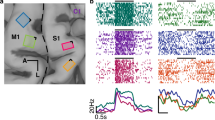
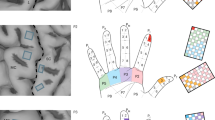
Abstract
Sensation plays a pivotal role in the orchestration of our daily lives. Intracortical microstimulation (ICMS) can elicit artificial sensations in persons who have lost sensation due to neurological injury or disease. Despite ongoing clinical studies to assess the safety and efficacy of ICMS, the mechanisms underlying neural activation by ICMS and their implications for perception are not well understood. This Review delves into the current understanding of ICMS mechanisms, drawing parallels with physiological sensory processing in the cortex. We explore emerging approaches and note challenges to current technologies, including resolution and the tissue response to electrode insertion. We conclude by highlighting the basic principles of ICMS, lingering questions and important focus areas for continued development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Foerster, O. Beitrage zur Pathophysiologie der Sehbahn und der Sehsphare. J. Psychol. Neurol. 39, 463–485 (1929).
Penfield, W. & Boldrey, E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389–443 (1937).
Dobelle, W. Artificial vision for the blind by connecting a television camera to the visual cortex. ASAIO J. 46, 3–9 (2000).
Dobelle, W. H. & Mladejovsky, M. G. Phosphenes produced by electrical stimulation of human occipital cortex, and their application to the development of a prosthesis for the blind. J. Physiol. 243, 553–576 (1974).
Dobelle, W. H., Quest, D. O., Antunes, J. L., Roberts, T. S. & Girvin, J. P. Artificial vision for the blind by electrical stimulation of the visual cortex. Neurosurgery 5, 521–527 (1979).
Brindley, G. S. & Lewin, W. S. The sensations produced by electrical stimulation of the visual cortex. J. Physiol. 196, 479–493 (1968).
Talalla, A., Bullara, L. & Pudenz, R. Electrical stimulation of the human visual cortex preliminary report. Can. J. Neurol. Sci. 1, 236–238 (1974).
Tolias, A. S. et al. Mapping cortical activity elicited with electrical microstimulation using fMRI in the macaque. Neuron 48, 901–911 (2005).
Girvin, J. P. Current status of artificial vision by electrocortical stimulation. Can. J. Neurol. Sci. 15, 58–62 (1988).
Bak, M. et al. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Med. Biol. Eng. Comput. 28, 257–259 (1990).
Schmidt, E. M. et al. Feasibility of a visual prosthesis for the blind based on intracortical microstimulation of the visual cortex. Brain 119, 507–522 (1996).
Flesher, S. N. et al. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 8, 361ra141 (2016).
Armenta Salas, M. et al. Proprioceptive and cutaneous sensations in humans elicited by intracortical microstimulation. eLife 7, e32904 (2018).
Fifer, M. S. et al. Intracortical somatosensory stimulation to elicit fingertip sensations in an individual with spinal cord injury. Neurology 98, E679–E687 (2022).
Greenspon, C. M. et al. Evoking stable and precise tactile sensations via multi-electrode intracortical microstimulation of the somatosensory cortex. Nat. Biomed. Eng. 9, 935–951 (2025).
Fernández, E. et al. Visual percepts evoked with an intracortical 96-channel microelectrode array inserted in human occipital cortex. J. Clin. Invest. 131, e151331 (2021).
Chen, X., Wang, F., Fernandez, E. & Roelfsema, P. R. Shape perception via a high-channel-count neuroprosthesis in monkey visual cortex. Science 370, 1191–1196 (2020).
Beauchamp, M. S. et al. Dynamic stimulation of visual cortex produces form vision in sighted and blind humans. Cell 181, 774–783.e5 (2020).
Christie, B. et al. Perceived timing of cutaneous vibration and intracortical microstimulation of human somatosensory cortex. Brain Stimul. 15, 881–888 (2022).
Osborn, L. E. et al. Intracortical microstimulation of somatosensory cortex enables object identification through perceived sensations. In 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 6259–6262 (IEEE, 2021).
Hughes, C. L., Flesher, S. N. & Gaunt, R. A. Effects of stimulus pulse rate on somatosensory adaptation in the human cortex. Brain Stimul. 15, 987–995 (2022).
Hughes, C. L. et al. Perception of microstimulation frequency in human somatosensory cortex. eLife 10, e65128 (2021).
Hughes, C. et al. Neural stimulation and recording performance in human sensorimotor cortex over 1500 days. J. Neural Eng. 18, 045012 (2021).
Flesher, S. N. et al. A brain–computer interface that evokes tactile sensations improves robotic arm control. Science 372, 831–836 (2021).
Shelchkova, N. D. et al. Microstimulation of human somatosensory cortex evokes task-dependent, spatially patterned responses in motor cortex. Nat. Commun. 14, 7270 (2023).
