Abstract
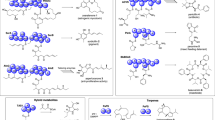
Studies of secondary metabolites (natural products) that cover their isolation, chemical synthesis and bioactivity investigation present myriad opportunities for discovery. For example, the isolation of novel secondary metabolites can inspire advances in chemical synthesis strategies to achieve their practical preparation for biological evaluation. In the process, chemical synthesis can also provide unambiguous structural characterization of the natural products. Although the isolation, chemical synthesis and bioactivity studies of natural products are mutually beneficial, they are often conducted independently. Here, we demonstrate the benefits of a collaborative study of the phomactins, diterpenoid fungal metabolites that serve as antagonists of the platelet activating factor receptor. Our isolation of novel phomactins has spurred the development of a bioinspired, unified approach that achieves the total syntheses of six congeners. We also demonstrate in vitro the beneficial effects of several phomactins in suppressing the rate of repopulation of tumour cells following gamma radiation therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Maier, M. E. Structural revisions of natural products by total synthesis. Nat. Prod. Rep. 26, 1105–1124 (2009).
Wilson, R. M. & Danishefsky, S. J. Small molecule natural products in the discovery of therapeutic agents: the synthesis connection. J. Org. Chem. 71, 8329–8351 (2006).
Maier, M. E. Design and synthesis of analogues of natural products. Org. Biomol. Chem. 13, 5302–5343 (2015).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 79, 629–661 (2016).
Sugano, M. et al. Phomactin A: a novel PAF antagonist from a marine fungus Phoma sp. J. Am. Chem. Soc. 113, 5463–5464 (1991).
Sugano, M. et al. Phomactins, novel PAF antagonists from marine fungus Phoma sp. J. Org. Chem. 59, 564–569 (1994).
Sugano, M. et al. Phomactin E, F, and G: new phomactin-group PAF antagonists from a marine fungus Phoma sp. J. Antibiot. 48, 1188–1190 (1995).
Chu, M. et al. A novel class of platelet activating factor antagonists from Phoma sp. J. Antibiot. 46, 554–563 (1993).
Koyama, K. et al. Phomactin H, a novel diterpene from an unidentified marine-derived fungus. Tetrahedron Lett. 45, 6947–6948 (2004).
Ishino, M. et al. Phomactin I, 13-epi-phomactin I, and phomactin J, three novel diterpenes from a marine-derived fungus. Tetrahedron 66, 2594–2597 (2010).
Ishino, M. et al. Phomactins K–M, three novel phomactin-type diterpenes from a marine-derived fungus. Tetrahedron 68, 8572–8576 (2012).
Ishino, M. et al. Three novel phomactin-type diterpenes from a marine-derived fungus. Tetrahedron Lett. 57, 4341–4344 (2016).
Passarini, M. R. Z., Santos, C., Lima, N., Berlinck, R. G. S. & Sette, L. D. Filamentous fungi from the Atlantic marine sponge Dragmacidon reticulatum. Arch. Microbiol. 195, 99–111 (2013).
Goldring, W. P. D. & Pattenden, G. The phomactins. A novel group of terpenoid platelet activating factor antagonists related biogenetically to the taxanes. Acc. Chem. Res. 39, 354–361 (2006).
Prescott, S. M., Zimmerman, G. A., Stafforini, D. M. & McIntyre, T. M. Platelet-activating factor and related lipid mediators. Annu. Rev. Biochem. 69, 419–445 (2000).
Onuchic, A. C. et al. Expression of PAFR as part of a pro-survival response to chemotherapy: a novel target for combination therapy in melanoma. Mediat. Inflamm. 2012, 175408 (2012).
Sahu, R. P. et al. Radiation therapy generates platelet-activating factor agonists. Oncotarget 7, 20788–20800 (2016).
Ciesielski, J. & Frontier, A. The phomactin natural products from isolation to total synthesis: a review. Org. Prep. Proced. Int 46, 214–251 (2014).
Goldring, W. P. D.., & Pattenden, G.. A total synthesis of phomactin. Chem. Commun. 2002, 1736–1737 (2002).
