Abstract
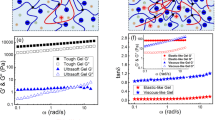
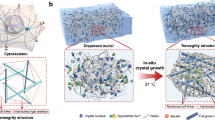
Soft biological tissues exhibit mechanical properties that reflect their composite structure of cells embedded within a biopolymer matrix. However, the microscopic mechanisms underlying their unique nonlinear mechanical response—characterized by strain stiffening in compression, but strain softening in shear or tension—remain poorly understood. Here we show that strain softening in composite systems can arise due to plastic dissipation, which is mediated by filler–polymer interactions. We characterize the nonlinear elasticity of composite hydrogels and soft tissues in isolation from these plastic effects, and show that their nonlinear elastic strain stiffening is driven by the stretching of the underlying biopolymer matrix. We thus show that strain stiffening in composite hydrogels and tissues is mediated by strain amplification factors that are universal in compression and shear. In doing so, we demonstrate the importance of fundamental composite properties such as filler concentration and filler–polymer interaction strength in mediating strain stiffening in composite systems. These findings highlight key structure–property relationships that underlie the nonlinear mechanics of biologically relevant soft solids such as composite gels and tissues.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Additional data regarding the study are available from the corresponding authors upon request.
References
Storm, C., Pastore, J. J., MacKintosh, F., Lubensky, T. & Janmey, P. A. Nonlinear elasticity in biological gels. Nature 435, 191–194 (2005).
Burla, F., Mulla, Y., Vos, B. E., Aufderhorst-Roberts, A. & Koenderink, G. H. From mechanical resilience to active material properties in biopolymer networks. Nat. Rev. Phys. 1, 249–263 (2019).
Van Oosten, A. S. et al. Emergence of tissue-like mechanics from fibrous networks confined by close-packed cells. Nature 573, 96–101 (2019).
Shadwick, R. E. Mechanical design in arteries. J. Exp. Biol. 202, 3305–3313 (1999).
Burla, F., Tauber, J., Dussi, S., van Der Gucht, J. & Koenderink, G. H. Stress management in composite biopolymer networks. Nat. Phys. 15, 549–553 (2019).
Andrei, D., Briscoe, B., Luckham, P. & Williams, D. in Modern Aspects of Colloidal Dispersions 15–24 (Springer, 1998).
Harding, S. E., Berth, G., Ball, A., Mitchell, J. R. & de la Torre, J. G. The molecular weight distribution and conformation of citrus pectins in solution studied by hydrodynamics. Carbohydr. Polym. 16, 1–15 (1991).
Shivers, J. L. et al. Compression stiffening of fibrous networks with stiff inclusions. Proc. Natl Acad. Sci. USA 117, 21037–21044 (2020).
Rubinstein, M. & Colby, R. H. Polymer Physics 263–315 (Oxford Univ. Press, 2003).
Song, Y. & Zheng, Q. A guide for hydrodynamic reinforcement effect in nanoparticle-filled polymers. Crit. Rev. Solid State Mater. Sci. 41, 318–346 (2016).
Smallwood, H. M. Limiting law of the reinforcement of rubber. J. Appl. Phys. 15, 758–766 (1944).
Guth, E. Theory of filler reinforcement. J. Appl. Phys. 16, 20–25 (1945).
Batchelor, G. K. & Green, J. T. The determination of the bulk stress in a suspension of spherical particles to order c2. J. Fluid Mech. 56, 401–427 (1972).
Deng, F. & Van Vliet, K. J. Prediction of elastic properties for polymer–particle nanocomposites exhibiting an interphase. Nanotechnology 22, 165703 (2011).
Medalia, A. I. Morphology of aggregates: VI. Effective volume of aggregates of carbon black from electron microscopy; application to vehicle absorption and to die swell of filled rubber. J. Colloid Interface Sci. 32, 115–131 (1970).
Merabia, S., Sotta, P. & Long, D. R. A microscopic model for the reinforcement and the nonlinear behavior of filled elastomers and thermoplastic elastomers (Payne and Mullins effects). Macromolecules 41, 8252–8266 (2008).
Chen, Q. et al. Mechanical reinforcement of polymer nanocomposites from percolation of a nanoparticle network. ACS Macro Lett. 4, 398–402 (2015).
Surve, M., Pryamitsyn, V. & Ganesan, V. Universality in structure and elasticity of polymer-nanoparticle gels. Phys. Rev. Lett. 96, 177805 (2006).
Thomas, C. U. & Muthukumar, M. Three‐body hydrodynamic effects on viscosity of suspensions of spheres. J. Chem. Phys. 94, 5180–5189 (1991).
Trappe, V., Prasad, V., Cipelletti, L., Segre, P. & Weitz, D. A. Jamming phase diagram for attractive particles. Nature 411, 772–775 (2001).
Koeze, D. J. & Tighe, B. P. Sticky matters: jamming and rigid cluster statistics with attractive particle interactions. Phys. Rev. Lett. 121, 188002 (2018).