Hughes, C. L., Herrera, A., Gaunt, R. & Collinger, J. Bidirectional brain–computer interfaces. Handb. Clin. Neurol. 168, 163–181 (2020).
Bjånes, D. A. et al. Charge density of multi-channel intra-cortical micro-stimulation modulates intensity and naturalness of evoked somatosensations. J. Neural Eng. 22, 066025 (2025).
Hughes, C. Biomimetic Intracortical Microstimulation in Human Somatosensory Cortex for a Bidirectional Brain–Computer Interface. PhD thesis, Univ. Pittsburgh (2021).
Hobbs, T. G. et al. Biomimetic stimulation patterns drive natural artificial touch percepts using intracortical microstimulation in humans. J. Neural Eng. 22, 036014 (2025).
Brocker, D. T. & Grill, W. M. in Handbook of Clinical Neurology Vol. 116 (eds Lozano, A. M. & Hallett, M.) 3–18 (Elsevier, 2013).
Gulledge, A. T., Kampa, B. M. & Stuart, G. J. Synaptic integration in dendritic trees. J. Neurobiol. 64, 75–90 (2005).
Tripathy, S. J., Savitskaya, J., Burton, S. D., Urban, N. N. & Gerkin, R. C. NeuroElectro: a window to the world’s neuron electrophysiology data. Front. Neuroinform. 8, 40 (2014).
Koch, C., Rapp, M. & Segev, I. A brief history of time (constants). Cereb. Cortex 6, 93–101 (1996).
Cogan, S. F. Neural stimulation and recording electrodes. Annu. Rev. Biomed. Eng. 10, 275–309 (2008).
Wikswo, J. P., Lin, S. F. & Abbas, R. A. Virtual electrodes in cardiac tissue: a common mechanism for anodal and cathodal stimulation. Biophys. J. 69, 2195–2210 (1995).
Stephanova, D. I. & Mileva, K. Different effects of blocked potassium channels on action potentials, accommodation, adaptation and anode break excitation in human motor and sensory myelinated nerve fibres: computer simulations. Biol. Cybern. 83, 161–167 (2000).
McIntyre, C. C. & Grill, W. M. Selective microstimulation of central nervous system neurons. Ann. Biomed. Eng. 28, 219–233 (2000).
Rattay, F. Analysis of models for external stimulation of axons. IEEE Trans. Biomed. Eng. BME-33, 974–977 (1986).
Geddes, L. A. Accuracy limitations of chronaxie values. IEEE Trans. Biomed. Eng. 51, 176–181 (2004).
IRNICH, W. The chronaxie time and its practical importance. Pacing Clin. Electrophysiol. 3, 292–301 (1980).
Stoney, S. D., Thompson, W. D. & Asanuma, H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J. Neurophysiol. 31, 659–669 (1968).
Kumaravelu, K., Sombeck, J., Miller, L. E., Bensmaia, S. J. & Grill, W. M. Stoney vs. Histed: quantifying the spatial effects of intracortical microstimulation. Brain Stimul. 15, 141–151 (2022).
Dalgleish, H. W. P. et al. How many neurons are sufficient for perception of cortical activity? eLife 9, e58889 (2020).
Urdaneta, M. E., Kunigk, N. G., Delgado, F., Fried, S. I. & Otto, K. J. Layer-specific parameters of intracortical microstimulation of the somatosensory cortex. J. Neural Eng. 18, 55007 (2021).
Altman, K. W. & Plonsey, R. Analysis of the longitudinal and radial resistivity measurements of the nerve trunk. Ann. Biomed. Eng. 17, 313–324 (1989).
Gustafsson, B. & Jankowska, E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J. Physiol. 258, 33–61 (1976).
Jankowska, E., Padel, Y. & Tanaka, R. The mode of activation of pyramidal tract cells by intracortical stimuli. J. Physiol. 249, 617–636 (1975).
Grill, W. M., Cantrell, M. B. & Robertson, M. S. Antidromic propagation of action potentials in branched axons: implications for the mechanisms of action of deep brain stimulation. J. Comput. Neurosci. 24, 81–93 (2008).
Tehovnik, E. J., Tolias, A. S., Sultan, F., Slocum, W. M. & Logothetis, N. K. Direct and indirect activation of cortical neurons by electrical microstimulation. J. Neurophysiol. 96, 512–521 (2006).
Tehovnik, E. J. Electrical stimulation of neural tissue to evoke behavioral responses. J. Neurosci. Methods 65, 1–17 (1996).
Baldissera, F., Lundberg, A. & Udo, M. Stimulation of pre- and postsynaptic elements in the red nucleus. Exp. Brain Res. 15, 151–167 (1972).
Butovas, S. & Schwarz, C. Spatiotemporal effects of microstimulation in rat neocortex: a parametric study using multielectrode recordings. J. Neurophysiol. 90, 3024–3039 (2003).
