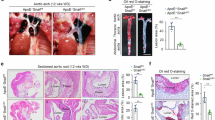
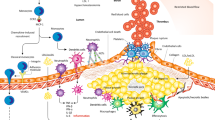
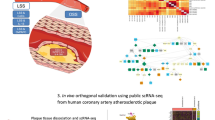
Abstract
Atherosclerotic diseases such as myocardial infarction, ischaemic stroke and peripheral artery disease continue to be leading causes of death worldwide despite the success of treatments with cholesterol-lowering drugs and drug-eluting stents, raising the need to identify additional therapeutic targets. Interestingly, atherosclerosis preferentially develops in curved and branching arterial regions, where endothelial cells are exposed to disturbed blood flow with characteristic low-magnitude oscillatory shear stress. By contrast, straight arterial regions exposed to stable flow, which is associated with high-magnitude, unidirectional shear stress, are relatively well protected from the disease through shear-dependent, atheroprotective endothelial cell responses. Flow potently regulates structural, functional, transcriptomic, epigenomic and metabolic changes in endothelial cells through mechanosensors and mechanosignal transduction pathways. A study using single-cell RNA sequencing and chromatin accessibility analysis in a mouse model of flow-induced atherosclerosis demonstrated that disturbed flow reprogrammes arterial endothelial cells in situ from healthy phenotypes to diseased ones characterized by endothelial inflammation, endothelial-to-mesenchymal transition, endothelial-to-immune cell-like transition and metabolic changes. In this Review, we discuss this emerging concept of disturbed-flow-induced reprogramming of endothelial cells (FIRE) as a potential pro-atherogenic mechanism. Defining the flow-induced mechanisms through which endothelial cells are reprogrammed to promote atherosclerosis is a crucial area of research that could lead to the identification of novel therapeutic targets to combat the high prevalence of atherosclerotic disease.
Key points
-
Atherosclerosis preferentially develops in curved and branching regions of arteries, which are sites of disturbed blood flow and low-magnitude oscillatory shear stress.
-
Disturbed flow delivers low-magnitude oscillatory shear stress to endothelial cells, which causes endothelial cells to adopt pro-atherogenic functions and gene transcription programmes.
-
Endothelial cells detect shear stress magnitudes and patterns through mechanosensory proteins and organelles and transmit these signals into intracellular changes via mechanotransduction pathways.
-
Advances in omics approaches and experimental models have helped to identify numerous novel potential therapeutic targets for atherosclerosis.
-
Disturbed-flow-induced reprogramming of endothelial cells (which we term FIRE) promotes endothelial inflammation, endothelial-to-mesenchymal transition and endothelial-to-immune-cell-like transition during atherogenesis.
-
Genes, proteins and pathways involved in FIRE are promising targets for anti-atherogenic therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Libby, P., Ridker, P. M. & Maseri, A. Inflammation and atherosclerosis. Circulation 105, 1135–1143 (2002).
Herrington, W., Lacey, B., Sherliker, P., Armitage, J. & Lewington, S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ. Res. 118, 535–546 (2016).
Davignon, J. & Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 109, III27–III32 (2004).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Libby, P. The changing landscape of atherosclerosis. Nature 592, 524–533 (2021).
Libby, P., Ridker, P. M., Hansson, G. K. & Atherothrombosis, L. T. N. O. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 54, 2129–2138 (2009).
Caro, C. G., Fitz-Gerald, J. M. & Schroter, R. C. Arterial wall shear and distribution of early atheroma in man. Nature 223, 1159–1160 (1969).
VanderLaan, P. A., Reardon, C. A. & Getz, G. S. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler Thromb. Vasc. Biol. 24, 12–22 (2004).
Tarbell, J. M. Mass transport in arteries and the localization of atherosclerosis. Annu. Rev. Biomed. Eng. 5, 79–118 (2003).
Fang, Y., Wu, D. & Birukov, K. G. Mechanosensing and mechanoregulation of endothelial cell functions. Compr. Physiol. 9, 873–904 (2019).