Dellatolas, I. et al. Local mechanism governs global reinforcement of nanofiller-hydrogel composites. ACS Nano 17, 20939–20948 (2023).
Huang, J., Zhou, J. & Liu, M. Interphase in polymer nanocomposites. JACS Au 2, 280–291 (2022).
Shen, J., Lin, X., Liu, J. & Li, X. Revisiting stress–strain behavior and mechanical reinforcement of polymer nanocomposites from molecular dynamics simulations. Phys. Chem. Chem. Phys. 22, 16760–16771 (2020).
Pogoda, K. et al. Compression stiffening of brain and its effect on mechanosensing by glioma cells. New J. Phys. 16, 075002 (2014).
Payne, A. R. The dynamic properties of carbon black‐loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 6, 57–63 (1962).
Mullins, L. Softening of rubber by deformation. Rubber Chem. Technol. 42, 339–362 (1969).
Hyun, K. et al. A review of nonlinear oscillatory shear tests: analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 36, 1697–1753 (2011).
Ewoldt, R. H., Hosoi, A. E. & McKinley, G. H. Nonlinear viscoelastic biomaterials: meaningful characterization and engineering inspiration. Integr. Comp. Biol. 49, 40–50 (2009).
Gardel, M. L. et al. Elastic behavior of cross-linked and bundled actin networks. Science 304, 1301–1305 (2004).
Donley, G. J., Singh, P. K., Shetty, A. & Rogers, S. A. Elucidating the G″ overshoot in soft materials with a yield transition via a time-resolved experimental strain decomposition. Proc. Natl Acad. Sci. USA 117, 21945–21952 (2020).
van Doorn, J. M., Verweij, J. E., Sprakel, J. & van der Gucht, J. Strand plasticity governs fatigue in colloidal gels. Phys. Rev. Lett. 120, 208005 (2018).
Kurniawan, N. A. et al. Fibrin networks support recurring mechanical loads by adapting their structure across multiple scales. Biophys. J. 111, 1026–1034 (2016).
Papon, A. et al. Solid particles in an elastomer matrix: impact of colloid dispersion and polymer mobility modification on the mechanical properties. Soft Matter 8, 4090–4096 (2012).
Walls, H., Caines, S. B., Sanchez, A. M. & Khan, S. A. Yield stress and wall slip phenomena in colloidal silica gels. J. Rheol. 47, 847–868 (2003).
Bueche, F. Molecular basis for the Mullins effect. J. Appl. Polym. Sci. 4, 107–114 (1960).
Gleissle, W. & Hochstein, B. Validity of the Cox–Merz rule for concentrated suspensions. J. Rheol. 47, 897–910 (2003).
Varol, H. S. et al. Nanoparticle amount, and not size, determines chain alignment and nonlinear hardening in polymer nanocomposites. Proc. Natl Acad. Sci. USA 114, E3170–E3177 (2017).
Mullins, L. & Tobin, N. Stress softening in rubber vulcanizates. Part I. Use of a strain amplification factor to describe the elastic behavior of filler‐reinforced vulcanized rubber. J. Appl. Polym. Sci. 9, 2993–3009 (1965).
Bergström, J. S. & Boyce, M. C. Large strain time-dependent behavior of filled elastomers. Mech. Mater. 32, 627–644 (2000).
Kuznetsova, T. G., Starodubtseva, M. N., Yegorenkov, N. I., Chizhik, S. A. & Zhdanov, R. I. Atomic force microscopy probing of cell elasticity. Micron 38, 824–833 (2007).
De Almeida, P. et al. Cytoskeletal stiffening in synthetic hydrogels. Nat. Commun. 10, 609 (2019).
Shupe, T., Williams, M., Brown, A., Willenberg, B. & Petersen, B. E. Method for the decellularization of intact rat liver. Organogenesis 6, 134–136 (2010).
de Cagny, H. C. et al. Porosity governs normal stresses in polymer gels. Phys. Rev. Lett. 117, 217802 (2016).
MacKintosh, F., Käs, J. & Janmey, P. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 75, 4425 (1995).
Bustamante, C., Marko, J. F., Siggia, E. D. & Smith, S. Entropic elasticity of λ-phage DNA. Science 265, 1599–1600 (1994).
Marszalek, P. E., Li, H., Oberhauser, A. F. & Fernandez, J. M. Chair-boat transitions in single polysaccharide molecules observed with force-ramp AFM. Proc. Natl Acad. Sci. USA 99, 4278–4283 (2002).
Haverkamp, R. G., Marshall, A. T. & Williams, M. Model for stretching elastic biopolymers which exhibit conformational transformations. Phys. Rev. E 75, 021907 (2007).
Bertula, K. et al. Strain-stiffening of agarose gels. ACS Macro Lett. 8, 670–675 (2019).
Liu, Y., Lin, S.-H., Chuang, W.-T., Dai, N.-T., & Hsu, S.-H Biomimetic strain-stiffening in chitosan self-healing hydrogels. ACS Appl. Mater. Interfaces 14, 16032–16046 (2022).