Romo, R., Hernández, A., Zainos, A. & Salinas, E. Somatosensory discrimination based on cortical microstimulation. Nature 392, 387–390 (1998).
Romo, R., Hernández, A., Zainos, A., Brody, C. D. & Lemus, L. Sensing without touching: psychophysical performance based on cortical microstimulation. Neuron 26, 273–278 (2000).
Binzegger, T., Douglas, R. J. & Martin, K. A. C. A quantitative map of the circuit of cat primary visual cortex. J. Neurosci. 24, 8441–8453 (2004).
Swanson, O. K. & Maffei, A. From hiring to firing: activation of inhibitory neurons and their recruitment in behavior. Front. Mol. Neurosci. 12, 168 (2019).
Blakemore, C. & Tobin, E. A. Lateral inhibition between orientation detectors in the cat’s visual cortex. Exp. Brain Res. 15, 439–440 (1972).
Logothetis, N. K., Kayser, C. & Oeltermann, A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron 55, 809–823 (2007).
Paulk, A. C. et al. Local and distant cortical responses to single pulse intracranial stimulation in the human brain are differentially modulated by specific stimulation parameters. Brain Stimul. 15, 491–508 (2022).
Histed, M. H., Bonin, V. & Reid, R. C. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron 63, 508–522 (2009).
Eles, J. R. & Kozai, T. D. Y. In vivo imaging of calcium and glutamate responses to intracortical microstimulation reveals distinct temporal responses of the neuropil and somatic compartments in layer II/III neurons. Biomaterials 234, 119767 (2020).
Markram, H. et al. Reconstruction and simulation of neocortical microcircuitry. Cell 163, 456–492 (2015).
Flesher, S. N. et al. Restoring touch through intracortical microstimulation of human somatosensory cortex. In New Generation of Circuits and Systems (NGCAS) 185–188 (IEEE, 2017).
McIntyre, C. C. & Grill, W. M. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 88, 1592–1604 (2002).
Grill, W. M. Model-based analysis and design of waveforms for efficient neural stimulation. Prog. Brain Res. 222, 147–162 (2015).
Stieger, K. C., Eles, J. R., Ludwig, K. A. & Kozai, T. D. Y. In vivo microstimulation with cathodic and anodic asymmetric waveforms modulates spatiotemporal calcium dynamics in cortical neuropil and pyramidal neurons of male mice. J. Neurosci. Res. 98, 2072–2095 (2020).
Stieger, K. C., Eles, J. R., Ludwig, K. A. & Kozai, T. D. Y. Intracortical microstimulation pulse waveform and frequency recruits distinct spatiotemporal patterns of cortical neuron and neuropil activation. J. Neural Eng. 19, 026024 (2022).
Bari, B. A., Ollerenshaw, D. R., Millard, D. C., Wang, Q. & Stanley, G. B. Behavioral and electrophysiological effects of cortical microstimulation parameters. PLoS ONE 8, e82170 (2013).
Karatum, O., Han, M., Erdogan, E. T., Karamursel, S. & Nizamoglu, S. Physical mechanisms of emerging neuromodulation modalities. J. Neural Eng. 20, 031001 (2023).
Plonsey, R. & Barr, R. C. Bioelectricity: A Quantitative Approach (Springer, 2007).
Eles, J. R., Stieger, K. C. & Kozai, T. D. Y. The temporal pattern of intracortical microstimulation pulses elicits distinct temporal and spatial recruitment of cortical neuropil and neurons. J. Neural Eng. 18, 15001 (2021).
Michelson, N. J., Eles, J. R., Vazquez, A. L., Ludwig, K. A. & Kozai, T. D. Y. Calcium activation of cortical neurons by continuous electrical stimulation: frequency dependence, temporal fidelity, and activation density. J. Neurosci. Res. 97, 620–638 (2019).
Callier, T., Brantly, N. W., Caravelli, A. & Bensmaia, S. J. The frequency of cortical microstimulation shapes artificial touch. Proc. Natl Acad. Sci. USA 117, 1191–1200 (2020).
Wu, G. K. et al. Amplitude- and frequency-dependent activation of layer II/III neurons by intracortical microstimulation. iScience 26, 108140 (2023).
Hughes, C. L., Stieger, K. C., Chen, K., Vazquez, A. L. & Kozai, T. D. Y. Spatiotemporal properties of cortical excitatory and inhibitory neuron activation by sustained and bursting electrical microstimulation. iScience 28, 112707 (2025).
O’Doherty, J. E. et al. Active tactile exploration using a brain–machine–brain interface. Nature 479, 228–231 (2011).
