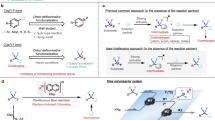
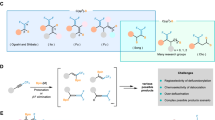
Abstract
Molecules that contain one or more fluorine atoms are crucial to drug discovery. There are protocols available for the selective synthesis of different organofluorine compounds, including those with a fluoro-substituted or a trifluoromethyl-substituted stereogenic carbon centre. However, approaches for synthesizing compounds with a trifluoromethyl- and fluoro-substituent stereogenic carbon centre are far less common. This potentially impactful set of molecules thus remains severely underdeveloped. Here we introduce a catalytic regio-, diastereo- and enantioselective strategy for the preparation of homoallylic alcohols bearing a stereogenic carbon centre bound to a trifluoromethyl group and a fluorine atom. The process, which involves a polyfluoroallyl boronate and is catalysed by an in situ-formed organozinc complex, can be used for diastereodivergent preparation of tetrafluoro-monosaccharides, including ribose core analogues of the antiviral drug sofosbuvir (Sovaldi). Unexpected reactivity/selectivity profiles, probably originating from the trifluoromethyl- and fluoro-substituted carbon site, are discovered, foreshadowing other unique chemistries that remain unknown.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data in support of the findings of this study are available within the Article and its Supplementary Information. X-ray crystallographic data for compounds R,R,S-3a, 3j, 5a, the p-nitrobenzoyl derivative of R,R,S-10, (ap)2Zn2, and the p-nitrobenzoyl derivative of R,R,R-13 are freely available from the Cambridge Crystallographic Data Center ((CCDC 2113846), (2113843), (2113794), (2113845), (2113842) and (2113792), respectively).
References
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals. Compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Meanwell, N. A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 61, 5822–5880 (2018).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Johnson, B. M., Shu, Y.-Z., Zhuo, X. & Meanwell, N. A. Metabolic and pharmaceutical aspects of fluorinated compounds. J. Med. Chem. 63, 6315–6386 (2020).
Inoue, M., Sumii, Y. & Shibata, N. Contribution of organofluorine compounds to pharmaceuticals. ACS Omega 5, 10633–10640 (2020).
Fujiwara, T. & O′Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 167, 16–29 (2014).
Berger, R., Resnati, G., Metrangolo, P., Weber, E. & Hulliger, J. Organic fluorine compounds: a great opportunity for enhanced materials properties. Chem. Soc. Rev. 40, 3496–3508 (2011).
Liu, P., Sharon, A. & Chu, C. K. Fluorinated nucleosides: synthesis and biological implication. J. Fluor. Chem. 129, 743–766 (2008).
Deleavy, G. F. & Damha, M. J. Designing chemically modified oligonucleotides for targeted gene silencing. Chem. Biol. 19, 937–954 (2012).
Hevey, R. The role of fluorine in glycomimetic drug design. Chem. Eur. J. 27, 2240–2253 (2020).
Kulkarni, J. A. et al. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 16, 630–643 (2021).
Sofia, M. J. et al. Discovery of a β-d-2′-deoxy-2′-α-fluoro-2′-β-C-methyluridine nucleotide prodrug (PSI-7977) for the treatment of hepatitis C virus. J. Med. Chem. 53, 7202–7218 (2010).
Fung, A. et al. Efficiency of incorporation and chain termination determines the inhibition potency of 2′-modified nucleotide analogs against hepatitis C virus polymerase. Antimicrob. Agents Chemother. 58, 3636–3645 (2014).
Keating, G. M. Sofosbuvir: a review of its use in patients with chronic hepatitis C. Drugs 74, 1127–1146 (2014).
Appleby, T. C. et al. Structural basis for RNA replication by the hepatitis C virus polymerase. Science 347, 771–775 (2015).
Mengshetti, S. et al. Discovery of a series of 2′-α-fluoro,2′-β-bromo-ribonucleosides and their phosphoramidate prodrugs as potent pan-genotypic inhibitors of hepatitis C virus. J. Med. Chem. 62, 1859–1874 (2019).
Rillahan, C. D. et al. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 8, 661–668 (2012).
Büll, C. et al. Targeted delivery of a sialic acid-blocking glycomimetic to cancer cells inhibits metastatic spread. ACS Nano 9, 733–745 (2015).
Baumann, A., Marchner, S., Daum, M. & Hoffmann-Röder, A. Synthesis of fluorinated Leishmania cap trisaccharides for diagnostic tool and vaccine development. Eur. J. Org. Chem. 2018, 3803–3815 (2018).
