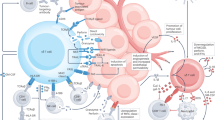
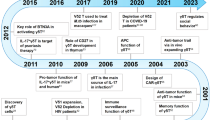
Abstract
γδT cell biology in cancer has been studied for decades but remains poorly understood mainly due to species differences in preclinical models and a lack of appropriate analytical tools. This lack of knowledge has hindered the clinical translation of promising therapeutic concepts. In recent years, advanced single-cell analysis techniques and comprehensive protein–protein interaction studies have transformed our understanding of γδT cells and their receptors. This insight has revealed new opportunities and challenges in harnessing γδT cells for therapeutic purposes. In this context, we will discuss the latest findings in γδT cell biology, with a special focus on their role in cancer immune therapies. We will explore strategies to overcome tolerance and shift the balance of γδT cells and their receptors towards antitumour efficacy, which has the potential to successfully translate into various engineering approaches in cancer immunotherapy.
Key points
-
Vγ9Vδ2 γδT cells and Vδ2– γδT cells have distinct transcriptomic profiles and different therapeutic potential in cancer therapy.
-
The unique γδT cell receptor–CD3 complex structure and its downstream signalling pathways have implications for the design of effective therapeutics.
-
Shifting the equilibrium between activating and inhibitory receptors is essential for the clinical success of both γδT cell-based biologics and engineered T cells.
-
Biomimetic models and Good Manufacturing Practice simulation will be necessary to accelerate the development process.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945 (2015).
Meraviglia, S. et al. Distinctive features of tumor-infiltrating γδ T lymphocytes in human colorectal cancer. Oncoimmunology 6, e1347742 (2017).
Bruni, E. et al. Intrahepatic CD69+Vδ1 T cells re-circulate in the blood of patients with metastatic colorectal cancer and limit tumor progression. J. Immunother. Cancer 10, e004579 (2022).
Wu, Y. et al. An innate-like Vδ1+ γδ T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci. Transl. Med. 11, eaax9364 (2019).
Willcox, B. E. & Willcox, C. R. γδ TCR ligands: the quest to solve a 500-million-year-old mystery. Nat. Immunol. 20, 121–128 (2019).
Davey, M. S. et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9– subsets. Nat. Commun. 9, 1760 (2018).
Pizzolato, G. et al. Single-cell RNA sequencing unveils the shared and the distinct cytotoxic hallmarks of human TCRVδ1 and TCRVδ2 γδ T lymphocytes. Proc. Natl Acad. Sci. USA 116, 11906–11915 (2019).
Vyborova, A. et al. γ9δ2 T-cell expansion and phenotypic profile are reflected in the CDR3δ repertoire of healthy adults. Front. Immunol. 13, 915366 (2022).
Davey, M. S. et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 8, 14760 (2017).
Yu, X. et al. Pan-cancer γδ TCR analysis uncovers clonotype diversity and prognostic potential. Cell Rep. Med. 5, 101764 (2024).
Scheper, W. et al. γδT cells elicited by CMV reactivation after allo-SCT cross-recognize CMV and leukemia. Leukemia 27, 1328–1338 (2013).
Janssen, A. et al. The role of γδ T cells as a line of defense in viral infections after allogeneic stem cell transplantation: opportunities and challenges. Viruses 14, 117 (2022).
Siblany, L. et al. Unconventional T cells influence clinical outcome after allogeneic hematopoietic cell transplantation. J. Clin. Immunol. 44, 139 (2024).
de Witte, M. A. et al. αβ T-cell graft depletion for allogeneic HSCT in adults with hematological malignancies. Blood Adv. 5, 240–249 (2021).
de Witte, M. A. et al. Activity of ex vivo graft and DLI engineering within the last decade increases, a survey from the EBMT Cellular Therapy & Immunobiology Working Party. Bone Marrow Transpl. 58, 719–722 (2023).
Sebestyen, Z., Prinz, I., Dechanet-Merville, J., Silva-Santos, B. & Kuball, J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat. Rev. Drug Discov. 19, 169–184 (2020).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602, 503–509 (2022). Study demonstrating CAR-expressing γδT cells persisting in long-term survivors who primarily received CAR-engineered αβT cells when using CD3+ T cells as starting material.
Anderson, N. D. et al. Transcriptional signatures associated with persisting CD19 CAR-T cells in children with leukemia. Nat. Med. 29, 1700–1709 (2023).
Gully, B. S. et al. Structure of a fully assembled γδ T-cell antigen receptor. Nature 634, 729–736 (2024). This study used the cryo-electron microscopy structure of the CD3–γδTCR complex to show that γδTCR activation requires higher ligand densities compared to αβTCR activation, owing to flexibility in the γδTCR connecting peptides.
Fisch, P. et al. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science 250, 1269–1273 (1990).
Constant, P. et al. Stimulation of human γδ T cells by nonpeptidic mycobacterial ligands. Science 264, 267–270 (1994).
Morita, C. T. et al. Recognition of nonpeptide prenyl pyrophosphate antigens by human γδ T cells. Microbes Infect. 1, 175–186 (1999).
Gruenbacher, G. & Thurnher, M. Mevalonate metabolism governs cancer immune surveillance. Oncoimmunology 6, e1342917 (2017).
Harly, C. et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human gammadelta T-cell subset. Blood 120, 2269–2279 (2012). This report detailing the identification of BTN3A as a key molecule for Vγ9Vδ2T cell activation was pivotal to many developments in the field over the last decade.
Rigau, M. et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by γδ T cells. Science 367, eaay5516 (2020). Published report that identified BTN2A1 as a ligand for the Vγ9Vδ2TCR.
Karunakaran, M. M. et al. Butyrophilin-2A1 directly binds germline-encoded regions of the Vγ9Vδ2 TCR and Is essential for phosphoantigen sensing. Immunity 52, 487–498.e6 (2020).
Compte, E., Pontarotti, P., Collette, Y., Lopez, M. & Olive, D. Frontline: characterization of BT3 molecules belonging to the B7 family expressed on immune cells. Eur. J. Immunol. 34, 2089–2099 (2004).