O’Doherty, J. E., Shokur, S., Medina, L. E., Lebedev, M. A. & Nicolelis, M. A. L. Creating a neuroprosthesis for active tactile exploration of textures. Proc. Natl Acad. Sci. USA 116, 21821–21827 (2019).
Friedman, R. M., Chen, L. M. & Roe, A. W. Modality maps within primate somatosensory cortex. Proc. Natl Acad. Sci. USA 101, 12724–12729 (2004).
Prsa, M., Morandell, K., Cuenu, G. & Huber, D. Feature-selective encoding of substrate vibrations in the forelimb somatosensory cortex. Nature 567, 384–388 (2019).
Wang, L. & Yau, J. M. Signatures of vibration frequency tuning in human neocortex. Preprint at bioRxiv https://doi.org/10.1101/2021.10.03.462923 (2021).
Chen, L. M., Friedman, R. M., Ramsden, B. M., LaMotte, R. H. & Roe, A. W. Fine-scale organization of SI (area 3b) in the squirrel monkey revealed with intrinsic optical imaging. J. Neurophysiol. 86, 3011–3029 (2001).
Sur, M., Wall, J. T. & Kaas, J. H. Modular distribution of neurons with slowly adapting and rapidly adapting responses in area 3b somatosensory cortex in monkeys. J. Neurophysiol. 51, 724–744 (1984).
Saal, H. P. & Bensmaia, S. J. Touch is a team effort: interplay of submodalities in cutaneous sensibility. Trends Neurosci. 37, 689–697 (2014).
Grill, W. M. Temporal pattern of electrical stimulation is a new dimension of therapeutic innovation. Curr. Opin. Biomed. Eng. 8, 1–6 (2018).
Hughes, C. L. & Gaunt, R. A. Changes in interpulse spacing changes tactile perception of microstimulation in human somatosensory cortex. In International IEEE/EMBS Conference on Neural Engineering, NER 660–663 (IEEE, 2021).
Birznieks, I. & Vickery, R. M. Spike timing matters in novel neuronal code involved in vibrotactile frequency perception. Curr. Biol. 27, 1485–1490.e2 (2017).
Ng, K. K. W. et al. Perceived frequency of aperiodic vibrotactile stimuli depends on temporal encoding. In International Conference on Human Haptic Sensing and Touch Enabled Computer Application Lecture Notes in Computer Science Vol. 10893 (eds Prattichizzo, D. et al.) 199–208 (Springer, 2018).
Aksöz, E. A., Laubacher, M., Binder-Macleod, S. & Hunt, K. J. Effect of stochastic modulation of inter-pulse interval during stimulated isokinetic leg extension. Eur. J. Transl. Myol. 26, 6160 (2016).
Aksöz, E. A. et al. Stochastically modulated inter-pulse intervals to increase the efficiency of functional electrical stimulation cycling. J. Rehabil. Assist. Technol. Eng. 5, 2055668318767364 (2018).
Bloodworth, D. M. et al. Comparison of stochastic vs. conventional transcutaneous electrical stimulation for pain modulation in patients with electromyographically documented radiculopathy. Am. J. Phys. Med. Rehabil. 83, 584–591 (2004).
Rubinstein, J. T. & Hong, R. Signal coding in cochlear implants: exploiting stochastic effects of electrical stimulation. Ann. Otol. Rhinol. Laryngol. 112, 14–19 (2003).
Tan, D. W. et al. A neural interface provides long-term stable natural touch perception. Sci. Transl. Med. 6, 257ra138 (2014).
McCreery, D. B., Bullara, L. A. & Agnew, W. F. Neuronal activity evoked by chronically implanted intracortical microelectrodes. Exp. Neurol. 92, 147–161 (1986).
Agnew, W. F. & McCreery, D. B. Considerations for safety with chronically implanted nerve electrodes. Epilepsia 31, S27–S32 (1990).
McCreery, D., Pikov, V. & Troyk, P. R. Neuronal loss due to prolonged controlled-current stimulation with chronically implanted microelectrodes in the cat cerebral cortex. J. Neural Eng. 7, 036005 (2010).
Agnew, W. F., Yuen, T. G. H., McCreery, D. B. & Bullara, L. A. Histopathologic evaluation of prolonged intracortical electrical stimulation. Exp. Neurol. 92, 162–185 (1986).
McCreery, D. B., Agnew, W. F., Yuen, T. G. H. & Bullara, L. Charge density and charge per phase as cofactors in neural injury induced by electrical stimulation. IEEE Trans. Biomed. Eng. 37, 996–1001 (1990).
McCreery, D. B., Agnew, W. F. & Bullara, L. A. The effects of prolonged intracortical microstimulation on the excitability of pyramidal tract neurons in the cat. Ann. Biomed. Eng. 30, 107–119 (2002).
Dong, Q. et al. Stability assessment of ultramicroelectrode arrays in neural stimulation: an electrochemical impedance spectroscopy analysis. In 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 1–4 (IEEE, 2024).