Oberbillig, T., Löwe, H. & Hoffmann-Röder, A. Synthesis of fluorinated glycosyl amino acid building blocks for MUCI cancer vaccine candidates by microreactor-assisted glycosylation. J. Flow Chem. 2, 83–86 (2012).
Klapars, A. et al. Efficient synthesis of antiviral agent uprifosbuvir enabled by new synthetic methods. Chem. Sci. 12, 9031–9036 (2021).
Beigel, J. H. et al. Remdesivir for the treatment of Covid-19 – final report. N. Engl. J. Med. 383, 1813–1826 (2020).
Jockusch, S. et al. Sofosbuvir terminated RNA is more resistant to SARS-CoV-2 proofreader than RNA terminated by remdesivir. Sci. Rep. 10, 16577 (2020).
Chien, M. et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J. Proteome Res. 19, 4690–4697 (2020).
Jeffries, B. et al. Systematic investigation of lipophilicity modulation by aliphatic fluorination motifs. J. Med. Chem. 63, 1002–1031 (2020).
Lindahl, T. Instability and decay of the primary structure of DNA. Nature 362, 709–715 (1993).
Mikkola, S., Lönnberg, T. & Lönnberg, H. Phosphodiester models for cleavage of nucleic acids. Beilstein J. Org. Chem. 14, 803–837 (2018).
Watts, J. K., Katolik, A., Viladoms, J. & Damha, M. J. Studies on the stability of 2′-fluoroarabinonucleic acid (2′F-ANA). Org. Biomol. Chem. 7, 1904–1910 (2009).
Wan, W. B. & Seth, P. P. The medicinal chemistry of therapeutic oligonucleotides. J. Med. Chem. 59, 9645–9667 (2016).
Krueger, A. C. et al. Synthesis and evaluation of 2′-dihalo ribonucleotide prodrugs with activity against hepatitis C virus. Bioorg. Med. Chem. 28, 115208 (2020).
Zhu, Y. et al. Modern approaches for asymmetric construction of carbon–fluorine quaternary stereogenic centers: synthetic challenges and pharmaceutical needs. Chem. Rev. 118, 3887–3964 (2018).
Shibata, N., Mizuta, S. & Kawai, H. Recent advances in enantioselective trifluoromethylation reactions. Tetrahedron Asymm. 19, 2633–2644 (2008).
Nie, J., Guo, H.-C., Cahard, D. & Ma, J.-A. Asymmetric construction of stereogenic carbon centers featuring a trifluoromethyl group from prochiral trifluoromethylated substrates. Chem. Rev. 111, 455–529 (2011).
Yang, X., Wu, T., Phipps, R. J. & Toste, F. D. Advances in catalytic enantioselective fluorination, mono-, di- and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015).
Brewitz, L. et al. Direct catalytic asymmetric Mannich-type reaction of α- and β-fluorinated amides. J. Am. Chem. Soc. 137, 15929–15939 (2015).
Brewitz, L., Kumagai, N. & Shibasaki, M. Catalytic asymmetric synthesis of 2,3,3,3-tetrafluoro-2-methyl-1-arylpropan-1-amines as useful building blocks for SAR-studies. J. Fluor. Chem. 194, 1–7 (2017).
Brewitz, L., Noda, H., Kumagai, N. & Shibasaki, M. (2R,3S)-3,4,4,4-tetrafluorovaline: a fluorinated bioisostere of isoleucine. Eur. J. Org. Chem. 2020, 1745–1752 (2020).
Meyer, S. et al. A chiral pentafluorinated isopropyl group via iodine(I)/(III) catalysis. Angew. Chem. Int. Ed. 60, 6430–6434 (2021).
Schäfer, M., Stünkel, T., Daniliuc, C. G. & Gilmour, R. Regio- and enantioselective intermolecular aminofluorination of alkenes via Iodine(I)/Iodine(III) catalysis. Angew. Chem. Int. Ed. 61, e202205508 (2022).
Chen, J. L.-Y. et al. Highly diastereo- and enantioselective allylboration of aldehydes using α-substituted allyl/crotyl pinacol boronic esters via in situ generated borinic esters. J. Am. Chem. Soc. 135, 5316–5319 (2013).
Silverio, D. L. et al. Simple organic molecules as catalysts for enantioselective synthesis of amines and alcohols. Nature 494, 216–221 (2013).
Van der Mei, F. W., Qin, C., Morrison, R. J. & Hoveyda, A. H. Practical, broadly applicable, α-selective, Z-selective, diastereoselective and enantioselective addition of allylboron compounds to mono-, di- and polyfluoroalkyl ketones. J. Am. Chem. Soc. 139, 9053–9065 (2017).