Gallego-Colon, E., Daum, A. & Yosefy, C. Statins and PCSK9 inhibitors: a new lipid-lowering therapy. Eur. J. Pharmacol. 878, 173114 (2020).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Kwak, B. R. et al. Biomechanical factors in atherosclerosis: mechanisms and clinical implications. Eur. Heart J. 35, 3013–3020 (2014).
Tarbell, J. M., Shi, Z. D., Dunn, J. & Jo, H. Fluid mechanics, arterial disease, and gene expression. Annu. Rev. Fluid Mech. 46, 591–614 (2014).
Andueza, A. et al. Endothelial reprogramming by disturbed flow revealed by single-cell RNA and chromatin accessibility study. Cell Rep. 33, 108491 (2020).
Chiu, J. J. & Chien, S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91, 327–387 (2011).
Simmons, R. D., Kumar, S. & Jo, H. The role of endothelial mechanosensitive genes in atherosclerosis and omics approaches. Arch. Biochem. Biophys. 591, 111–131 (2016).
Fernandez-Friera, L. et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: the PESA (progression of early subclinical atherosclerosis) study. Circulation 131, 2104–2113 (2015).
Laclaustra, M. et al. Femoral and carotid subclinical atherosclerosis association with risk factors and coronary calcium: the AWHS study. J. Am. Coll. Cardiol. 67, 1263–1274 (2016).
Nam, D. et al. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 297, H1535–H1543 (2009).
Cheng, C. et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation 113, 2744–2753 (2006).
Kumar, S., Kang, D. W., Rezvan, A. & Jo, H. Accelerated atherosclerosis development in C57Bl6 mice by overexpressing AAV-mediated PCSK9 and partial carotid ligation. Lab. Invest. 97, 935–945 (2017).
Kim, C. W. et al. Disturbed flow promotes arterial stiffening through thrombospondin-1. Circulation 136, 1217–1232 (2017).
Kuhlmann, M. T. et al. Implantation of a carotid cuff for triggering shear-stress induced atherosclerosis in mice. J. Vis. Exp. https://doi.org/10.3791/3308 (2012).
Tang, D., Geng, F., Yu, C. & Zhang, R. Recent application of zebrafish models in atherosclerosis research. Front. Cell Dev. Biol. 9, 643697 (2021).
Schlegel, A. Zebrafish models for dyslipidemia and atherosclerosis research. Front. Endocrinol. 7, 159 (2016).
Baek, K. I. et al. Vascular injury in the zebrafish tail modulates blood flow and peak wall shear stress to restore embryonic circular network. Front. Cardiovasc. Med. 9, 841101 (2022).
Hsu, J. J. et al. Contractile and hemodynamic forces coordinate Notch1b-mediated outflow tract valve formation. JCI Insight 4, e124460 (2019).
Lee, J. et al. 4-Dimensional light-modulation of cardiac trabeculation. J. Clin. Investig. 126, 1679–1690 (2016).
Lee, J. et al. Spatial and temporal variations in hemodynamic forces initiate cardiac trabeculation. JCI Insight https://doi.org/10.1172/jci.insight.96672 (2018).
Baek, K. I. et al. Flow-responsive vascular endothelial growth factor receptor-protein kinase C isoform epsilon signaling mediates glycolytic metabolites for vascular repair. Antioxid. Redox Signal. 28, 31–43 (2018).
Dewey, C. F. Jr., Bussolari, S. R., Gimbrone, M. A. Jr. & Davies, P. F. The dynamic response of vascular endothelial cells to fluid shear stress. J. Biomech. Eng. 103, 177–185 (1981).
Lawrence, M. B., McIntire, L. V. & Eskin, S. G. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood 70, 1284–1290 (1987).
Rezvan, A., Ni, C.-W., Alberts-Grill, N. & Jo, H. Animal, in vitro, and ex vivo models of flow-dependent atherosclerosis: role of oxidative stress. Antioxid. Redox Signal. 15, 1433–1448 (2011).