Cubillos-Ruiz, J. R. et al. CD277 is a negative co-stimulatory molecule universally expressed by ovarian cancer microenvironmental cells. Oncotarget 1, 329–338 (2010).
Benyamine, A. et al. BTN3A is a prognosis marker and a promising target for Vγ9Vδ2 T cells based-immunotherapy in pancreatic ductal adenocarcinoma (PDAC). Oncoimmunology 7, e1372080 (2017).
Dang, A. T. et al. NLRC5 promotes transcription of BTN3A1-3 genes and Vγ9Vδ2 T cell-mediated killing. iScience 24, 101900 (2021).
Liang, F. et al. Comprehensive analysis of BTN3A1 in cancers: mining of omics data and validation in patient samples and cellular models. FEBS Open Bio. 11, 2586–2599 (2021).
Yang, W. et al. BTN3A1 promotes tumor progression and radiation resistance in esophageal squamous cell carcinoma by regulating ULK1-mediated autophagy. Cell Death Dis. 13, 984 (2022).
Mamedov, M. R. et al. CRISPR screens decode cancer cell pathways that trigger gammadelta T cell detection. Nature 621, 188–195 (2023).
Wu, Z. et al. Unsynchronized butyrophilin molecules dictate cancer cell evasion of Vγ9Vδ2 T-cell killing. Cell Mol. Immunol. 21, 362–373 (2024).
Sebestyen, Z. et al. RhoB mediates phosphoantigen recognition by Vγ9Vδ2 T cell receptor. Cell Rep. 15, 1973–1985 (2016).
Sandstrom, A. et al. The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 40, 490–500 (2014). This study resolved the outstanding question of how pAgs are involved in the recognition of tumour cells by Vγ9Vδ2T cells. The authors showed that pAgs bind to the intracellular B30.2 domain of BTN3A1 and were not able to bind to the extracellular domains of BTN3A1.
Hsiao, C. C., Nguyen, K., Jin, Y., Vinogradova, O. & Wiemer, A. J. Ligand-induced interactions between butyrophilin 2A1 and 3A1 internal domains in the HMBPP receptor complex. Cell Chem. Biol. 29, 985–995.e5 (2022).
Yuan, L. et al. Phosphoantigens glue butyrophilin 3A1 and 2A1 to activate Vγ9Vδ2 T cells. Nature 621, 840–848 (2023). This study describes in molecular detail how the binding of pAgs to the intracellular B30.2 domain of BTN3A1 leads to an interaction with the intracellular B30.2 domain of BTN2A1, leading to Vγ9Vδ2T cell activation.
Wiemer, A. J. Structure-activity relationships of butyrophilin 3 ligands. ChemMedChem 15, 1030–1039 (2020).
Karunakaran, M. M. et al. A distinct topology of BTN3A IgV and B30.2 domains controlled by juxtamembrane regions favors optimal human γδ T cell phosphoantigen sensing. Nat. Commun. 14, 7617 (2023). A comprehensive and elegant analysis of how the BTN3A isoform heterodimer composition is important for Vγ9Vδ2T cell activation.
Willcox, C. R. et al. Phosphoantigen sensing combines TCR-dependent recognition of the BTN3A IgV domain and germline interaction with BTN2A1. Cell Rep. 42, 112321 (2023).
Fulford, T. S. et al. Vγ9Vδ2 T cells recognize butyrophilin 2A1 and 3A1 heteromers. Nat. Immunol. 25, 1355–1366 (2024).
Zhang, M. et al. Cryo-EM structural insights into Vγ9Vδ2 TCR activation via multiple butyrophilins. Preprint at bioRxiv https://doi.org/10.1101/2024.10.02.616253 (2024).
van Diest, E. et al. Gamma delta TCR anti-CD3 bispecific molecules (GABs) as novel immunotherapeutic compounds. J. Immunother. Cancer 9, e003850corr1 (2021). Paper showing that Vγ9Vδ2TCR–anti-CD3 TCEs can be effectively used in in vivo preclinical models.
Grunder, C. et al. γ9 and δ2CDR3 domains regulate functional avidity of T cells harboring γ9δ2TCRs. Blood 120, 5153–5162 (2012).
Ampuero, J. et al. Fine-mapping butyrophilin family genes revealed several polymorphisms influencing viral genotype selection in hepatitis C infection. Genes Immun. 16, 297–300 (2015).
Cleven, A. et al. Sensitivity to Vγ9Vδ2TCR T cells is imprinted after single mutations during early oncogenesis. Preprint at bioRxiv https://doi.org/10.1101/2024.11.19.624272 (2024).
Raute, K. et al. Breast cancer stem cell-derived tumors escape from γδ T-cell immunosurveillance in vivo by modulating γδ T-cell ligands. Cancer Immunol. Res. 11, 810–829 (2023).
Willcox, C. R. et al. Butyrophilin-like 3 directly binds a human Vγ4+ T cell receptor using a modality distinct from closnally-restricted antigen. Immunity 51, 813–825.e4 (2019).
Melandri, D. et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 19, 1352–1365 (2018). Report of the ‘superantigen’-like interaction between BTNL molecules with Vγ-domains in γδTCRs, which is conserved between mice and men.
Dart, R. J. et al. Conserved γδ T cell selection by BTNL proteins limits progression of human inflammatory bowel disease. Science 381, eadh0301 (2023).
Hazini, A., Fisher, K. & Seymour, L. Deregulation of HLA-I in cancer and its central importance for immunotherapy. J. Immunother. Cancer 9, e002899 (2021).
Xie, N. et al. Neoantigens: promising targets for cancer therapy. Signal. Transduct. Target. Ther. 8, 9 (2023).
Kierkels, G. J. J. et al. Identification of a tumor-specific allo-HLA-restricted γδTCR. Blood Adv. 3, 2870–2882 (2019).
Deseke, M. et al. A CMV-induced adaptive human Vδ1+ γδ T cell clone recognizes HLA-DR. J. Exp. Med. 219, e20212525 (2022).
Look, A., Burns, D., Tews, I., Roghanian, A. & Mansour, S. Towards a better understanding of human iNKT cell subpopulations for improved clinical outcomes. Front. Immunol. 14, 1176724 (2023).