McCreery, D. B., Yuen, T. G. H., Agnew, W. F. & Bullara, L. A. A characterization of the effects on neuronal excitability due to prolonged microstimulation with chronically implanted microelectrodes. IEEE Trans. Biomed. Eng. 44, 931–939 (1997).
Berglund, U. & Berglund, B. Adaption and recovery in vibrotactile perception. Percept. Mot. Skills 30, 843–853 (1970).
Hollins, M., Goble, A. K., Whitsel, B. L. & Tommerdahl, M. Time course and action spectrum of vibrotactile adaptation. Somatosens. Mot. Res. 7, 205–221 (1990).
Whitmire, C. J. & Stanley, G. B. Rapid sensory adaptation redux: a circuit perspective. Neuron 92, 298–315 (2016).
Guo, Z., Feng, Z., Wang, Y. & Wei, X. Simulation study of intermittent axonal block and desynchronization effect induced by high-frequency stimulation of electrical pulses. Front. Neurosci. 12, 858 (2018).
Sombeck, J. et al. Characterizing the short-latency evoked response to intracortical microstimulation across a multi-electrode array. J. Neural Eng. 19, 026044 (2022).
Hughes, C. & Kozai, T. Dynamic amplitude modulation of microstimulation evokes biomimetic onset and offset transients and reduces depression of evoked calcium responses in sensory cortices. Brain Stimul. 16, 939–965 (2023).
Dadarlat, M. C., Sun, Y. J. & Stryker, M. P. Activity-dependent recruitment of inhibition and excitation in the awake mammalian cortex during electrical stimulation. Neuron 112, 821–834.e4 (2024).
Suematsu, N., Vazquez, A. L. & Kozai, T. D. Y. Activation and depression of neural and hemodynamic responses induced by the intracortical microstimulation and visual stimulation in the mouse visual cortex. J. Neural Eng. 21, 026033 (2024).
Glasser, M. F. et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016).
Roux, F.-E., Djidjeli, I. & Durand, J.-B. Functional architecture of the somatosensory homunculus detected by electrostimulation. J. Physiol. 596, 941–956 (2018).
Penfield, W. & Jasper, H. Epilepsy and the Functional Anatomy of the Human Brain 692–738 (Little Brown, 1954).
Sur, M., Wall, J. T. & Kaas, J. H. Modular segregation of functional cell classes within the postcentral somatosensory cortex of monkeys. Science 212, 1059–1061 (1981).
Kaas, J. H., Nelson, R. J., Sur, M., Lin, C. S. & Merzenich, M. M. Multiple representations of the body within the primary somatosensory cortex of primates. Science 204, 521–523 (1979).
McMullen, D. P. et al. Novel intraoperative online functional mapping of somatosensory finger representations for targeted stimulating electrode placement. J. Neurosurg. https://doi.org/10.3171/2020.9.JNS202675 (2021).
Mountcastle, V. B. The columnar organization of the neocortex. Brain 120, 701–722 (1997).
Hubel, D. H. & Wiesel, T. N. Shape and arrangement of columns in cat’s striate cortex. J. Physiol. 165, 559–568 (1963).
DeYoe, E. A., Lewine, J. D. & Doty, R. W. Laminar variation in threshold for detection of electrical excitation of striate cortex by macaques. J. Neurophysiol. 94, 3443–3450 (2005).
Tehovnik, E. J. & Slocum, W. M. Depth-dependent detection of microampere currents delivered to monkey V1. Eur. J. Neurosci. 29, 1477–1489 (2009).
Millard, D. C., Whitmire, C. J., Gollnick, C. A., Rozell, C. J. & Stanley, G. B. Electrical and optical activation of mesoscale neural circuits with implications for coding. J. Neurosci. 35, 15702–15715 (2015).
Narayanan, R. T., Udvary, D. & Oberlaender, M. Cell type-specific structural organization of the six layers in rat barrel cortex. Front. Neuroanat. 11, 91 (2017).
Obermayer, J. et al. Lateral inhibition by Martinotti interneurons is facilitated by cholinergic inputs in human and mouse neocortex. Nat. Commun. 9, 4101 (2018).
Clark, G. A., Ledbetter, N. M., Warren, D. J. & Harrison, R. R. Recording sensory and motor information from peripheral nerves with Utah Slanted Electrode Arrays. In Proc. Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS 4641–4644 (IEEE, 2011).
Wendelken, S. et al. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves. J. Neuroeng. Rehabil. 14, 121 (2017).
Zhao, Z. et al. Ultraflexible electrode arrays for months-long high-density electrophysiological mapping of thousands of neurons in rodents. Nat. Biomed. Eng. 7, 520–532 (2022).