Colgan, O. C. et al. Regulation of bovine brain microvascular endothelial tight junction assembly and barrier function by laminar shear stress. Am. J. Physiol. Heart Circ. Physiol. 292, H3190–H3197 (2007).
Orsenigo, F. et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat. Commun. 3, 1208 (2012).
Caolo, V. et al. Shear stress and VE-cadherin. Arterioscler. Thromb. Vasc. Biol. 38, 2174–2183 (2018).
Levesque, M. J., Nerem, R. M. & Sprague, E. A. Vascular endothelial cell proliferation in culture and the influence of flow. Biomaterials 11, 702–707 (1990).
Lin, K. et al. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc. Natl Acad. Sci. USA 97, 9385–9389 (2000).
Dimmeler, S., Haendeler, J., Rippmann, V., Nehls, M. & Zeiher, A. M. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 399, 71–74 (1996).
Dimmeler, S., Assmus, B., Hermann, C., Haendeler, J. & Zeiher, A. M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ. Res. 83, 334–341 (1998).
Liu, J. et al. Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis. 6, e1827 (2015).
Warboys, C. M. et al. Disturbed flow promotes endothelial senescence via a p53-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 34, 985–995 (2014).
Doddaballapur, A. et al. Laminar shear stress inhibits endothelial cell metabolism via KLF2-mediated repression of PFKFB3. Arterioscler. Thromb. Vasc. Biol. 35, 137–145 (2015).
Yamamoto, K., Imamura, H. & Ando, J. Shear stress augments mitochondrial ATP generation that triggers ATP release and Ca2+ signaling in vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 315, H1477–H1485 (2018).
Go, Y. M. et al. Disturbed flow induces systemic changes in metabolites in mouse plasma: a metabolomics study using ApoE−/− mice with partial carotid ligation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308, R62–R72 (2015).
Hong, S. G. et al. Flow pattern-dependent mitochondrial dynamics regulates the metabolic profile and inflammatory state of endothelial cells. JCI Insight https://doi.org/10.1172/jci.insight.159286 (2022).
Heo, K.-S., Fujiwara, K. & Abe, J.-I. Disturbed-flow-mediated vascular reactive oxygen species induce endothelial dysfunction. Circ. J. 75, 2722–2730 (2011).
Hwang, J. et al. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ. Res. 93, 1225–1232 (2003).
Hwang, J. et al. Oscillatory shear stress stimulates endothelial production of O2− from p47phox-dependent NAD(P)H oxidases, leading to monocyte adhesion. J. Biol. Chem. 278, 47291–47298 (2003).
Jo, H., Song, H. & Mowbray, A. Role of NADPH oxidases in disturbed flow- and BMP4- induced inflammation and atherosclerosis. Antioxid. Redox Signal. 8, 1609–1619 (2006).
Zhang, J. X. et al. Low shear stress induces vascular eNOS uncoupling via autophagy-mediated eNOS phosphorylation. Biochim. Biophys. Acta Mol. Cell. Res. 1865, 709–720 (2018).
Chachisvilis, M., Zhang, Y. L. & Frangos, J. A. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc. Natl Acad. Sci. USA 103, 15463–15468 (2006).
Li, L. et al. GTP cyclohydrolase I phosphorylation and interaction with GTP cyclohydrolase feedback regulatory protein provide novel regulation of endothelial tetrahydrobiopterin and nitric oxide. Circ. Res. 106, 328–336 (2010).
McNally, J. S. et al. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Heart Circ. Physiol. 285, H2290–H2297 (2003).
Meyer, J. W. & Schmitt, M. E. A central role for the endothelial NADPH oxidase in atherosclerosis. FEBS Lett. 472, 1–4 (2000).
Sorescu, G. P. et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ. Res. 95, 773–779 (2004).
Nagel, T., Resnick, N., Dewey, C. F. Jr. & Gimbrone, M. A. Jr. Vascular endothelial cells respond to spatial gradients in fluid shear stress by enhanced activation of transcription factors. Arterioscler. Thromb. Vasc. Biol. 19, 1825–1834 (1999).
