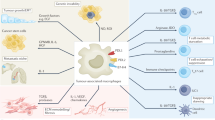
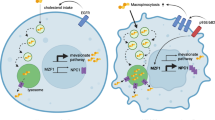
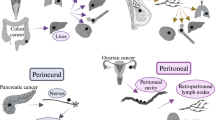
Abstract
Macropinocytosis is a nutrient-scavenging process that enables cells to engulf large volumes of extracellular fluid and solutes through dynamic plasma membrane ruffling. In cancer, this evolutionarily conserved process is frequently hijacked to meet the heightened metabolic demands of malignant cells, particularly under conditions of nutrient deprivation. Through macropinocytosis, tumour cells internalize diverse extracellular components — including proteins, nucleotides, lipids, ions and debris from dead cells — which are subsequently degraded in lysosomes and recycled to support biosynthesis and energy production. This process is tightly regulated by oncogenic signalling pathways and cues from the tumour microenvironment, including those associated with oncogene activation, loss of tumour suppressors and hypoxia. Beyond facilitating tumour growth and metabolic adaptation, macropinocytosis is implicated in resistance to chemotherapy, radiotherapy, targeted therapy and immunotherapy. When excessively activated, it can also lead to methuosis, a form of non-apoptotic cell death characterized by macropinosome overload. This Review outlines the molecular mechanisms and functional consequences of macropinocytosis in cancer, highlighting its dual potential as a metabolic vulnerability and a route for therapeutic delivery. Continued investigation into its regulation, context-specific roles and pharmacological modulation may uncover new opportunities for combination therapies and precision cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Amstutz, B. et al. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 27, 956–969 (2008).
Francis, C. L., Ryan, T. A., Jones, B. D., Smith, S. J. & Falkow, S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364, 639–642 (1993).
Sallusto, F., Cella, M., Danieli, C. & Lanzavecchia, A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182, 389–400 (1995).
Norbury, C. C., Hewlett, L. J., Prescott, A. R., Shastri, N. & Watts, C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 3, 783–791 (1995).
Schmees, C. et al. Macropinocytosis of the PDGF β-receptor promotes fibroblast transformation by H-RasG12V. Mol. Biol. Cell 23, 2571–2582 (2012).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013). This study shows that macropinocytosis contributes to the scavenging of extracellular nutrients in mutant RAS-driven cancers.
Kim, S. M. et al. PTEN deficiency and AMPK activation promote nutrient scavenging and anabolism in prostate cancer cells. Cancer Discov. 8, 866–883 (2018). This study demonstrates that loss of PTEN and AMPK activation induces macropinocytosis in prostate cancer.
Yao, W. et al. Syndecan 1 is a critical mediator of macropinocytosis in pancreatic cancer. Nature 568, 410–414 (2019).
Racoosin, E. L. & Swanson, J. A. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J. Cell Sci. 102, 867–880 (1992).
Bar-Sagi, D. & Feramisco, J. R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science 233, 1061–1068 (1986). This study demonstrates that HRAS protein injection induces macropinocytosis in fibroblasts.
Li, C. et al. AGER-dependent macropinocytosis drives resistance to KRAS-G12D-targeted therapy in advanced pancreatic cancer. Sci. Transl. Med. 17, eadp4986 (2025). This study demonstrates that AGER-dependent macropinocytosis contributes to drug resistance during RAS-targeted therapy.
Wang, Y. et al. Inhibition of tumor cell macropinocytosis driver DHODH reverses immunosuppression and overcomes anti-PD1 resistance. Immunity 58, 2456–2471 (2025).
Zhang, Y. et al. Macropinocytosis maintains CAF subtype identity under metabolic stress in pancreatic cancer. Cancer Cell 43, 1677–1696 (2025).
Puccini, J., Badgley, M. A. & Bar-Sagi, D. Exploiting cancer’s drinking problem: regulation and therapeutic potential of macropinocytosis. Trends Cancer 8, 54–64 (2022).
Zhang, Y. & Commisso, C. Macropinocytosis in cancer: a complex signaling network. Trends Cancer 5, 332–334 (2019).
Lambies, G. et al. Cell polarity proteins promote macropinocytosis in response to metabolic stress. Nat. Commun. 15, 10541 (2024).
Bryant, D. M. et al. EGF induces macropinocytosis and SNX1-modulated recycling of E-cadherin. J. Cell Sci. 120, 1818–1828 (2007).