Orlemann, C. et al. Flexible polymer electrodes for stable prosthetic visual perception in mice. Adv. Health. Mater. 13, e2304169 (2024).
Lycke, R. et al. Low-threshold, high-resolution, chronically stable intracortical microstimulation by ultraflexible electrodes. Cell Rep. 42, 112554 (2023).
Jadi, M. P. & Sejnowski, T. J. Regulating cortical oscillations in an inhibition-stabilized network. Proc. IEEE 102, 830–842 (2014).
Ozeki, H., Finn, I. M., Schaffer, E. S., Miller, K. D. & Ferster, D. Inhibitory stabilization of the cortical network underlies visual surround suppression. Neuron 62, 578–592 (2009).
O’Rawe, J. F. et al. Excitation creates a distributed pattern of cortical suppression due to varied recurrent input. Neuron 111, 4086–4101.e5 (2023).
Rich, S., Hutt, A., Skinner, F. K., Valiante, T. A. & Lefebvre, J. Neurostimulation stabilizes spiking neural networks by disrupting seizure-like oscillatory transitions. Sci. Rep. 10, 15408 (2020).
Osorio, I. et al. Automated seizure abatement in humans using electrical stimulation. Ann. Neurol. 57, 258–268 (2005).
Velasco, A. L. et al. Electrical stimulation of the hippocampal epileptic foci for seizure control: a double-blind, long-term follow-up study. Epilepsia 48, 1895–1903 (2007).
Aberra, A. S., Peterchev, A. V. & Grill, W. M. Biophysically realistic neuron models for simulation of cortical stimulation. J. Neural Eng. 15, 066023 (2018).
Yavorska, I. & Wehr, M. Somatostatin-expressing inhibitory interneurons in cortical circuits. Front. Neural Circuits 10, 76 (2016).
Ma, Y., Hu, H. & Agmon, A. Short-term plasticity of unitary inhibitory-to-inhibitory synapses depends on the presynaptic interneuron subtype. J. Neurosci. 32, 983–988 (2012).
Reyes, A. et al. Target-cell-specific facilitation and depression in neocortical circuits. Nat. Neurosci. 1, 279–284 (1998).
Mruczek, R. E. B. & Sheinberg, D. L. Stimulus selectivity and response latency in putative inhibitory and excitatory neurons of the primate inferior temporal cortex. J. Neurophysiol. 108, 2725–2736 (2012).
Li, L. Y. et al. Differential receptive field properties of parvalbumin and somatostatin inhibitory neurons in mouse auditory cortex. Cereb. Cortex 25, 1782–1791 (2015).
Jang, H. J. et al. Distinct roles of parvalbumin and somatostatin interneurons in gating the synchronization of spike times in the neocortex. Sci. Adv. 6, eaay5333 (2020).
Tremblay, R., Lee, S. & Rudy, B. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292 (2016).
Goldberg, E. M. et al. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron 58, 387–400 (2008).
Erisir, A., Lau, D., Rudy, B. & Leonard, C. S. Function of specific K+ channels in sustained high-frequency firing of fast-spiking neocortical interneurons. J. Neurophysiol. 82, 2476–2489 (1999).
Rudy, B. et al. Contributions of Kv3 channels to neuronal excitability. Ann. N. Y. Acad. Sci. 868, 304–343 (1999).
Kawaguchi, Y. & Kubota, Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. J. Neurosci. 16, 2701–2715 (1996).
Kawaguchi, Y. & Kubota, Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486 (1997).
Benardo, L. S. Separate activation of fast and slow inhibitory postsynaptic potentials in rat neocortex in vitro. J. Physiol. 476, 203–215 (1994).
Mateo, C. et al. In vivo optogenetic stimulation of neocortical excitatory neurons drives brain-state-dependent inhibition. Curr. Biol. 21, 1593–1602 (2011).
Avermann, M., Tomm, C., Mateo, C., Gerstner, W. & Petersen, C. C. H. Microcircuits of excitatory and inhibitory neurons in layer 2/3 of mouse barrel cortex. J. Neurophysiol. 107, 3116–3134 (2012).
Li, N. et al. Spatiotemporal constraints on optogenetic inactivation in cortical circuits. eLife 8, e48622 (2019).
Margalit, S. N. & Slovin, H. Spatio-temporal activation patterns of neuronal population evoked by optostimulation and the comparison to electrical microstimulation. Sci. Rep. 13, 12689 (2023).
Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Polikov, V. S., Tresco, P. A. & Reichert, W. M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 148, 1–18 (2005).
Chen, K., Wellman, S. M., Yaxiaer, Y., Eles, J. R. & Kozai, T. D. In vivo spatiotemporal patterns of oligodendrocyte and myelin damage at the neural electrode interface. Biomaterials 268, 120526 (2021).