Sterpetti, A. V. et al. Shear stress increases the release of interleukin-1 and interleukin-6 by aortic endothelial cells. Surgery 114, 911–914 (1993).
Souilhol, C., Harmsen, M. C., Evans, P. C. & Krenning, G. Endothelial–mesenchymal transition in atherosclerosis. Cardiovasc. Res. 114, 565–577 (2018).
Tardy, Y., Resnick, N., Nagel, T., Gimbrone, M. A. Jr. & Dewey, C. F. Jr. Shear stress gradients remodel endothelial monolayers in vitro via a cell proliferation-migration-loss cycle. Arterioscler. Thromb. Vasc. Biol. 17, 3102–3106 (1997).
Tressel, S. L. et al. Angiopoietin-2 stimulates blood flow recovery after femoral artery occlusion by inducing inflammation and arteriogenesis. Arterioscler. Thromb. Vasc. Biol. 28, 1989–1995 (2008).
Tressel, S. L., Huang, R. P., Tomsen, N. & Jo, H. Laminar shear inhibits tubule formation and migration of endothelial cells by an angiopoietin-2 dependent mechanism. Arterioscler. Thromb. Vasc. Biol. 27, 2150–2156 (2007).
Nerem, R. M., Levesque, M. J. & Cornhill, J. F. Vascular endothelial morphology as an indicator of the pattern of blood flow. J. Biomech. Eng. 103, 172–176 (1981).
Flaherty, J. T. et al. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ. Res. 30, 23–33 (1972).
Langille, B. L. & Adamson, S. L. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ. Res. 48, 481–488 (1981).
Franke, R. P. et al. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature 307, 648–649 (1984).
Kim, D. W., Langille, B. L., Wong, M. K. & Gotlieb, A. I. Patterns of endothelial microfilament distribution in the rabbit aorta in situ. Circ. Res. 64, 21–31 (1989).
Tzima, E. et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005).
Steward, R. Jr., Tambe, D., Hardin, C. C., Krishnan, R. & Fredberg, J. J. Fluid shear, intercellular stress, and endothelial cell alignment. Am. J. Physiol. Cell Physiol. 308, C657–C664 (2015).
Coan, D. E., Wechezak, A. R., Viggers, R. F. & Sauvage, L. R. Effect of shear stress upon localization of the Golgi apparatus and microtubule organizing center in isolated cultured endothelial cells. J. Cell Sci. 104, 1145–1153 (1993).
Kwon, H. B. et al. In vivo modulation of endothelial polarization by Apelin receptor signalling. Nat. Commun. 7, 11805 (2016).
Demos, C., Tamargo, I. & Jo, H. Biomechanical regulation of endothelial function in atherosclerosis. Biomech. Coron. Atheroscler. Plaque 4, 3–47 (2021).
Albarrán-Juárez, J. et al. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 215, 2655–2672 (2018).
Bartosch, A. M. W., Mathews, R. & Tarbell, J. M. Endothelial glycocalyx-mediated nitric oxide production in response to selective AFM pulling. Biophys. J. 113, 101–108 (2017).
Caolo, V. et al. Shear stress activates ADAM10 sheddase to regulate Notch1 via the Piezo1 force sensor in endothelial cells. eLife https://doi.org/10.7554/eLife.50684 (2020).
Chuntharpursat-Bon, E. et al. PIEZO1 and PECAM1 interact at cell–cell junctions and partner in endothelial force sensing. Commun. Biol. 6, 358 (2023).
Dela Paz, N. G. & Frangos, J. A. Rapid flow-induced activation of Gαq/11 is independent of Piezo1 activation. Am. J. Physiol. Cell Physiol. 316, C741–c752 (2019).
Florian, J. A. et al. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 93, e136–e142 (2003).
Li, J. et al. Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282 (2014).
Mack, J. J. et al. NOTCH1 is a mechanosensor in adult arteries. Nat. Commun. 8, 1620 (2017).