Kim, S., Cho, S. & Kim, J. H. CD1-mediated immune responses in mucosal tissues: molecular mechanisms underlying lipid antigen presentation system. Exp. Mol. Med. 55, 1858–1871 (2023).
Nelson, A., Lukacs, J. D. & Johnston, B. The current landscape of NKT cell immunotherapy and the hills ahead. Cancers 13, 5174 (2021).
Lee, M. S. & Webb, T. J. Novel lipid antigens for NKT cells in cancer. Front. Immunol. 14, 1173375 (2023).
Shahine, A., Van Rhijn, I., Rossjohn, J. & Moody, D. B. CD1 displays its own negative regulators. Curr. Opin. Immunol. 83, 102339 (2023).
McWilliam, H. E. G. & Villadangos, J. A. MR1 antigen presentation to MAIT cells and other MR1-restricted T cells. Nat. Rev. Immunol. 24, 178–192 (2024).
Lepore, M. et al. Functionally diverse human T cells recognize non-microbial antigens presented by MR1. eLife 6, e29743 (2017).
Crowther, M. D. et al. Genome-wide CRISPR-Cas9 screening reveals ubiquitous T cell cancer targeting via the monomorphic MHC class I-related protein MR1. Nat. Immunol. 21, 178–185 (2020).
Constantin, D. et al. MR1 gene and protein expression are enhanced by inhibition of the extracellular signal-regulated kinase ERK. Cancer Immunol. Res 12, 1452–1467 (2024).
Willcox, C. R. et al. Cytomegalovirus and tumor stress surveillance by binding of a human γδ T cell antigen receptor to endothelial protein C receptor. Nat. Immunol. 13, 872–879 (2012). This study shows a γδT cell clone that expanded after CMV reactivation bearing a γδTCR that recognizes EPCR, providing evidence that γδT cells are adaptive T cells that can expand in a γδTCR–antigen-specific manner.
Janssen, A. et al. γδ T-cell receptors derived from breast cancer-infiltrating T lymphocytes mediate antitumor reactivity. cancer. Immunol. Res. 8, 530–543 (2020).
Wojtukiewicz, M. Z., Hempel, D., Sierko, E., Tucker, S. C. & Honn, K. V. Endothelial protein C receptor (EPCR), protease activated receptor-1 (PAR-1) and their interplay in cancer growth and metastatic dissemination. Cancers 11, 51 (2019).
Lal, N. et al. Endothelial protein C receptor is overexpressed in colorectal cancer as a result of amplification and hypomethylation of chromosome 20q. J. Pathol. Clin. Res. 3, 155–170 (2017).
Christensen, M. V., Hogdall, C. K., Jochumsen, K. M. & Hogdall, E. V. S. Annexin A2 and cancer: a systematic review. Int. J. Oncol. 52, 5–18 (2018).
Marlin, R. et al. Sensing of cell stress by human γδ TCR-dependent recognition of annexin A2. Proc. Natl Acad. Sci. USA 114, 3163–3168 (2017).
Harly, C. et al. Human γδ T cell sensing of AMPK-dependent metabolic tumor reprogramming through TCR recognition of EphA2. Sci. Immunol. 6, eaba9010 (2021).
Wilson, K., Shiuan, E. & Brantley-Sieders, D. M. Oncogenic functions and therapeutic targeting of EphA2 in cancer. Oncogene 40, 2483–2495 (2021).
Veiga, R. N., de Azevedo, A. L. K., de Oliveira, J. C. & Gradia, D. F. Targeting EphA2: a promising strategy to overcome chemoresistance and drug resistance in cancer. J. Mol. Med. 102, 479–493 (2024).
Benveniste, P. M. et al. Generation and molecular recognition of melanoma-associated antigen-specific human γδ T cells. Sci. Immunol. 3, eaav4036 (2018).
Luoma, A. M. et al. Crystal structure of Vδ1 T cell receptor in complex with CD1d-sulfatide shows MHC-like recognition of a self-lipid by human γδ T cells. Immunity 39, 1032–1042 (2013).
Uldrich, A. P. et al. CD1d-lipid antigen recognition by the γδ TCR. Nat. Immunol. 14, 1137–1145 (2013).
Le Nours, J. et al. A class of γδ T cell receptors recognize the underside of the antigen-presenting molecule MR1. Science 366, 1522–1527 (2019). Prior to this study, all determined γδTCR–ligand complex structures were consistent with those observed for αβTCR–ligand complexes. However, this study reported a γδTCR–ligand complex that was completely different.
Rice, M. T. et al. Recognition of the antigen-presenting molecule MR1 by a Vδ3+ γδ T cell receptor. Proc. Natl Acad. Sci. USA 118, e2110288118 (2021).
Wegrecki, M. et al. Atypical sideways recognition of CD1a by autoreactive γδ T cell receptors. Nat. Commun. 13, 3872 (2022).
Siegers, G. M. et al. Different composition of the human and the mouse gammadelta T cell receptor explains different phenotypes of CD3γ and CD3δ immunodeficiencies. J. Exp. Med. 204, 2537–2544 (2007).
Susac, L. et al. Structure of a fully assembled tumor-specific T cell receptor ligated by pMHC. Cell 185, 3201–3213.e19 (2022).
Xin, W. et al. Structures of human γδ T cell receptor-CD3 complex. Nature 630, 222–229 (2024).
Hoque, M. et al. Structural characterization of two γδ TCR/CD3 complexes. Nat. Commun. 16, 318 (2025).
Mallis, R. J. et al. Molecular design of the γδT cell receptor ectodomain encodes biologically fit ligand recognition in the absence of mechanosensing. Proc. Natl Acad. Sci. USA 118, e2023050118 (2021).
Chen, Y. et al. Cholesterol inhibits TCR signaling by directly restricting TCR-CD3 core tunnel motility. Mol. Cell 82, 1278–1287.e5 (2022).
Swamy, M. et al. A cholesterol-based allostery model of T cell receptor phosphorylation. Immunity 44, 1091–1101 (2016).
Figueroa, M. G., Parker, L. M., Krol, K. & Zhao, M. Distal lck promoter-driven cre shows cell type-specific function in innate-like T cells. Immunohorizons 5, 772–781 (2021).