Roitbak, A. I. & Fanardjian, V. V. Depolarization of cortical glial cells in response to electrical stimulation of the cortical surface. Neuroscience 6, 2529–2537 (1981).
Ma, Z. et al. Two-photon calcium imaging of neuronal and astrocytic responses: the influence of electrical stimulus parameters and calcium signaling mechanisms. J. Neural Eng. 18, 046096 (2021).
Cha, M., Lee, K. H. & Lee, B. H. Astroglial changes in the zona incerta in response to motor cortex stimulation in a rat model of chronic neuropathy. Sci. Rep. 10, 943 (2020).
Gary, D. S., Malone, M., Capestany, P., Houdayer, T. & Mcdonald, J. W. Electrical stimulation promotes the survival of oligodendrocytes in mixed cortical cultures. J. Neurosci. Res. 90, 72–83 (2012).
Chen, K., Stieger, K. C. & Kozai, T. D. Challenges and opportunities of advanced gliomodulation technologies for excitation–inhibition balance of brain networks. Curr. Opin. Biotechnol. 72, 112–120 (2021).
Bahr-Hosseini, M. & Bikson, M. Neurovascular-modulation: a review of primary vascular responses to transcranial electrical stimulation as a mechanism of action. Brain Stimul. 14, 837–847 (2021).
Gellner, A. K., Frase, S., Reis, J. & Fritsch, B. Direct current stimulation increases blood flow and permeability of cortical microvasculature in vivo. Eur. J. Neurol. 30, 362–371 (2023).
Cauli, B. & Hamel, E. Brain perfusion and astrocytes. Trends Neurosci. 41, 409–413 (2018).
Wellman, S. M. et al. Dynamic changes in the structure and function of brain mural cells around chronically implanted microelectrodes. Biomaterials 315, 122963 (2025).
Vazquez, A., Fukuda, M. & Kim, S. Inhibitory neuron activity contributions to hemodynamic responses and metabolic load examined using an inhibitory optogenetic mouse model. Cereb. Cortex 28, 4105–4119 (2018).
Krawchuk, M. B., Ruff, C. F., Yang, X., Ross, S. E. & Vazquez, A. L. Optogenetic assessment of VIP, PV, SOM and NOS inhibitory neuron activity and cerebral blood flow regulation in mouse somato-sensory cortex. J. Cereb. Blood Flow. Metab. 40, 1427–1440 (2020).
Anenberg, E., Chan, A. W., Xie, Y., LeDue, J. M. & Murphy, T. H. Optogenetic stimulation of GABA neurons can decrease local neuronal activity while increasing cortical blood flow. J. Cereb. Blood Flow Metab. 35, 1579–1586 (2015).
Kozai, T. D. Y., Jaquins-Gerstl, A. S., Vazquez, A. L., Michael, A. C. & Cui, X. T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 6, 48–67 (2015).
Chen, X. et al. Chronic stability of a neuroprosthesis comprising multiple adjacent Utah arrays in monkeys. J. Neural Eng. 20, 036039 (2023).
Woeppel, K. et al. Explant analysis of Utah electrode arrays implanted in human cortex for brain-computer-interfaces. Front. Bioeng. Biotechnol. 9, 759711 (2021).
Barrese, J. C. et al. Failure mode analysis of silicon-based intracortical microelectrode arrays in non-human primates. J. Neural Eng. 10, 066014 (2013).
Piech, D. K. et al. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 4, 207–222 (2020).
Lee, A. H., Lee, J., Leung, V., Larson, L. & Nurmikko, A. Patterned electrical brain stimulation by a wireless network of implantable microdevices. Nat. Commun. 15, 10093 (2024).
Burton, A. et al. Wireless, battery-free, and fully implantable electrical neurostimulation in freely moving rodents. Microsyst. Nanoeng. 7, 62 (2021).
Kim, S. et al. Integrated wireless neural interface based on the Utah electrode array. Biomed. Microdevices 11, 453–466 (2009).
Zhang, Z. & Dai, J. Fully implantable wireless brain–computer interface for humans: advancing toward the future. Innovation 5, 100595 (2024).
Simeral, J. D. et al. Home use of a percutaneous wireless intracortical brain–computer interface by individuals with tetraplegia. IEEE Trans. Biomed. Eng. 68, 2313–2325 (2021).
Weiss, J. M., Gaunt, R. A., Franklin, R., Boninger, M. L. & Collinger, J. L. Demonstration of a portable intracortical brain–computer interface. Brain Comput. Interfaces 6, 106–117 (2019).
Wellman, S. M. et al. A materials roadmap to functional neural interface design. Adv. Funct. Mater. 28, 1701269 (2018).
Opie, N. in Brain–Computer Interface Research (eds Guger, C. et al.) 127–132 (Springer, 2021).