Morrison, R. J., van der Mei, F. W., Romiti, F. & Hoveyda, A. H. A catalytic approach for enantioselective synthesis of homoallylic alcohols bearing a Z-alkenyl chloride or trifluoromethyl group. A concise and protecting group-free synthesis of mycothiazole. J. Am. Chem. Soc. 142, 436–447 (2020).
Van der Mei, F. W., Miyamoto, H., Silverio, D. L. & Hoveyda, A. H. Lewis acid catalyzed borotropic shifts in the design of diastereo- and enantioselective γ-additions of allylboron moieties to aldimines. Angew. Chem. Int. Ed. 55, 4701–4706 (2016).
Paioti, P. H. S. et al. Catalytic enantioselective boryl and silyl substitution with trifluoromethyl alkenes: scope, utility and mechanistic nuances of Cu–F β-elimination. J. Am. Chem. Soc. 141, 19917–19934 (2019).
Butcher, T. W., Yang, J. L. & Hartwig, J. F. Copper-catalyzed defluorinative borylation and silylation of gem-difluoroallyl groups. Org. Lett. 22, 6805–6809 (2020).
Krishna, P. R., Reddy, V. V. R. & Srinivas, R. A new synthetic route to oxazole and pyrrole 2-deoxy-C-ribosides. Tetrahedron 63, 9871–9880 (2007).
Gu, Y., Kar, T. & Scheiner, S. Fundamental properties of the CH•••O interaction: is it a true hydrogen bond? J. Am. Chem. Soc. 121, 9411–9422 (1999).
Sessler, C. D. et al. CF2H, a hydrogen bond donor. J. Am. Chem. Soc. 139, 9325–9332 (2017).
Gruner, S. A. W., Locardi, E., Lohof, E. & Kessler, H. Carbohydrate-based mimetics in drug design: sugar amino acid and carbohydrate scaffolds. Chem. Rev. 102, 491–514 (2002).
Tacar, O., Sriamornsak, P. & Dass, C. R. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J. Pharmacy Pharmacol. 65, 157–170 (2012).
Boiarska, Z., Braga, T., Silvani, A. & Passarella, D. Brown allylation: application to the synthesis of natural products. Eur. J. Org. Chem. 2021, 3214–3222 (2021).
Acknowledgements
This research was supported by a grant from the National Institutes of Health (R35 GM-130395 to A.H.H. and R35 GM-128779 to P.L.). S.X. and M.J.K. were supported as LaMattina Family and Bristol-Myers Squibb Graduate Fellows, respectively. DFT calculations were performed at the Center for Research Computing at the University of Pittsburgh, the Frontera supercomputer at the Texas Advanced Computing Center, and the Extreme Science and Engineering Discovery Environment (XSEDE) supported by the National Science Foundation.
Author information
Authors and Affiliations
Contributions
S.X., J.d.P., F.R., R.J.M., K.L., S.H., M.J.K. and J.L. developed the catalytic method and carried out the applications to the synthesis of polyfluoro monosaccharides. F.R. designed the applications to polyfluoro monosaccharide syntheses. S.X., J.d.P., Y.F., B.K.M. and X.L. designed and performed the mechanistic and DFT studies. The investigations were directed by A.H.H. DFT studies were directed by P.L. A.H.H. wrote the manuscript with revisions provided by the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks A. Boese and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
All experimental, analytical, crystallographic and computational data. The methods by which various starting materials were prepared are presented. The 1H and 13C NMR spectra for each compound are reproduced (one per page). This document also contains the source for each reagent used, as well as an extended bibliography. There are a total of 18 tables and 20 figures.
Supplementary Data 1
Crystallographic data for compound 3j; CCDC reference 2113843.
Supplementary Data 2
Crystallographic data for compound (R,R,S)-3a; CCDC reference 2113846.
Supplementary Data 3
Crystallographic data for compound 5a; CCDC reference 2113794.
Supplementary Data 4
Crystallographic data for p-nitro-benozoate derivative of (R,R,R)-13; CCDC reference 2113792.
Supplementary Data 5
Data for X-ray structure of p-nitro-benozoate derivative of (R,R,S)-10; CCDC reference 2113845.
Supplementary Data 6
Data for X-ray structure of compound (ap)2Zn2; CCDC reference 2113842.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, S., del Pozo, J., Romiti, F. et al. Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre. Nat. Chem. 14, 1459–1469 (2022). https://doi.org/10.1038/s41557-022-01054-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-022-01054-4
This article is cited by
-
Site-selective chemical reactions by on-water surface sequential assembly
Nature Communications (2023)