Tan, L. et al. Single-cell transcriptomics identifies the adaptation of Scart1+ Vγ6+ T cells to skin residency as activated effector cells. Cell Rep. 27, 3657–3671.e4 (2019).
Lui, V. G. et al. A partial human LCK defect causes a T cell immunodeficiency with intestinal inflammation. J. Exp. Med. 221, e20230927 (2024).
Hartl, F. A. et al. Noncanonical binding of Lck to CD3ε promotes TCR signaling and CAR function. Nat. Immunol. 21, 902–913 (2020).
Schamel, W. W., Alarcon, B., Hofer, T. & Minguet, S. The allostery model of TCR regulation. J. Immunol. 198, 47–52 (2017).
Schamel, W. W., Alarcon, B. & Minguet, S. The TCR is an allosterically regulated macromolecular machinery changing its conformation while working. Immunol. Rev. 291, 8–25 (2019).
Rancan, C. et al. Exhausted intratumoral Vδ2− γδ T cells in human kidney cancer retain effector function. Nat. Immunol. 24, 612–624 (2023).
Gherardin, N. A. et al. γδ T cells in Merkel cell carcinomas have a proinflammatory profile prognostic of patient survival. Cancer Immunol. Res. 9, 612–623 (2021).
Lien, S. C. et al. Tumor reactive γδ T cells contribute to a complete response to PD-1 blockade in a Merkel cell carcinoma patient. Nat. Commun. 15, 1094 (2024).
Vyborova, A. et al. γ9δ2T cell diversity and the receptor interface with tumor cells. J. Clin. Invest. 130, 4637–4651 (2020).
Cazzetta, V. et al. NKG2A expression identifies a subset of human Vδ2 T cells exerting the highest antitumor effector functions. Cell Rep. 37, 109871 (2021).
Silva-Santos, B. & Strid, J. Working in “NK mode”: natural killer group 2 member D and natural cytotoxicity receptors in stress-surveillance by γδ T cells. Front. Immunol. 9, 851 (2018).
Toutirais, O. et al. DNAX accessory molecule-1 (CD226) promotes human hepatocellular carcinoma cell lysis by Vγ9Vδ2 T cells. Eur. J. Immunol. 39, 1361–1368 (2009).
Choi, H. et al. Human allogenic γδ T cells kill patient-derived glioblastoma cells expressing high levels of DNAM-1 ligands. Oncoimmunology 11, 2138152 (2022).
Wang, X. et al. Diminished cytolytic activity of γδ T cells with reduced DNAM-1 expression in neuroblastoma patients. Clin. Immunol. 203, 63–71 (2019).
Jin, Z. et al. Characteristic of TIGIT and DNAM-1 expression on Foxp3+ γδ T cells in AML patients. Biomed. Res. Int. 2020, 4612952 (2020).
Mensurado, S. et al. CD155/PVR determines acute myeloid leukemia targeting by delta one T cells. Blood 143, 1488–1495 (2024). The development of Delta One T cells led to the profound insight that the therapeutic efficacy of γδT cells does not necessarily need to rely on γδTCR but can be effectively mediated by other activating immune receptors such as DNAM1.
Rincon-Orozco, B. et al. Activation of Vγ9Vδ2 T cells by NKG2D. J. Immunol. 175, 2144–2151 (2005).
Wolf, N. K., Kissiov, D. U. & Raulet, D. H. Roles of natural killer cells in immunity to cancer, and applications to immunotherapy. Nat. Rev. Immunol. 23, 90–105 (2023).
Lopes, N. et al. Distinct metabolic programs established in the thymus control effector functions of γδ T cell subsets in tumor microenvironments. Nat. Immunol. 22, 179–192 (2021).
Goldberg, E. L. et al. Ketogenesis activates metabolically protective γδ T cells in visceral adipose tissue. Nat. Metab. 2, 50–61 (2020).
Wang, H. et al. CD36-mediated metabolic adaptation supports regulatory T cell survival and function in tumors. Nat. Immunol. 21, 298–308 (2020).
Lim, S. A. et al. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature 591, 306–311 (2021).
Harmon, C. et al. γδ T cell dichotomy with opposing cytotoxic and wound healing functions in human solid tumors. Nat. Cancer 4, 1122–1137 (2023). This study shows that not all γδT cells are alike; depending on their phenotype, γδT cells can be cytotoxic and thus have the ability to kill tumour cells or can secrete amphiregulin, which promotes tumour cell proliferation.
Mu, X. et al. Glucose metabolism controls human γδ T-cell-mediated tumor immunosurveillance in diabetes. Cell Mol. Immunol. 19, 944–956 (2022).
Park, J. H. et al. Tumor hypoxia represses γδ T cell-mediated antitumor immunity against brain tumors. Nat. Immunol. 22, 336–346 (2021).
Zhao, S., Peralta, R. M., Avina-Ochoa, N., Delgoffe, G. M. & Kaech, S. M. Metabolic regulation of T cells in the tumor microenvironment by nutrient availability and diet. Semin. Immunol. 52, 101485 (2021).
Lim, A. R., Rathmell, W. K. & Rathmell, J. C. The tumor microenvironment as a metabolic barrier to effector T cells and immunotherapy. eLife 9, e55185 (2020).
Wu, Y. et al. A local human Vδ1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat. Cancer 3, 696–709 (2022).
Chauvin, C. et al. NKG2D controls natural reactivity of Vγ9Vδ2 T lymphocytes against mesenchymal glioblastoma cells. Clin. Cancer Res. 25, 7218–7228 (2019).
Knight, A. et al. CMV-independent lysis of glioblastoma by ex vivo expanded/activated Vδ1+ γδ T cells. PLoS One 8, e68729 (2013).
Kunzmann, V., Bauer, E. & Wilhelm, M. γ/δ T-cell stimulation by pamidronate. N. Engl. J. Med. 340, 737–738 (1999).
Kakimi, K. et al. Adoptive transfer of zoledronate-expanded autologous Vγ9Vδ2 T-cells in patients with treatment-refractory non-small-cell lung cancer: a multicenter, open-label, single-arm, phase 2 study. J. Immunother. Cancer 8, e001185 (2020).
Xu, Y. et al. Allogeneic Vγ9Vδ2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol. Immunol. 18, 427–439 (2021).