Lee, S. W. et al. EGFR–Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Dev. Cell 50, 381–392.e5 (2019). This study demonstrates that the EGFR–PAK pathway promotes macropinocytosis in response to glutamine starvation.
Zhang, Y. F. et al. A low amino acid environment promotes cell macropinocytosis through the YY1–FGD6 axis in Ras-mutant pancreatic ductal adenocarcinoma. Oncogene 41, 1203–1215 (2022). This study demonstrates that CDC42 promotes macropinocytosis in mutant RAS-driven cancers.
Kamphorst, J. J. et al. Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Res. 75, 544–553 (2015).
Wahi, K. et al. Macropinocytosis mediates resistance to loss of glutamine transport in triple-negative breast cancer. EMBO J. 43, 5857–5882 (2024).
Hodakoski, C. et al. Rac-mediated macropinocytosis of extracellular protein promotes glucose independence in non-small cell lung cancer. Cancers 11, 37 (2019).
Ridley, A. J., Paterson, H. F., Johnston, C. L., Diekmann, D. & Hall, A. The small GTP-binding protein Rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 (1992).
Dharmawardhane, S. et al. Regulation of macropinocytosis by p21-activated kinase-1. Mol. Biol. Cell 11, 3341–3352 (2000).
Chen, B. et al. Rac1 GTPase activates the WAVE regulatory complex through two distinct binding sites. eLife 6, e29795 (2017).
Mettlen, M. et al. Src triggers circular ruffling and macropinocytosis at the apical surface of polarized MDCK cells. Traffic 7, 589–603 (2006).
Innocenti, M. et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat. Cell Biol. 7, 969–976 (2005).
Singla, B., Lin, H. P., Ghoshal, P., Cherian-Shaw, M. & Csanyi, G. PKCδ stimulates macropinocytosis via activation of SSH1–cofilin pathway. Cell Signal. 53, 111–121 (2019).
Campa, C. C., Ciraolo, E., Ghigo, A., Germena, G. & Hirsch, E. Crossroads of PI3K and Rac pathways. Small GTPases 6, 71–80 (2015).
Liao, Y. N. et al. Progesterone receptor potentiates macropinocytosis through CDC42 in pancreatic ductal adenocarcinoma. Oncogenesis 13, 10 (2024).
Edwards, D. C., Sanders, L. C., Bokoch, G. M. & Gill, G. N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1, 253–259 (1999).
Hewlett, L. J., Prescott, A. R. & Watts, C. The coated pit and macropinocytic pathways serve distinct endosome populations. J. Cell Biol. 124, 689–703 (1994).
Zhang, B. et al. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron 21, 1465–1475 (1998).
Maxson, M. E., Sarantis, H., Volchuk, A., Brumell, J. H. & Grinstein, S. Rab5 regulates macropinocytosis by recruiting the inositol 5-phosphatases OCRL and Inpp5b that hydrolyse PtdIns(4,5)P2. J. Cell Sci. 134, jcs252411 (2021).
Hamasaki, M., Araki, N. & Hatae, T. Association of early endosomal autoantigen 1 with macropinocytosis in EGF-stimulated A431 cells. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 277, 298–306 (2004).
Balaji, K. et al. RIN1 orchestrates the activation of RAB5 GTPases and ABL tyrosine kinases to determine the fate of EGFR. J. Cell Sci. 125, 5887–5896 (2012).
Tall, G. G., Barbieri, M. A., Stahl, P. D. & Horazdovsky, B. F. Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73–82 (2001).
Feliciano, W. D., Yoshida, S., Straight, S. W. & Swanson, J. A. Coordination of the Rab5 cycle on macropinosomes. Traffic 12, 1911–1922 (2011).
Jayashankar, V. & Edinger, A. L. Macropinocytosis confers resistance to therapies targeting cancer anabolism. Nat. Commun. 11, 1121 (2020). This study demonstrates that macropinocytosis mediates chemotherapy and radiation resistance in cancer cells.
Salloum, G. et al. PI3Kβ is selectively required for growth factor-stimulated macropinocytosis. J. Cell Sci. 132, jcs231639 (2019).
Amyere, M. et al. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol. Biol. Cell 11, 3453–3467 (2000).
Tisdale, E. J., Shisheva, A. & Artalejo, C. R. Overexpression of atypical protein kinase C in HeLa cells facilitates macropinocytosis via Src activation. Cell Signal. 26, 1235–1242 (2014).