Liu, X. & Pollack, G. H. Mechanics of F-actin characterized with microfabricated cantilevers. Biophys. J. 83, 2705–2715 (2002).
Bozec, L. & Horton, M. Topography and mechanical properties of single molecules of type I collagen using atomic force microscopy. Biophys. J. 88, 4223–4231 (2005).
Liu, X., Sun, J. Q., Heggeness, M. H., Yeh, M.-L. & Luo, Z.-P. Force-mediated dissociation of proteoglycan aggregate in articular cartilage. Biorheology 43, 183–190 (2006).
Lindström, S. B., Kulachenko, A., Jawerth, L. M. & Vader, D. A. Finite-strain, finite-size mechanics of rigidly cross-linked biopolymer networks. Soft Matter 9, 7302–7313 (2013).
Gutsmann, T. et al. Force spectroscopy of collagen fibers to investigate their mechanical properties and structural organization. Biophys. J. 86, 3186–3193 (2004).
Licup, A. J., Sharma, A. & MacKintosh, F. C. Elastic regimes of subisostatic athermal fiber networks. Phys. Rev. E 93, 012407 (2016).
Sharma, A. et al. Strain-controlled criticality governs the nonlinear mechanics of fibre networks. Nat. Phys. 12, 584–587 (2016).
Puxkandl, R. et al. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos. Trans. R. Soc. Lond. B 357, 191–197 (2002).
Soetens, J., Van Vijven, M., Bader, D., Peters, G. & Oomens, C. A model of human skin under large amplitude oscillatory shear. J. Mech. Behav. Biomed. Mater. 86, 423–432 (2018).
Darvish, K. & Crandall, J. Nonlinear viscoelastic effects in oscillatory shear deformation of brain tissue. Med. Eng. Phys. 23, 633–645 (2001).
Han, Y. L. et al. Cell contraction induces long-ranged stress stiffening in the extracellular matrix. Proc. Natl Acad. Sci. USA 115, 4075–4080 (2018).
Hall, M. S. et al. Fibrous nonlinear elasticity enables positive mechanical feedback between cells and ECMs. Proc. Natl Acad. Sci. USA 113, 14043–14048 (2016).
Yuan, H. et al. Synthetic fibrous hydrogels as a platform to decipher cell–matrix mechanical interactions. Proc. Natl Acad. Sci. USA 120, e2216934120 (2023).
Moeendarbary, E. et al. The cytoplasm of living cells behaves as a poroelastic material. Nat. Mater. 12, 253–261 (2013).
Frantz, C., Stewart, K. M. & Weaver, V. M. The extracellular matrix at a glance. J. Cell Sci. 123, 4195–4200 (2010).
Kratochvil, M. J. et al. Engineered materials for organoid systems. Nat. Rev. Mater. 4, 606–622 (2019).
Chin, L., Xia, Y., Discher, D. E. & Janmey, P. A. Mechanotransduction in cancer. Curr. Opin. Chem. Eng. 11, 77–84 (2016).
Kai, F., Drain, A. P. & Weaver, V. M. The extracellular matrix modulates the metastatic journey. Dev. Cell 49, 332–346 (2019).
Punter, M. T., Vos, B. E., Mulder, B. M. & Koenderink, G. H. Poroelasticity of (bio)polymer networks during compression: theory and experiment. Soft Matter 16, 1298–1305 (2020).
Hashemnejad, S. M. & Kundu, S. Strain stiffening and negative normal stress in alginate hydrogels. J. Polym. Sci. Part B: Polym. Phys. 54, 1767–1775 (2016).
Kong, H. J., Kim, C. J., Huebsch, N., Weitz, D. & Mooney, D. J. Noninvasive probing of the spatial organization of polymer chains in hydrogels using fluorescence resonance energy transfer (FRET). J. Am. Chem. Soc. 129, 4518–4519 (2007).
Ewoldt, R. H., Hosoi, A. & McKinley, G. H. New measures for characterizing nonlinear viscoelasticity in large amplitude oscillatory shear. J. Rheol. 52, 1427–1458 (2008).
Acknowledgements
J.S. acknowledges financial support from the MIT Lemelson-Vest award and the MIT MathWorks fellowship. J.S. and G.H.M. acknowledge helpful discussions with P. Janmey (U Penn), I. Dellatolas (MIT), I. Bischofberger (MIT), E. Del Gado (Georgetown) and J. Shivers (U Chicago).
Author information
Authors and Affiliations
Contributions
J.S. conceived the project. J.S. and G.H.M. designed the study. J.S. and S.Y. performed the experiments. E.D.-Y. prepared the tissue specimens for the study. G.H.M. supervised the study. J.S. and G.H.M. analysed the data and wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Physics thanks Jasper van der Gucht and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, J., Deiss-Yehiely, E., Yesilata, S. et al. Strain-stiffening universality in composite hydrogels and soft tissues. Nat. Phys. 21, 1125–1133 (2025). https://doi.org/10.1038/s41567-025-02869-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41567-025-02869-x