Mehta, V. et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature 578, 290–295 (2020).
Nauli, S. M. et al. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117, 1161–1171 (2008).
Shin, H. et al. Fine control of endothelial VEGFR-2 activation: caveolae as fluid shear stress shelters for membrane receptors. Biomech. Model Mechanobiol. 18, 5–16 (2019).
Wang, S. et al. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Invest. 126, 4527–4536 (2016).
Yamamoto, K., Korenaga, R., Kamiya, A. & Ando, J. Fluid shear stress activates Ca2+ influx into human endothelial cells via P2X4 purinoceptors. Circ. Res. 87, 385–391 (2000).
Zheng, Q. et al. Mechanosensitive channel PIEZO1 senses shear force to induce KLF2/4 expression via CaMKII/MEKK3/ERK5 axis in endothelial cells. Cells https://doi.org/10.3390/cells11142191 (2022).
Ridone, P., Vassalli, M. & Martinac, B. Piezo1 mechanosensitive channels: what are they and why are they important. Biophys. Rev. 11, 795–805 (2019).
Ranade, S. S. et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl Acad. Sci. USA 111, 10347–10352 (2014).
Xu, J. et al. GPR68 senses flow and is essential for vascular physiology. Cell 173, 762–775.e16 (2018).
Tzima, E., del Pozo, M. A., Shattil, S. J., Chien, S. & Schwartz, M. A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 20, 4639–4647 (2001).
Zhang, C. et al. Coupling of integrin α5 to annexin A2 by flow drives endothelial activation. Circ. Res. 127, 1074–1090 (2020).
Schwarz, U. S. & Gardel, M. L. United we stand: integrating the actin cytoskeleton and cell–matrix adhesions in cellular mechanotransduction. J. Cell Sci. 125, 3051–3060 (2012).
Mohan, S., Mohan, N. & Sprague, E. A. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am. J. Physiol. 273, C572–C578 (1997).
Murthy, S. E., Dubin, A. E. & Patapoutian, A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18, 771–783 (2017).
Wang, S. et al. P2Y2 and Gq/G11 control blood pressure by mediating endothelial mechanotransduction. J. Clin. Invest. 125, 3077–3086 (2015).
Iring, A. et al. Shear stress-induced endothelial adrenomedullin signaling regulates vascular tone and blood pressure. J. Clin. Invest. 129, 2775–2791 (2019).
Harry, B. L. et al. Endothelial cell PECAM-1 promotes atherosclerotic lesions in areas of disturbed flow in ApoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 28, 2003–2008 (2008).
Tzima, E. Role of small GTPases in endothelial cytoskeletal dynamics and the shear stress response. Circ. Res. 98, 176–185 (2006).
Wang, L. et al. Integrin-YAP/TAZ-JNK cascade mediates atheroprotective effect of unidirectional shear flow. Nature 540, 579–582 (2016).
Civelekoglu-Scholey, G. et al. Model of coupled transient changes of Rac, Rho, adhesions and stress fibers alignment in endothelial cells responding to shear stress. J. Theor. Biol. 232, 569–585 (2005).
Tzima, E., Kiosses, W. B., del Pozo, M. A. & Schwartz, M. A. Localized cdc42 activation, detected using a novel assay, mediates microtubule organizing center positioning in endothelial cells in response to fluid shear stress. J. Biol. Chem. 278, 31020–31023 (2003).
Palazzo, A. F. et al. Cdc42, dynein, and dynactin regulate MTOC reorientation independent of Rho-regulated microtubule stabilization. Curr. Biol. 11, 1536–1541 (2001).
Orr, A. W. et al. The subendothelial extracellular matrix modulates NF-κB activation by flow: a potential role in atherosclerosis. J. Cell Biol. 169, 191–202 (2005).
Chen, J. et al. αvβ3 Integrins mediate flow-induced NF-κB activation, proinflammatory gene expression, and early atherogenic inflammation. Am. J. Pathol. 185, 2575–2589 (2015).
Stupack, D. G. & Cheresh, D. A. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci. STKE 2002, pe7 (2002).