Reis, B. S. et al. TCR-Vγδ usage distinguishes protumor from antitumor intestinal γδ T cell subsets. Science 377, 276–284 (2022).
Yang, J., Chen, Y., Jing, Y., Green, M. R. & Han, L. Advancing CAR T cell therapy through the use of multidimensional omics data. Nat. Rev. Clin. Oncol. 20, 211–228 (2023).
Weiss, H. M. et al. Biodistribution and plasma protein binding of zoledronic acid. Drug Metab. Dispos. 36, 2043–2049 (2008).
Di Mascolo, D. et al. Nanoformulated zoledronic acid boosts the Vδ2 T cell immunotherapeutic potential in colorectal cancer. Cancers 12, 104 (2019).
Li, X. et al. Zoledronic acid-containing nanoparticles with minimum premature release show enhanced activity against extraskeletal tumor. ACS Appl. Mater. Interfaces 11, 7311–7319 (2019).
Belisario, D. C. et al. ABCA1/ABCB1 ratio determines chemo- and immune-sensitivity in human osteosarcoma. Cells 9, 647 (2020).
Akman, M. et al. TFEB controls sensitivity to chemotherapy and immuno-killing in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 43, 219 (2024).
Johanna, I. et al. Evaluating in vivo efficacy — toxicity profile of TEG001 in humanized mice xenografts against primary human AML disease and healthy hematopoietic cells. J. Immunother. Cancer 7, 69 (2019).
Ridgley, L. A., Caron, J., Dalgleish, A. & Bodman-Smith, M. Releasing the restraints of Vγ9Vδ2 T-cells in cancer immunotherapy. Front. Immunol. 13, 1065495 (2022).
Laskowski, T. J., Biederstadt, A. & Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 22, 557–575 (2022).
Zakeri, N. et al. Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma. Nat. Commun. 13, 1372 (2022).
Hu, Y. et al. γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal. Transduct. Target. Ther. 8, 434 (2023).
Wang, H. et al. Interleukin-15 enhanced the survival of human γδT cells by regulating the expression of Mcl-1 in neuroblastoma. Cell Death Discov. 8, 139 (2022).
de Vries, N. L. et al. γδ T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature 613, 743–750 (2023). This study demonstrates that patients with β2-microglobulin-deficient cancers being treated with immune-checkpoint blockade therapy have increased tumour infiltration of γδT cells, resulting in positive clinical responses.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05886491 (2023).
Wang, R. N. et al. Optimized protocols for γδ T cell expansion and lentiviral transduction. Mol. Med. Rep. 19, 1471–1480 (2019).
Ferry, G. M. et al. A simple and robust single-step method for CAR-Vδ1 γδT cell expansion and transduction for cancer immunotherapy. Front. Immunol. 13, 863155 (2022).
Rohaan, M. W. et al. Tumor-infiltrating lymphocyte therapy or ipilimumab in advanced melanoma. N. Engl. J. Med. 387, 2113–2125 (2022).
Cappell, K. M. & Kochenderfer, J. N. Long-term outcomes following CAR T cell therapy: what we know so far. Nat. Rev. Clin. Oncol. 20, 359–371 (2023).
Ribeiro, S. T., Ribot, J. C. & Silva-Santos, B. Five layers of receptor signaling in gammadelta T-cell differentiation and activation. Front. Immunol. 6, 15 (2015).
Goulmy, E. et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N. Engl. J. Med. 334, 281–285 (1996).
Bordoni, V. et al. Antiviral potential of Vδ2 T-cells in children given TCR αβ/CD19 cell depleted HLA-haploidentical HSCT. Blood Adv. 9, 990–1002 (2024).
Singh, A. et al. A novel bioinformatics pipeline for the identification of immune inhibitory receptors as potential therapeutic targets. eLife 13, RP92870 (2024).
Loh, L. et al. Unraveling the phenotypic states of human innate-like T cells: comparative insights with conventional T cells and mouse models. Cell Rep. 43, 114705 (2024).
De Gassart, A. et al. Development of ICT01, a first-in-class, anti-BTN3A antibody for activating Vγ9Vδ2 T cell-mediated antitumor immune response. Sci. Transl. Med. 13, eabj0835 (2021). This paper describes the preclinical development of the agonistic BTN3A antibody, including preliminary safety data with this antibody in the first treated patients.
Girard, P., Ponsard, B., Charles, J., Chaperot, L. & Aspord, C. Potent bidirectional cross-talk between plasmacytoid dendritic cells and γδT cells through BTN3A, type I/II IFNs and immune checkpoints. Front. Immunol. 11, 861 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05307874 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04243499 (2020).
Oberg, H. H. et al. Novel bispecific antibodies increase gammadelta T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 74, 1349–1360 (2014).
de Bruin, R. C. G. et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology 7, e1375641 (2017).
Ganesan, R. et al. Selective recruitment of gammadelta T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia 35, 2274–2284 (2021).
Boutin, L. et al. Camelid-derived Tcell engagers harnessing human γδ T cells as promising antitumor immunotherapeutic agents. Eur. J. Immunol. 54, e2350773 (2024).
Yang, R. et al. Vγ2 x PD-L1, a bispecific antibody targeting both the Vγ2 TCR and PD-L1, improves the anti-tumor response of Vγ2Vδ2 T cell. Front. Immunol. 13, 923969 (2022).
LAVA-Therapeutics. LAVA Therapeutics Provides Updates on Clinical Programs and Extends the Cash Runway https://ir.lavatherapeutics.com/news-releases/news-release-details/lava-therapeutics-provides-updates-clinical-programs-and-extends/ (2023).
Marcu-Malina, V. et al. Redirecting αβ T cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood 118, 50–59 (2011). Report of the TEG concept relying on high-affinity Vγ9Vδ2TCRs, which have been successfully developed into first-in-human studies.
Lai, A. Y. et al. Cutting edge: bispecific γδ T cell engager containing heterodimeric BTN2A1 and BTN3A1 promotes targeted activation of Vγ9Vδ2+ T cells in the presence of costimulation by CD28 or NKG2D. J. Immunol. 209, 1475–1480 (2022).