Goldring, W. P. D. & Pattenden, G. Total synthesis of (±)-phomactin G, a platelet activating factor antagonist from the marine fungus Phoma sp. Org. Biomol. Chem. 2, 466–473 (2004).
Huang, J., Wu, C. & Wulff, W. D. Total synthesis of (±)-phomactin B2 via an intramolecular cyclohexadienone annulation of a chromium carbene complex. J. Am. Chem. Soc. 129, 13366–13367 (2007).
Tang, Y., Cole, K. P., Buchanan, G. S., Li, G. & Hsung, R. P. Total synthesis of phomactin A. Org. Lett. 11, 1591–1594 (2009).
Miyaoka, H., Saka, Y., Miura, S. & Yamada, Y. Total synthesis of phomactin D. Tetrahedron Lett. 37, 7107–7110 (1996).
Mohr, P. J. & Halcomb, R. L. Total synthesis of (+)-phomactin A using a B-alkyl Suzuki macrocyclization. J. Am. Chem. Soc. 125, 1712–1713 (2003).
Tokiwano, T., Fukushi, E., Endo, T. & Oikawa, H. Biosynthesis of phomactins: common intermediate phomactatriene and taxadiene. Chem. Commun. 1324–1325 (2004).
Tokiwano, T. et al. Proposed mechanism for diterpene synthases in the formation of phomactatriene and taxadiene. Org. Biomol. Chem. 3, 2713–2722 (2005).
Masarwa, A., Weber, M. & Sarpong, R. Selective C–C and C–H bond activation/cleavage of pinene derivatives: synthesis of enantiopure cyclohexenone scaffolds and mechanistic insights. J. Am. Chem. Soc. 137, 6327–6334 (2015).
Bermejo, F. A. et al. Ti(iii)-promoted cyclizations. Application to the synthesis of (E)-endo-bergamoten-12-oic acids. Moth oviposition stimulants isolated from Lycopersicon hirsutum. Tetrahedron 62, 8933–8942 (2002).
Murakami, M., Makino, M., Ashida, S. & Matsuda, T. Construction of carbon frameworks through β-carbon elimination mediated by transition metals. Bull. Chem. Soc. Jpn 79, 1315–1321 (2006).
Cramer, N. & Seiser, T. β-Carbon elimination from cyclobutanols: a clean access to alkylrhodium intermediates bearing a quaternary stereogenic center. Synlett 449–460 (2011).
Lipshutz, B. H. & Miller, T. A. Deprotection of ‘SEM’ ethers: a convenient, general procedure. Tetrahedron Lett. 30, 7149–7152 (1989).
Nicolaou, K. C. & Harrison, S. T. Total synthesis of abyssomicin C and atrop-abyssomicin C. Angew. Chem. Int. Ed. 45, 3256–3260 (2006).
Catino, A. J., Forslund, R. E. & Doyle, M. P. Dirhodium(ii) caprolactamate: an exceptional catalyst for allylic oxidation. J. Am. Chem. Soc. 126, 13622–13623 (2004).
Honda, T. & Mizutani, H. Regioselective ring-opening of 2,3-epoxy alcohols with tetramethylammonium triacetoxyborohydride. Heterocycles 48, 1753–1757 (1998).
Evans, D. A., Chapman, K. T. & Carreira, E. M. Directed reduction of β-hydroxy ketones employing tetramethylammonium tracetoxyborohydride. J. Am. Chem. Soc. 110, 3560–3578 (1988).
Jancar, S. & Chammas, R. PAF receptor and tumor growth. Curr. Drug Targets 15, 982–987 (2014).
Bussolati, B. et al. PAF produced by human breast cancer cells promotes migration and proliferation of tumor cells and neo-angiogenesis. Am. J. Pathol. 157, 1713–1725 (2000).
Chan, F. K.-M., Moriwaki, K. & De Rosa, M. J. in Immune Homeostasis. Methods and Protocols Vol. 979 (eds Snow, A. & Lenardo, M.) 65–70 (Humana, Totowa, 2013).
da Silva-Jr, I. A., Chammas, R., Lepique, A. P. & Jancar, S. Platelet-activating factor (PAF) receptor as a promising target for cancer cell repopulation after radiotherapy. Oncogenesis 6, e296 (2017).