Li, B. et al. c-Abl regulates YAPY357 phosphorylation to activate endothelial atherogenic responses to disturbed flow. J. Clin. Invest. 129, 1167–1179 (2019).
Dekker, R. J. et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am. J. Pathol. 167, 609–618 (2005).
Lin, Z. et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ. Res. 96, e48–e57 (2005).
van Thienen, J. V. et al. Shear stress sustains atheroprotective endothelial KLF2 expression more potently than statins through mRNA stabilization. Cardiovasc. Res. 72, 231–240 (2006).
Young, A. et al. Flow activation of AMP-activated protein kinase in vascular endothelium leads to Kruppel-like factor 2 expression. Arterioscler. Thromb. Vasc. Biol. 29, 1902–1908 (2009).
Wu, C. et al. Mechanosensitive PPAP2B regulates endothelial responses to atherorelevant hemodynamic forces. Circ. Res. 117, e41–e53 (2015).
SenBanerjee, S. et al. KLF2 is a novel transcriptional regulator of endothelial proinflammatory activation. J. Exp. Med. 199, 1305–1315 (2004).
Hsieh, C. Y. et al. Regulation of shear-induced nuclear translocation of the Nrf2 transcription factor in endothelial cells. J. Biomed. Sci. 16, 12 (2009).
Fledderus, J. O. et al. KLF2 primes the antioxidant transcription factor Nrf2 for activation in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28, 1339–1346 (2008).
Berk, B. C. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation 117, 1082–1089 (2008).
Abe, J. & Berk, B. C. Novel mechanisms of endothelial mechanotransduction. Arterioscler. Thromb. Vasc. Biol. 34, 2378–2386 (2014).
Kasler, H. G., Victoria, J., Duramad, O. & Winoto, A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol. Cell Biol. 20, 8382–8389 (2000).
Sohn, S. J., Li, D., Lee, L. K. & Winoto, A. Transcriptional regulation of tissue-specific genes by the ERK5 mitogen-activated protein kinase. Mol. Cell Biol. 25, 8553–8566 (2005).
Woo, C. H. et al. ERK5 activation inhibits inflammatory responses via peroxisome proliferator-activated receptor δ (PPARδ) stimulation. J. Biol. Chem. 281, 32164–32174 (2006).
Akaike, M. et al. The hinge–helix 1 region of peroxisome proliferator-activated receptor γ1 (PPARγ1) mediates interaction with extracellular signal-regulated kinase 5 and PPARγ1 transcriptional activation: involvement in flow-induced PPARγ activation in endothelial cells. Mol. Cell Biol. 24, 8691–8704 (2004).
Woo, C. H. et al. Extracellular signal-regulated kinase 5 SUMOylation antagonizes shear stress-induced antiinflammatory response and endothelial nitric oxide synthase expression in endothelial cells. Circ. Res. 102, 538–545 (2008).
Parmar, K. M. et al. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 280, 26714–26719 (2005).
Nakajima, H. & Mochizuki, N. Flow pattern-dependent endothelial cell responses through transcriptional regulation. Cell Cycle 16, 1893–1901 (2017).
Kempe, S., Kestler, H., Lasar, A. & Wirth, T. NF-κB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 33, 5308–5319 (2005).
van Uden, P., Kenneth, N. S. & Rocha, S. Regulation of hypoxia-inducible factor-1α by NF-κB. Biochem. J. 412, 477–484 (2008).
Feng, S. et al. Mechanical activation of hypoxia-inducible factor 1α drives endothelial dysfunction at atheroprone sites. Arterioscler. Thromb. Vasc. Biol. 37, 2087–2101 (2017).
Fernandez Esmerats, J. et al. Disturbed flow increases UBE2C (ubiquitin E2 ligase C) via loss of miR-483-3p, inducing aortic valve calcification by the pVHL (von Hippel-Lindau protein) and HIF-1α (hypoxia-inducible factor-1α) pathway in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 39, 467–481 (2019).