Orgaz, J. L. et al. Myosin II reactivation and cytoskeletal remodeling as a hallmark and a vulnerability in melanoma therapy resistance. Cancer Cell 37, 85–103.e9 (2020).
Brzeska, H., Koech, H., Pridham, K. J., Korn, E. D. & Titus, M. A. Selective localization of myosin-I proteins in macropinosomes and actin waves. Cytoskeleton 73, 68–82 (2016).
Schink, K. O. et al. The phosphoinositide coincidence detector Phafin2 promotes macropinocytosis by coordinating actin organisation at forming macropinosomes. Nat. Commun. 12, 6577 (2021).
Williamson, C. D. & Donaldson, J. G. Arf6, JIP3, and dynein shape and mediate macropinocytosis. Mol. Biol. Cell 30, 1477–1489 (2019).
Le, A. H. et al. CYRI-A limits invasive migration through macropinosome formation and integrin uptake regulation. J. Cell Biol. 220, e202012114 (2021).
Nikolaou, S. et al. CYRI-B-mediated macropinocytosis drives metastasis via lysophosphatidic acid receptor uptake. eLife 13, e83712 (2024).
Puccini, J., Wei, J., Tong, L. & Bar-Sagi, D. Cytoskeletal association of ATP citrate lyase controls the mechanodynamics of macropinocytosis. Proc. Natl Acad. Sci. USA 120, e2213272120 (2023).
Freeman, S. A. et al. Lipid-gated monovalent ion fluxes regulate endocytic traffic and support immune surveillance. Science 367, 301–305 (2020).
Wang, X. et al. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell 151, 372–383 (2012).
Zeziulia, M., Blin, S., Schmitt, F. W., Lehmann, M. & Jentsch, T. J. Proton-gated anion transport governs macropinosome shrinkage. Nat. Cell Biol. 24, 885–895 (2022).
Krishna, S. et al. PIKfyve regulates vacuole maturation and nutrient recovery following engulfment. Dev. Cell 38, 536–547 (2016).
Kerr, M. C. et al. Visualisation of macropinosome maturation by the recruitment of sorting nexins. J. Cell Sci. 119, 3967–3980 (2006).
Lim, J. P., Wang, J. T., Kerr, M. C., Teasdale, R. D. & Gleeson, P. A. A role for SNX5 in the regulation of macropinocytosis. BMC Cell Biol. 9, 58 (2008).
Alonso-Curbelo, D. et al. RAB7 counteracts PI3K-driven macropinocytosis activated at early stages of melanoma development. Oncotarget 6, 11848–11862 (2015).
Swanson, J. A. & Araki, N. Roles for 3′ phosphoinositides in macropinocytosis. Subcell. Biochem. 98, 119–141 (2022).
Bucci, C., Thomsen, P., Nicoziani, P., McCarthy, J. & van Deurs, B. Rab7: a key to lysosome biogenesis. Mol. Biol. Cell 11, 467–480 (2000).
Weber, T. et al. SNAREpins: minimal machinery for membrane fusion. Cell 92, 759–772 (1998).
Dolat, L. & Spiliotis, E. T. Septins promote macropinosome maturation and traffic to the lysosome by facilitating membrane fusion. J. Cell Biol. 214, 517–527 (2016).
Xu, D. et al. Repression of Septin9 and Septin2 suppresses tumor growth of human glioblastoma cells. Cell Death Dis. 9, 514 (2018).
Jain, A. et al. Leucine aminopeptidase LyLAP enables lysosomal degradation of membrane proteins. Science 387, eadq8331 (2025). This study demonstrates that lysosomal leucine aminopeptidase is the protease responsible for degrading hydrophobic transmembrane domains.
Buckley, C. M. et al. WASH drives early recycling from macropinosomes and phagosomes to maintain surface phagocytic receptors. Proc. Natl Acad. Sci. USA 113, E5906–E5915 (2016).
Bienvenu, A. et al. The multifunction coxiella effector vice stimulates macropinocytosis and interferes with the ESCRT machinery. Proc. Natl Acad. Sci. USA 121, e2315481121 (2024).
Maltese, W. A. & Overmeyer, J. H. Methuosis: nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments. Am. J. Pathol. 184, 1630–1642 (2014).
Qian, Y. et al. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett. 351, 242–251 (2014).