Kuball, J., Beringer, D. X. & Van Diest, E. Btn2a1 Binding Peptide https://patents.google.com/patent/WO2024133301A1/en (2023).
van Diest, E. et al. The making of multivalent gamma delta TCR anti-CD3 bispecific T cell engagers. Front. Immunol. 13, 1052090 (2022).
Stary, V. et al. Dysfunctional tumor-infiltrating Vδ1+ T lymphocytes in microsatellite-stable colorectal cancer. Nat. Commun. 15, 6949 (2024).
Schamel, W. W. et al. The potential of γδ CAR and TRuC T cells: an unearthed treasure. Eur. J. Immunol. 54, e2451074 (2024).
Ganapathy, T., Radhakrishnan, R., Sakshi, S. & Martin, S. CAR γδ T cells for cancer immunotherapy. Is the field more yellow than green? Cancer Immunol. Immunother. 72, 277–286 (2023).
Marin, D. et al. Safety, efficacy and determinants of response of allogeneic CD19-specific CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial. Nat. Med. 30, 772–784 (2024).
Sanchez Martinez, D. et al. Generation and proof-of-concept for allogeneic CD123 CAR-Delta One T (DOT) cells in acute myeloid leukemia. J. Immunother. Cancer 10, e005400 (2022).
Le Floch, A. C. et al. Low frequency of Vγ9Vδ2 T cells predicts poor survival in newly diagnosed acute myeloid leukemia. Blood Adv. 8, 4262–4275 (2024).
Lv, Z., Luo, F. & Chu, Y. Strategies for overcoming bottlenecks in allogeneic CAR-T cell therapy. Front. Immunol. 14, 1199145 (2023).
Velasco Cardenas, R. M. et al. Harnessing CD3 diversity to optimize CAR T cells. Nat. Immunol. 24, 2135–2149 (2023).
Ding, J. et al. Mesothelin-targeting T cells bearing a novel T cell receptor fusion construct (TRuC) exhibit potent antitumor efficacy against solid tumors. Oncoimmunology 12, 2182058 (2023).
Juraske, C. et al. Reprogramming of human γδ T cells by expression of an anti-CD19 TCR fusion construct (εTRuC) to enhance tumor killing. J. Leukoc. Biol. 115, 293–305 (2024).
Li, H. K. et al. A novel allogeneic rituximab-conjugated gamma delta T cell therapy for the treatment of relapsed/refractory B-cell lymphoma. Cancers 15, 4844 (2023).
Fisher, J. et al. Avoidance of on-target off-tumor activation using a co-stimulation-only chimeric antigen receptor. Mol. Ther. 25, 1234–1247 (2017).
Fisher, J. et al. Engineering γδT cells limits tonic signaling associated with chimeric antigen receptors. Sci. Signal. 12, eaax1872 (2019).
van der Veken, L. T. et al. αβ T-cell receptor engineered γδ T cells mediate effective antileukemic reactivity. Cancer Res. 66, 3331–3337 (2006).
Immatics. Next-generation ACT https://immatics.com/next-generation-act/ (2023).
Wang, Y. et al. Anti-PD-1 antibody armored γδ T cells enhance anti-tumor efficacy in ovarian cancer. Signal. Transduct. Target. Ther. 8, 399 (2023).
Fowler, D. et al. Payload-delivering engineered γδ T cells display enhanced cytotoxicity, persistence, and efficacy in preclinical models of osteosarcoma. Sci. Transl. Med. 16, eadg9814 (2024). An interesting example of the current strategies for developing engineered γδT cells for the treatment of cancer.
Huang, S. W. et al. BiTE-secreting CAR-γδT as a dual targeting strategy for the treatment of solid tumors. Adv. Sci. 10, e2206856 (2023).
Branella, G. M. et al. Ligand-based targeting of c-kit using engineered γδ T cells as a strategy for treating acute myeloid leukemia. Front. Immunol. 14, 1294555 (2023).
Becker, S. A. et al. Enhancing the effectiveness of γδ T cells by mRNA transfection of chimeric antigen receptors or bispecific T cell engagers. Mol. Ther. Oncolytics 29, 145–157 (2023).
Hernandez-Lopez, P. et al. Dual targeting of cancer metabolome and stress antigens affects transcriptomic heterogeneity and efficacy of engineered T cells. Nat. Immunol. 25, 88–101 (2024). This paper describes the superior activity of T cells engineered with a Vγ9Vδ2TCR and a CCR in preclinical in vivo models for the treatment of both haematological malignancies and solid tumours.
Chabannon, C. et al. Celebrating the registration of 9.000 patients treated with CAR T cells in the EBMT registry: collection of real-world data in the context of hematopoietic cellular therapies. Best Pract. Res. Clin. Haematol. 37, 101557 (2024).
Peng, J. J., Wang, L., Li, Z., Ku, C. L. & Ho, P. C. Metabolic challenges and interventions in CAR T cell therapy. Sci. Immunol. 8, eabq3016 (2023).
Good, Z. et al. Post-infusion CAR TReg cells identify patients resistant to CD19-CAR therapy. Nat. Med. 28, 1860–1871 (2022).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Siddiqi, T. et al. CD19-directed CAR T-cell therapy for treatment of primary CNS lymphoma. Blood Adv. 5, 4059–4063 (2021).
Straetemans, T. et al. Untouched GMP-ready purified engineered immune cells to treat cancer. Clin. Cancer Res. 21, 3957–3968 (2015).
de Witte, M. et al. First in human clinical responses and persistence data on TEG001: a next generation of engineered Αβ T cells targeting AML and MM with a high affinity γ9δ2TCR. Blood 140, 12737–12739 (2022).
Davies, D. M. et al. Engineering a dual specificity γδ T-cell receptor for cancer immunotherapy. Biology 13, 196 (2024).
Xu, Y. et al. A novel antibody-TCR (AbTCR) platform combines fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 4, 62 (2018).
Li, C. et al. Novel CD19-specific γ/δ TCR-T cells in relapsed or refractory diffuse large B-cell lymphoma. J. Hematol. Oncol. 16, 5 (2023).
Dao, T. et al. A dual-receptor T-cell platform with Ab-TCR and costimulatory receptor achieves specificity and potency against AML. Blood 143, 507–521 (2024).
