducible expression of IL-12 enhances antitumor activity of glypican-3-targeted chimeric antigen receptor-engineered T cells in hepatocellular carcinoma. J. Immunol. 203, 198–207 (2019).
Pegram, H. J. et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141 (2012).
Avanzi, M. P. et al. Engineered tumor-targeted T cells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Rep. 23, 2130–2141 (2018).
Wang, D., Shao, Y., Zhang, X., Lu, G. & Liu, B. IL-23 and PSMA-targeted duo-CAR T cells in prostate cancer eradication in a preclinical model. J. Transl. Med 18, 23 (2020).
Li, X., Daniyan, A. F., Lopez, A. V., Purdon, T. J. & Brentjens, R. J. Cytokine IL-36γ improves CAR T-cell functionality and induces endogenous antitumor response. Leukemia 35, 506–521 (2021).
Seo, H. et al. BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells. Nat. Immunol. 22, 983–995 (2021).
Acknowledgements
The study was supported by the Innovative Medicines Initiative 2 Joint Undertaking, from the European Union’s Horizon 2020 research and innovation programme and EFPIA (grant agreement no. 116026, T2EVOLVE to M.H. and M.L.), the Wilhelm-Sander-Stiftung (grant no. 2022.134.1 to A.V., K.Z.-M. and M.L.), ERA-NET TRANSCAN-3 (EC co-funded call 2021, SmartCAR-T to T.T.N., J.C., M.H., K.Z.-M. and M.L.), the Paula & Rodger Riney Foundation (to M.H. and M.L.), IZKF Würzburg (S-511 to S.S. and M.L., C-543 to M.L.), the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, TRR 221 (subproject A03 to M.H., H.E. and M.L.); TRR338 (subproject A02 to M.H. and M.L., C04 to M.L.); and CRC1525 (seed grant to M.L.)), the Bavarian Cancer Research Center (BZKF; TANGO to M.L. and M.H.), INCA Award by Novartis (to M.L.), the Fonds de la Recherche Scientifique (Postdoctoral fellowship to T.T.N. and funding to J.C.), Fondation Contre le Cancer (C/2020/1447), Université de Liège (J.C.) and FIRS CHU de Liège (J.C.).
Author information
Authors and Affiliations
Contributions
Article design was carried out by T.T.N. and M.L. Figures and tables were produced by M.J., A.B. and T.T.N. The paper was written by C.G., J.J.M., J.C., S.S., K.Z.-M., T.T.N. and M.L. Editing was carried out by C.G., J.J.M., M.H., P.H. and M.L.
Corresponding author
Ethics declarations
Competing interests
M.L. and M.H. are listed as inventors on patent application WO2021/058811A1. M.H. is listed as an inventor on patent applications and granted patents related to CAR T technologies that have been filed by the Fred Hutchinson Cancer Research Center, Seattle, WA and by the University of Würzburg, Würzburg, Germany. M.H. is a co-founder and equity owner of T-CURX GmbH, Würzburg, Germany. M.H. received honoraria from Celgene/BMS, Janssen, Kite/Gilead.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, T.T., Ho, P., Staudt, S. et al. Fine tuning towards the next generation of engineered T cells. Nat. Biomed. Eng 9, 1610–1631 (2025). https://doi.org/10.1038/s41551-025-01492-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41551-025-01492-8