Cao, Y. et al. Extracellular and macropinocytosis internalized ATP work together to induce epithelial–mesenchymal transition and other early metastatic activities in lung cancer. Cancer Cell Int. 19, 254 (2019).
Olivares, O. et al. Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nat. Commun. 8, 16031 (2017).
Nakase, I., Kobayashi, N. B., Takatani-Nakase, T. & Yoshida, T. Active macropinocytosis induction by stimulation of epidermal growth factor receptor and oncogenic Ras expression potentiates cellular uptake efficacy of exosomes. Sci. Rep. 5, 10300 (2015).
Zhao, H. et al. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. eLife 5, e10250 (2016).
Skorda, A. et al. Activation of invasion by oncogenic reprogramming of cholesterol metabolism via increased NPC1 expression and macropinocytosis. Oncogene 42, 2495–2506 (2023).
Aubert, L. et al. Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat. Commun. 11, 3701 (2020).
King, B., Araki, J., Palm, W. & Thompson, C. B. Yap/Taz promote the scavenging of extracellular nutrients through macropinocytosis. Genes Dev. 34, 1345–1358 (2020).
Tang, D., Kroemer, G. & Kang, R. Oncogenic KRAS blockade therapy: renewed enthusiasm and persistent challenges. Mol. Cancer 20, 128 (2021).
Mukhopadhyay, S., Vander Heiden, M. G. & McCormick, F. The metabolic landscape of RAS-driven cancers from biology to therapy. Nat. Cancer 2, 271–283 (2021).
Davidson, S. M. et al. Direct evidence for cancer-cell-autonomous extracellular protein catabolism in pancreatic tumors. Nat. Med. 23, 235–241 (2017).
Liu, H. et al. KRAS-enhanced macropinocytosis and reduced FcRn-mediated recycling sensitize pancreatic cancer to albumin-conjugated drugs. J. Control. Rel. 296, 40–53 (2019).
Seguin, L. et al. An integrin β3–KRAS–RalB complex drives tumour stemness and resistance to EGFR inhibition. Nat. Cell Biol. 16, 457–468 (2014).
Seguin, L. et al. Galectin-3, a druggable vulnerability for KRAS-addicted cancers. Cancer Discov. 7, 1464–1479 (2017). This study demonstrates that LGALS3 can induce macropinocytosis in RAS-mutant cancer cells.
Palm, W. et al. The utilization of extracellular proteins as nutrients is suppressed by mTORC1. Cell 162, 259–270 (2015). This study demonstrates that mTORC1 inhibits macropinocytosis through a feedback mechanism.
Nofal, M., Zhang, K., Han, S. & Rabinowitz, J. D. mTOR inhibition restores amino acid balance in cells dependent on catabolism of extracellular protein. Mol. Cell 67, 936–946.e5 (2017).
Rodriguez-Viciana, P. et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell 89, 457–467 (1997).
Meng, D. et al. SNAT7 regulates mTORC1 via macropinocytosis. Proc. Natl Acad. Sci. USA 119, e2123261119 (2022).
Chen, F., Kang, R., Liu, J. & Tang, D. The V-ATPases in cancer and cell death. Cancer Gene Ther. 29, 1529–1541 (2022).
Ramirez, C., Hauser, A. D., Vucic, E. A. & Bar-Sagi, D. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature 576, 477–481 (2019). This study demonstrates that plasma membrane V-ATPase is required for macropinocytosis in RAS-mutant cancer cells.
Zhou, Z. et al. Acetyl-coenzyme A synthetase 2 potentiates macropinocytosis and muscle wasting through metabolic reprogramming in pancreatic cancer. Gastroenterology 163, 1281–1293.e1 (2022).
Hobbs, G. A. et al. Atypical KRASG12R mutant is impaired in PI3K signaling and macropinocytosis in pancreatic cancer. Cancer Discov. 10, 104–123 (2020).
Garg, B. et al. MICAL2 promotes pancreatic cancer growth and metastasis. Cancer Res. 85, 1049–1063 (2025).
Zhang, Y. et al. Macropinocytosis in cancer-associated fibroblasts is dependent on CaMKK2/ARHGEF2 signaling and functions to support tumor and stromal cell fitness. Cancer Discov. 11, 1808–1825 (2021). This study demonstrates that macropinocytosis mediates nutrient transfer between CAFs and PDAC cells.