Rios, F. J. O., Koga, M. M., Ferracini, M. & Jancar, S. Co-stimulation of PAFR and CD36 is required for oxLDL-induced human macrophages activation. PLoS ONE 7, e36632 (2012).
Huang, Q. et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 17, 860–866 (2011).
Acknowledgements
The authors thank K. Koyama (Meiji Pharmaceutical University) for providing spectral data for phomactin P and S. Dreher and A. Buevich (Merck Pharmaceuticals) for the 1H NMR spectrum of Sch 49027. R.S. thanks the National Science Foundation (CHE-1566430) for financial support. Y.K. thanks the Japan Society for the Promotion of Science (JSPS) for an Overseas Research Fellowship. P.R.L. thanks the National Science Foundation for a graduate research fellowship. S.C. acknowledges a National Science and Engineering Council–Canada (NSERC) Postdoctoral Fellowship. R.G.S.B and J.R.G. thank FAPESP (Fundaçao de Amparo a Pesquisa de São Paulo) for financial support (BIOTA-BIOprospecTA 2013/50228-8, 2015/01017-0 and 2017/06014-4) and K.J.N. thanks CNPq for a PhD scholarship. S.J. and I.A.S.J. are grateful to FAPESP for financial support and a graduate research fellowship. N.N. thanks JSPS for a travel fellowship. K.B. is grateful to the Amgen Scholar Program (UC Berkeley) for support. R.J.A. thanks the NSERC for funding. The KBP cells were supplied by J.B. Travers (Indiana University, Indianapolis, IN) and the TC-1 cell line was donated to us by T.-C. Wu (Johns Hopkins, Baltimore). The authors thank K. Owens for insightful discussions regarding the structure of Sch 49027.
Author information
Authors and Affiliations
Contributions
R.G.S.B. (isolation and identification), Y.K. (chemical synthesis), R.S. (chemical synthesis) and S.J. (biological assays) wrote each of the corresponding sections and R.S. composed the manuscript. P.R.L., S.C. and R.S. conceived the general plan for the chemical synthesis of phomactin R. Y.K. and R.S. designed the plan for the chemical syntheses of phomactins A, K, P, T and Sch 49027. P.R.L. and S.C. carried out the initial studies on the cyclobutanol opening, cyclohexenone functionalization, and macrocyclization that provided a part of the basis of the reported syntheses. Y.K. conducted the chemical reactions reported herein and compiled the Supplementary Information. N.N. conducted the large-scale preparation of 18 and 20 to support front-line synthetic studies. A.R. and K.B. carried out exploratory studies on the cyclobutanol intermediates and subsequent functionalizations. K.J.N. isolated the new phomactins and elucidated their structures jointly with J.R.G. V.M.D. performed X-ray diffraction analysis. A.G.F., D.E.W. and R.J.A. provided support and performed NMR analyses of the new phomactins. L.D.S. provided and identified the fungal strain. I.A.S.J. and S.J. performed the assays for PAFR antagonistic activity and the repopulation assays with cancer cell lineages.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary experimental details and compound characterization data
Crystallographic data
CIF for compound 3; CCDC reference: 1830519
Rights and permissions
About this article
Cite this article
Kuroda, Y., Nicacio, K.J., da Silva-Jr, I.A. et al. Isolation, synthesis and bioactivity studies of phomactin terpenoids. Nature Chem 10, 938–945 (2018). https://doi.org/10.1038/s41557-018-0084-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-018-0084-x
This article is cited by
-
Late-stage benzenoid-to-troponoid skeletal modification of the cephalotanes exemplified by the total synthesis of harringtonolide
Nature Communications (2024)
-
Total synthesis of nine longiborneol sesquiterpenoids using a functionalized camphor strategy
Nature Chemistry (2022)
-
Fungal-derived compounds and mycogenic nanoparticles with antimycobacterial activity: a review
SN Applied Sciences (2022)
-
Synthetic biology based construction of biological activity-related library of fungal decalin-containing diterpenoid pyrones
Nature Communications (2020)