Liu, Y. et al. Chimeric STAR receptors using TCR machinery mediate robust responses against solid tumors. Sci. Transl. Med. 13, eabb5191 (2021).
Mansilla-Soto, J. et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat. Med. 28, 345–352 (2022).
Mensurado, S., Blanco-Dominguez, R. & Silva-Santos, B. The emerging roles of γδ T cells in cancer immunotherapy. Nat. Rev. Clin. Oncol. 20, 178–191 (2023).
Riano, F. et al. Vγ9Vδ2 TCR-activation by phosphorylated antigens requires butyrophilin 3 A1 (BTN3A1) and additional genes on human chromosome 6. Eur. J. Immunol. 44, 2571–2576 (2014).
Braham, M. V. J. et al. Cellular immunotherapy on primary multiple myeloma expanded in a 3D bone marrow niche model. Oncoimmunology 7, e1434465 (2018).
Alieva, M. et al. BEHAV3D: a 3D live imaging platform for comprehensive analysis of engineered T cell behavior and tumor response. Nat. Protoc. 19, 2052–2084 (2024).
Dekkers, J. F. et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 41, 60–69 (2023).
Johanna, I. et al. Basics of advanced therapy medicinal product development in academic pharma and the role of a GMP simulation unit. Immunooncol. Technol. 20, 100411 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05369000 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04887259 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05983133 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06372236 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06092047 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04735471 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06480565 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06193486 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06150885 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04107142 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05302037 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04702841 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06018363 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06592092 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06415487 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05653271 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT06404281 (2024).
CCMO. Safety of TEG001 in Patients with r/r AML, High-risk MDS or MM https://onderzoekmetmensen.nl/en/trial/25189 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04014894 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04864054 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04502082 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04634357 (2022).
Allison, T. J., Winter, C. C., Fournie, J. J., Bonneville, M. & Garboczi, D. N. Structure of a human γδ T-cell antigen receptor. Nature 411, 820–824 (2001).
Weng, R. R. et al. Epigenetic modulation of immune synaptic-cytoskeletal networks potentiates γδ T cell-mediated cytotoxicity in lung cancer. Nat. Commun. 12, 2163 (2021).
Legut, M., Cole, D. K. & Sewell, A. K. The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy. Cell Mol. Immunol. 12, 656–668 (2015).
Dimova, T. et al. Effector Vγ9Vδ2 T cells dominate the human fetal γδ T-cell repertoire. Proc. Natl Acad. Sci. USA 112, E556–E565 (2015).
Perriman, L. et al. A three-stage developmental pathway for human Vγ9Vδ2 T cells within the postnatal thymus. Sci. Immunol. 8, eabo4365 (2023).
Papadopoulou, M. et al. TCR sequencing reveals the distinct development of fetal and adult human Vγ9Vδ2 T cells. J. Immunol. 203, 1468–1479 (2019).
Fichtner, A. S., Ravens, S. & Prinz, I. Human γδ TCR repertoires in health and disease. Cells 9, 800 (2020).
Tieppo, P. et al. The human fetal thymus generates invariant effector γδ T cells. J. Exp. Med. 217, jem.20190580 (2020).
Gray, J. I. et al. Human γδ T cells in diverse tissues exhibit site-specific maturation dynamics across the life span. Sci. Immunol. 9, eadn3954 (2024).
Pitard, V. et al. Long-term expansion of effector/memory Vδ2− γδ T cells is a specific blood signature of CMV infection. Blood 112, 1317–1324 (2008).
Ravens, S. et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 18, 393–401 (2017).
Acknowledgements
Funding for this study was provided by Dutch Cancer Society (KWF: 12586, 13043, 13493, 15614, 15570) and KIKA473 to J.K. National Growth Fund Oncode Accelerator (https://www.oncodeaccelerator.nl) provided support to J.K., Z.S., T.S. and D.X.B. S.M. is supported by the German Research Foundation (DFG) under Germany’s Excellence Strategy – EXC-2189 Project ID: 390939984 and under the Excellence Initiative of the German Federal and State Governments – EXC-294, and in part by the Ministry for Science, Research and Arts of the State of Baden-Württemberg. Further support is given by the DFG under FOR2799 (MI1942/3-1), SFB1479 (project ID: 441891347 - P15), SFB1160 (project ID: 256073931 - B01), and projects MI1942/4-1 (project ID: 501418856) and MI1942/5-1 (project ID: 501436442). S.M. is also supported by the Dutch Cancer Society (KWF project ID: 13043). T.S. is supported by grants provided by the Dutch Cancer Society (KWF: 12586 and 13876) and National Growth Fund RegMed XB pilot Factory UMCU Innovation Center of Advanced Therapies. D.X.B. is supported by a grant provided by the Dutch Cancer Society (KWF: 15570).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. D.X.B., T.S., S.M., L.L., C.R., Z.S. and J.K. contributed substantially to discussion of the content. All authors wrote the article. D.X.B., Z.S., S.M. and J.K. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.K. was a shareholder of Gadeta and is a shareholder of Gadeta Founder BV. J.K., Z.S., L.L. and D.X.B. are inventors on patents with γδTCR-related topics. J.K., Z.S. and D.X.B. are inventors on patents with CD277-related topics. L.L. is on the SAB of MiNK Therapeutics, Faeth Therapeutics and Deciduous Therapeutics. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Michal Besser who co-reviewed with Ilan Bank, Mary Poupot and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Innovation Center for Advanced Therapies (ICAT): https://icat-utrecht.nl/en
Glossary
- Activation-induced cell death
-
Apoptosis of T cells due to strong or prolonged activation of the T cell receptor.
- Advanced therapy medicinal products
-
(ATMPs). Innovative medical therapies based on engineered genes, cells or tissue designed to treat or prevent disease.
- Aminobisphosphonates
-
(NBPs). A nitrogen-containing class of drugs that inhibits the mevalonate pathway, leading to an accumulation of phosphoantigens in tumour cells.
- Biomimetic models
-
Artificial systems or structures designed to imitate biological processes, functions or materials found in nature. These models are used in research and development to study complex biological phenomena, develop new technologies or create therapeutic solutions.