Alvarez-Garcia, V., Tawil, Y., Wise, H. M. & Leslie, N. R. Mechanisms of PTEN loss in cancer: it’s all about diversity. Semin. Cancer Biol. 59, 66–79 (2019).
Kim, J. et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141 (2011).
Mihaylova, M. M. & Shaw, R. J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 13, 1016–1023 (2011).
Mense, S. M. et al. PTEN inhibits PREX2-catalyzed activation of RAC1 to restrain tumor cell invasion. Sci. Signal. 8, ra32 (2015).
Voloshanenko, O. et al. Wnt secretion is required to maintain high levels of Wnt activity in colon cancer cells. Nat. Commun. 4, 2610 (2013).
Wu, X., Que, H., Li, Q. & Wei, X. Wnt/β-catenin mediated signaling pathways in cancer: recent advances, and applications in cancer therapy. Mol. Cancer 24, 171 (2025).
Albrecht, L. V. et al. GSK3 inhibits macropinocytosis and lysosomal activity through the Wnt destruction complex machinery. Cell Rep. 32, 107973 (2020).
Tejeda-Munoz, N. et al. The PMA phorbol ester tumor promoter increases canonical Wnt signaling via macropinocytosis. eLife 12, RP89141 (2023).
Tejeda-Munoz, N., Albrecht, L. V., Bui, M. H. & De Robertis, E. M. Wnt canonical pathway activates macropinocytosis and lysosomal degradation of extracellular proteins. Proc. Natl Acad. Sci. USA 116, 10402–10411 (2019).
Redelman-Sidi, G. et al. The canonical Wnt pathway drives macropinocytosis in cancer. Cancer Res. 78, 4658–4670 (2018).
Malliri, A. et al. The rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J. Biol. Chem. 281, 543–548 (2006).
Fu, M. et al. The Hippo signalling pathway and its implications in human health and diseases. Signal. Transduct. Target. Ther. 7, 376 (2022).
Zdzalik-Bielecka, D. et al. The GAS6–AXL signaling pathway triggers actin remodeling that drives membrane ruffling, macropinocytosis, and cancer-cell invasion. Proc. Natl Acad. Sci. USA 118, e2024596118 (2021).
Zeng, J., Li, M., Shi, H. & Guo, J. Upregulation of FGD6 predicts poor prognosis in gastric cancer. Front. Med. 8, 672595 (2021).
Goyette, M. A. & Cote, J. F. AXL receptor tyrosine kinase as a promising therapeutic target directing multiple aspects of cancer progression and metastasis. Cancers 14, 466 (2022).
Zhang, M. S. et al. Hypoxia-induced macropinocytosis represents a metabolic route for liver cancer. Nat. Commun. 13, 954 (2022). This study demonstrates that hypoxia induces macropinocytosis in liver cancer.
Blume, J. J., Halbach, A., Behrendt, D., Paulsson, M. & Plomann, M. EHD proteins are associated with tubular and vesicular compartments and interact with specific phospholipids. Exp. Cell Res. 313, 219–231 (2007).
Garcia-Bermudez, J. et al. Adaptive stimulation of macropinocytosis overcomes aspartate limitation in cancer cells under hypoxia. Nat. Metab. 4, 724–738 (2022). This study demonstrates that hypoxia induces macropinocytosis in PDAC.
DeNicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011).
Li, N. et al. Loss of acinar cell IKKα triggers spontaneous pancreatitis in mice. J. Clin. Invest. 123, 2231–2243 (2013).
Todoric, J. et al. Stress-activated NRF2-MDM2 cascade controls neoplastic progression in pancreas. Cancer Cell 32, 824–839.e8 (2017).
Su, H. et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell 39, 678–693.e11 (2021).
Hosogi, S. et al. An inhibitor of Na+/H+ exchanger (NHE), ethyl-isopropyl amiloride (EIPA), diminishes proliferation of MKN28 human gastric cancer cells by decreasing the cytosolic Cl– concentration via DIDS-sensitive pathways. Cell Physiol. Biochem. 30, 1241–1253 (2012).
Cerrato, G. et al. AI-based classification of anticancer drugs reveals nucleolar condensation as a predictor of immunogenicity. Mol. Cancer 23, 275 (2024).
Huang, Z. et al. ATM inhibition drives metabolic adaptation via induction of macropinocytosis. J. Cell Biol. 222, e202007026 (2023).
Xia, H. et al. Inhibition of macropinocytosis enhances the sensitivity of osteosarcoma cells to benzethonium chloride. Cancers 15, 961 (2023).