Zheng, X. S., Tan, C., Castagnola, E. & Cui, X. T. Electrode materials for chronic electrical microstimulation. Adv. Health. Mater. 10, 2100119 (2021).
Kozai, T. D. Y. et al. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 11, 1065–1073 (2012).
Cogan, S. F., Ludwig, K. A., Welle, C. G. & Takmakov, P. Tissue damage thresholds during therapeutic electrical stimulation. J. Neural Eng. 13, 021001 (2016).
Johnson, M. D., Otto, K. J. & Kipke, D. R. Repeated voltage biasing improves unit recordings by reducing resistive tissue impedances. IEEE Trans. Neural Syst. Rehabil. Eng. 13, 160–165 (2005).
Otto, K. J., Johnson, M. D. & Kipke, D. R. Voltage pulses change neural interface properties and improve unit recordings with chronically implanted microelectrodes. IEEE Trans. Biomed. Eng. 53, 333–340 (2006).
Eles, J. R., Vazquez, A. L., Kozai, T. D. Y. & Cui, X. T. In vivo imaging of neuronal calcium during electrode implantation: spatial and temporal mapping of damage and recovery. Biomaterials 174, 79–94 (2018).
Bjånes, D. A. et al. Quantifying physical degradation alongside recording and stimulation performance of 980 intracortical microelectrodes chronically implanted in three humans for 956–2130 days. Acta Biomater. 198, 188–206 (2025).
Szymanski, L. J. et al. Neuropathological effects of chronically implanted, intracortical microelectrodes in a tetraplegic patient. J. Neural Eng. 18, 0460b9 (2021).
Urdaneta, M. E. et al. The long-term stability of intracortical microstimulation and the foreign body response are layer dependent. Front. Neurosci. 16, 908858 (2022).
Okorokova, E. V., He, Q. & Bensmaia, S. J. Biomimetic encoding model for restoring touch in bionic hands through a nerve interface. J. Neural Eng. 15, 066033 (2018).
Saal, H. P., Delhaye, B. P., Rayhaun, B. C. & Bensmaia, S. J. Simulating tactile signals from the whole hand with millisecond precision. Proc. Natl Acad. Sci. USA 114, E5693–E5702 (2017).
Valle, G. et al. Biomimetic intraneural sensory feedback enhances sensation naturalness, tactile sensitivity, and manual dexterity in a bidirectional prosthesis. Neuron 100, 37–45 (2018).
van der Grinten, M. et al. Towards biologically plausible phosphene simulation for the differentiable optimization of visual cortical prostheses. eLife 13, e85812 (2024).
Kumaravelu, K. & Grill, W. M. Neural mechanisms of the temporal response of cortical neurons to intracortical microstimulation. Brain Stimul. 17, 365–381 (2024).
Kunigk, N. G., Urdaneta, M. E., Malone, I. G., Delgado, F. & Otto, K. J. Reducing behavioral detection thresholds per electrode via synchronous, spatially-dependent intracortical microstimulation. Front. Neurosci. 16, 876142 (2022).
Meikle, S. J., Hagan, M. A., Price, N. S. C. & Wong, Y. T. Intracortical current steering shifts the location of evoked neural activity. J. Neural Eng. 19, 035003 (2022).
Meikle, S. J., Hagan, M. A., Price, N. S. C. & Wong, Y. T. Cortical layering disrupts multi-electrode current steering. J. Neural Eng. 20, 036031 (2023).
Hokanson, J. A., Gaunt, R. A. & Weber, D. J. Effects of synchronous electrode pulses on neural recruitment during multichannel microstimulation. Sci. Rep. 8, 13067 (2018).
Shannon, R. V.A model of safe levels for electrical stimulation. IEEE Trans. Biomed. Eng. 39, 424–426 (1992).
Acknowledgements
We dedicate this Review to the late Sliman Bensmaia, whose work on somatosensation and neuroprostheses in both non-human primates and humans helped lay the foundation for much of our current understanding of ICMS in sensory cortices and strongly motivated this work. We also thank F. Li and N. Suematsu for providing feedback on the manuscript. This Review was supported by NIH F32MH130022, T32NS086749, R01NS105691, R01NS115707, R01NS129632, NSF CAREER CBET 1943906, NIH U01 NS126052, R01 NS117405 and R37 NS040894.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
X.C. is the co-founder and shareholder of Phosphoenix BV. Since the preparation of this Review, C.H. has become employed by Blackrock Neurotech, which has developed technology discussed herein. This employment did not influence the content or conclusions of the Review. T.D.Y.K. and W.G. declare no competing interests.
Peer review
Peer review information
Nature Biomedical Engineering thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hughes, C., Chen, X., Grill, W. et al. Neural mechanisms underlying intracortical microstimulation for sensory restoration. Nat. Biomed. Eng (2026). https://doi.org/10.1038/s41551-025-01583-6
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41551-025-01583-6