- Bispecific T cell engagers
-
(TCEs). Engineered molecules designed to simultaneously bind to a specific protein on tumour cells and to another protein, normally CD3, on T cells. This dual binding directs the T cells to attack and destroy the cancer cells.
- Chimeric antigen receptors
-
(CARs). Genetically engineered receptors that enhance the capacity of immune effector cells to recognize and kill specific cancer cells.
- Clonal expansion
-
The rapid proliferation of genetically identical T cells from a single parental cell, enhancing the immune response to specific pathogens or abnormal cells.
- Complementarity-determining region
-
(CDR). Specific portion of the variable regions of an antibody or T cell receptor that determines antigen-binding specificity.
- CRISPR–Cas9
-
Genome-editing technology that uses a guide RNA to direct the Cas9 nuclease to specific DNA sequences creating double-strand breaks for precise gene modification.
- Cryo-electron microscopy
-
Technique that captures high-resolution images of biological samples cooled at cryogenic temperature, preserving their native structure.
- Cytokine release syndrome
-
Rapid release of cytokines by immune effector cells caused by immunotherapy treatment, leading to systemic inflammation.
- Exhaustion
-
A dysfunctional state of immune cells caused by chronic exposure to antigen and characterized by increased inhibitory receptor expression and reduced effector function.
- Fab
-
Antibody fragment consisting of variable heavy and first constant heavy domains paired with variable light and constant light domains that binds to antigens.
- Functional avidity
-
The capability of a specific T cell receptor (TCR) to activate T cell functions, such as cytotoxicity, cytokine secretion and proliferation, as a function of ligand concentration. A TCR with high functional avidity is capable of activating T cells at low ligand concentrations.
- Glutamine
-
Most abundant amino acid in the serum essential for T cell activation, proliferation, cytokine production and nucleotide synthesis.
- Graft-versus-host
-
The reactivity of transplanted donor-derived immune cells to healthy cells of the recipient.
- Graft-versus-leukaemia
-
The reactivity of transplanted donor-derived immune cells to leukaemia cells in the patient.
- Immune effector cell-associated neurotoxicity syndrome
-
A common adverse event after immunotherapy affecting neurological functions, caused by inflammation in the central nervous system.
- Immunoreceptor tyrosine-based activation motifs
-
(ITAMs). Highly conserved regions located in the cytoplasmic tail of immune receptors, crucial for conveying activation signals in immune cells.
- Lipidome
-
The complete array of lipids within a biological system representing its metabolic state and function.
- Mevalonate pathway
-
Essential metabolic pathway for the synthesis of important molecules like cholesterol, ubiquinone and dolichol. The enzymes of the mevalonate pathway are frequently overexpressed and highly active in cancer cells, leading to the promotion of tumour growth. One of the central metabolites in the mevalonate pathway is isopentenyl pyrophosphate, the key endogenous phosphoantigen required for Vγ9Vδ2T cell activation.
- Nanoparticles
-
Ultra-small particles, ranging from 1 to 100 nm, characterized by unique physical and chemical properties.
- Opsonins
-
Molecules that enhance the process of phagocytosis by tagging pathogens or cells for recognition by the immune system.
- Phosphoantigens
-
(pAg). Metabolites containing a pyrophosphate group, such as isopentenyl pyrophosphate, dimethylallyl pyrophosphate and (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate, and sensed by BTN2A1 and BTN3A, which subsequently induce Vγ9Vδ2T cell activation.
- Post-marketing authorization studies
-
Also known as post-authorization safety studies; clinical studies carried out after a drug has received marketing authorization in order to acquire additional safety and/or efficacy data or to evaluate the effectiveness of risk management measures.
- Purine synthesis
-
A biosynthesis pathway for purine molecules essential for the synthesis of adenine and adenosine monophosphate and guanine and guanosine monophosphate.
- Pyroglutamate
-
A post-translational modification of an N-terminal glutamine of a protein, where the side chain of glutamine reacts with a free amino group, resulting in the cyclic amino acid pyroglutamate. Several enzymes can catalyse this reaction, for example, glutaminyl-peptide cyclotransferase-like protein in the Golgi apparatus and glutaminyl-peptide cyclotransferase in the extracellular space.
- Shedding
-
Highly regulated proteolytic release of extracellular domains of membrane proteins from the cell surface into the surrounding environment.
- Single-chain variable fragment
-
(ScFv). Fusion protein composed of the variable heavy and light chains of immunoglobulins connected by a short linker.
- Single nucleotide polymorphisms
-
Genetic variation involving a change in a single nucleotide in the DNA sequence, potentially influencing phenotypic characteristics or disease susceptibility.
- Superantigen
-
Superantigens are a class of pathogen-derived proteins that bind to TCRs outside of their conventional recognition sites, such as the CDRs, triggering a polyclonal T cell response.
- T cell repertoires
-
Diverse and unique arrays of TCRs present in an individual, enabling the detection of a broad range of antigens.
- TCR constant domains
-
Extracellular membrane-proximal domains of the TCR not involved in antigen recognition. αβTCR constant domains interact with the extracellular domains of CD3, whilst γδTCR constant domains have no interaction with the extracellular domains of CD3.
- TCR variable domains
-
Extracellular membrane distal domains of the TCR important for antigen binding and specificity through their highly variable CDR loops.
- Tonic signalling
-
A sustained, non-specific activation of T cells that occurs independently of ligand binding.
- Tumour organoids
-
3D cell cultures derived from patient tumour samples that recapitulate the complexity of the structure and function of the original tumour.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Beringer, D.X., Straetemans, T., Minguet, S. et al. Disrupting the balance between activating and inhibitory receptors of γδT cells for effective cancer immunotherapy. Nat Rev Cancer 25, 590–612 (2025). https://doi.org/10.1038/s41568-025-00830-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41568-025-00830-x
This article is cited by
-
Cis- and trans-binding chimeric costimulatory receptors enhance T-cell fitness and tumor control
Cellular & Molecular Immunology (2025)
-
Usage of rabbit anti-thymocyte / anti-T-lymphocyte globulins (ATG / ATLG) for hematological malignancies in allogeneic hematopoietic cell transplantation: Best practice recommendations from the EBMT Practice Harmonisation and Guidelines Committee
Bone Marrow Transplantation (2025)