Cullis, J. et al. Macropinocytosis of nab-paclitaxel drives macrophage activation in pancreatic cancer. Cancer Immunol. Res. 5, 182–190 (2017).
Redelman-Sidi, G., Iyer, G., Solit, D. B. & Glickman, M. S. Oncogenic activation of Pak1-dependent pathway of macropinocytosis determines BCG entry into bladder cancer cells. Cancer Res. 73, 1156–1167 (2013).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008).
Hudes, G. et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N. Engl. J. Med. 356, 2271–2281 (2007).
Nazio, F. et al. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 15, 406–416 (2013).
Han, L. et al. Macroautophagy/autophagy promotes resistance to KRASG12D-targeted therapy through glutathione synthesis. Cancer Lett. 604, 217258 (2024).
Song, X. et al. SRC kinase drives multidrug resistance induced by KRAS-G12C inhibition. Sci. Adv. 10, eadq4274 (2024).
Yanada, H. et al. Sotorasib resistance in KRAS G12C-mutant invasive mucinous adenocarcinoma with implications for VEGF-A. NPJ Precis. Oncol. 9, 154 (2025).
Mohanty, A. et al. Acquired resistance to KRAS G12C small-molecule inhibitors via genetic/nongenetic mechanisms in lung cancer. Sci. Adv. 9, eade3816 (2023).
Yaeger, R. & Solit, D. B. Overcoming adaptive resistance to KRAS inhibitors through vertical pathway targeting. Clin. Cancer Res. 26, 1538–1540 (2020).
Chen, R. et al. DAMPs in the immunogenicity of cell death. Mol. Cell 85, 3874–3889 (2025).
Kang, R. et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc. Natl Acad. Sci. USA 109, 7031–7036 (2012).
Kang, R. et al. RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death Dis. 5, e1480 (2014).
Li, R. et al. Therapeutically reprogrammed nutrient signalling enhances nanoparticulate albumin bound drug uptake and efficacy in KRAS-mutant cancer. Nat. Nanotechnol. 16, 830–839 (2021).
Hu, H. et al. Thyroid cancers exhibit oncogene-enhanced macropinocytosis that is restrained by IGF1R and promote albumin–drug conjugate response. Clin. Cancer Res. 29, 3457–3470 (2023).
Desai, A. S., Hunter, M. R. & Kapustin, A. N. Using macropinocytosis for intracellular delivery of therapeutic nucleic acids to tumour cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20180156 (2019).
Bern, M., Sand, K. M., Nilsen, J., Sandlie, I. & Andersen, J. T. The role of albumin receptors in regulation of albumin homeostasis: implications for drug delivery. J. Control. Rel. 211, 144–162 (2015).
Redka, D. S., Gutschow, M., Grinstein, S. & Canton, J. Differential ability of proinflammatory and anti-inflammatory macrophages to perform macropinocytosis. Mol. Biol. Cell 29, 53–65 (2018).
Charpentier, J. C. et al. Macropinocytosis drives T cell growth by sustaining the activation of mTORC1. Nat. Commun. 11, 180 (2020).
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000).
Yang, S. et al. Pancreatic cancers require autophagy for tumor growth. Genes Dev. 25, 717–729 (2011).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 25, 460–470 (2011).
Chi, S. et al. Oncogenic Ras triggers cell suicide through the activation of a caspase-independent cell death program in human cancer cells. Oncogene 18, 2281–2290 (1999).
Mao, Z. & Chai, G. Oral delivery of MOMIPP lipid nanoparticles for methuosis-induced cancer chemotherapy. Nanoscale 17, 4082–4098 (2025).
Chen, Y. et al. Discovery of potent and selective phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) inhibitors as methuosis inducers. J. Med. Chem. 67, 165–179 (2024).
Chen, X. et al. International consensus guidelines for the definition, detection, and interpretation of autophagy-dependent ferroptosis. Autophagy 20, 1213–1246 (2024).
Lim, R. M. et al. CARMIL1-AA selectively inhibits macropinocytosis while sparing autophagy. Mol. Biol. Cell 36, ar4 (2025).
Acknowledgements
The authors thank all of the pioneers in the field and their colleagues who have contributed to advancing the understanding of macropinocytosis. They apologize to those whose work could not be cited due to space limitations.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
G.K. has held research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sutro, Tollys and Vascage; is on the Board of Directors of the Bristol Myers Squibb Foundation France; is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio; is on the scientific advisory boards of Hevolution, Institut Servier, Longevity Vision Funds and Rejuveron Life Sciences/Centenara Labs AG; and is the inventor of patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders (among these patents, ‘Methods for weight reduction’ (US11905330B1) is relevant to this study). G.K.’s brother, R. Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. G.K.’s wife, L. Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9m, Tusk and Roche; was on the on the Board of Directors of Transgene; is a co-founder of everImmune; and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Joel Swanson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Actin polymerization
-
The dynamic assembly of actin filaments that drive membrane protrusions, including ruffles and lamellipodia, that is essential for processes such as macropinocytosis, migration and intracellular trafficking.
- Anchorage-independent growth
-
The ability of cells to proliferate without attachment to the extracellular matrix, often associated with tumorigenicity and supported by metabolic scavenging pathways.
- Antigen presentation
-
The process by which antigen-presenting cells display processed peptide fragments on major histocompatibility complex (MHC) molecules to T cells, linking innate and adaptive immunity.
- Autolysosomes
-
Vesicular structures formed from the fusion of an autophagosome with a lysosome, where enzymatic degradation of the sequestered material occurs.
- Autophagosomes
-
Double-membrane vesicles that sequester cellular cargo for delivery to the lysosome during macroautophagy.
- Autophagy
-
A catabolic process that delivers cytoplasmic components, including damaged organelles and macromolecules, to lysosomes for degradation and nutrient recycling.
- Circular dorsal ruffle
-
A transient, ring-like membrane structure formed on the dorsal surface of cells that can function as an entry site for macropinosomes.
- DAMPs
-
(Damage-associated molecular patterns). Endogenous molecules released from stressed or dying cells that activate immune responses by engaging pattern recognition receptors.
- Endosomes
-
Membrane-bound intracellular vesicles that sort internalized cargo from the plasma membrane and direct it towards recycling, degradation or other trafficking routes.
- ESCRT
-
(Endosomal sorting complex required for transport). A group of protein complexes involved in membrane remodelling events, such as multivesicular body formation, cytokinetic abscission and membrane repair.
- Filamentous scaffolds
-
Cytoskeletal structures, primarily composed of actin or microtubules, that provide mechanical support and spatial organization for membrane trafficking and signalling processes.
- Fluid-phase uptake
-
A non-selective internalization mechanism by which cells ingest solutes and nutrients dissolved in extracellular fluid.
- Lysosomes
-
Acidic organelles containing hydrolytic enzymes that degrade macromolecules delivered by endocytosis, phagocytosis or autophagy to facilitate cellular recycling.
- Macropinocytosis
-
An actin-driven, clathrin-independent endocytic process by which cells engulf extracellular fluid and solutes into large vesicles called macropinosomes.
- Macropinolysosomes
-
Specialized intracellular vesicles formed by the fusion of macropinosomes and lysosomes.
- Macropinosomes
-
Large, irregularly shaped intracellular vesicles formed by plasma membrane ruffling and closure, facilitating bulk nutrient uptake.
- Membrane ruffling
-
Dynamic, actin-rich protrusions of the plasma membrane that initiate macropinocytosis in response to external stimuli such as growth factors or oncogenic signals.
- Methuosis
-
A caspase-independent, non-apoptotic form of cell death caused by uncontrolled accumulation of large, fluid-filled macropinosomes and cytoplasmic vacuoles.
- Necrocytosis
-
A macropinocytosis-mediated process to engulf necrotic debris, facilitating the acquisition of carbohydrates and fatty acids for energy production.
- Nutrient scavenging
-
A metabolic adaptation that enables cancer cells to acquire essential nutrients from the extracellular environment under nutrient-deprived conditions.
- Trophic support
-
The supply of essential growth factors, nutrients or metabolic signals that sustain cell viability, proliferation and differentiation.
- WAVE regulatory complexes
-
(WASP family verprolin-homologous protein regulatory complexes). Multiprotein assemblies that activate the actin-related protein 2/3 (ARP2/3) complex to initiate branched actin polymerization, facilitating membrane ruffling and lamellipodia formation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, D., Wang, J., Kroemer, G. et al. Targeting macropinocytosis for cancer therapy. Nat Rev Cancer (2025). https://doi.org/10.1038/s41568-025-00892-x
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41568-025-00892-x