Villa-Roel, N. et al. Hypoxia inducible factor 1α inhibitor PX-478 reduces atherosclerosis in mice. Atherosclerosis 344, 20–30 (2022).
Wu, D. et al. HIF-1alpha is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. eLife https://doi.org/10.7554/eLife.25217 (2017).
Wang, K.-C. et al. Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis. Proc. Natl Acad. Sci. USA 113, 11525–11530 (2016).
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP/TAZ at the roots of cancer. Cancer cell 29, 783–803 (2016).
Piccolo, S., Dupont, S. & Cordenonsi, M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 (2014).
Demos, C. et al. Sox13 is a novel flow-sensitive transcription factor that prevents inflammation by repressing chemokine expression in endothelial cells. Front. Cardiovasc. Med. 9, 979745 (2022).
Ni, C.-W. et al. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood 116, e66–e73 (2010).
Dunn, J. et al. Flow-dependent epigenetic DNA methylation regulates endothelial gene expression and atherosclerosis. J. Clin. Invest. 124, 3187–3199 (2014).
Chen, X. et al. Plasma metabolomics reveals biomarkers of the atherosclerosis. J. Sep. Sci. 33, 2776–2783 (2010).
Goveia, J., Stapor, P. & Carmeliet, P. Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol. Med. 6, 1105–1120 (2014).
Wu, J. et al. Proteomic identification of endothelial proteins isolated in situ from atherosclerotic aorta via systemic perfusion. J. Proteome Res. 6, 4728–4736 (2007).
Ajami, N. E. et al. Systems biology analysis of longitudinal functional response of endothelial cells to shear stress. Proc. Natl Acad. Sci. USA 114, 10990–10995 (2017).
Kumar, S., Williams, D., Sur, S., Wang, J.-Y. & Jo, H. Role of flow-sensitive microRNAs and long noncoding RNAs in vascular dysfunction and atherosclerosis. Vasc. Pharmacol. 114, 76–92 (2019).
Simmons, R. D., Kumar, S., Thabet, S. R., Sur, S. & Jo, H. Omics‐based approaches to understand mechanosensitive endothelial biology and atherosclerosis. Wiley Interdiscip. Rev. Syst. Biol. Med. 8, 378–401 (2016).
Williams, D. et al. Stable flow-induced expression of KLK10 inhibits endothelial inflammation and atherosclerosis. eLife https://doi.org/10.7554/eLife.72579 (2022).
Liu, R., Qu, S., Xu, Y., Jo, H. & Dai, Z. Spatial control of robust transgene expression in mouse artery endothelium under ultrasound guidance. Signal Transduct. Target. Ther. 7, 225 (2022).
Jiang, Y. Z. et al. Hemodynamic disturbed flow induces differential DNA methylation of endothelial Kruppel-like factor 4 promoter in vitro and in vivo. Circ. Res. 115, 32–43 (2014).
Zhou, J., Li, Y.-S., Wang, K.-C. & Chien, S. Epigenetic mechanism in regulation of endothelial function by disturbed flow: induction of DNA hypermethylation by DNMT1. Cell. Mol. Bioeng. 7, 218–224 (2014).
Firasat, S., Hecker, M., Binder, L. & Asif, A. R. Advances in endothelial shear stress proteomics. Expert. Rev. Proteom. 11, 611–619 (2014).
Burghoff, S. & Schrader, J. R. Secretome of human endothelial cells under shear stress. J. Proteome Res. 10, 1160–1169 (2011).
Bibli, S. I. et al. Mapping the endothelial cell S-sulfhydrome highlights the crucial role of integrin sulfhydration in vascular function. Circulation 143, 935–948 (2021).
Son, D. J. et al. The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis. Nat. Commun. 4, 3000 (2013).
Weiler, P., Van den Berge, K., Street, K. & Tiberi, S. A guide to trajectory inference and RNA velocity. Methods Mol. Biol. 2584, 269–292 (2023).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902.e21 (2019).
Chen, P. Y. et al. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Invest. 125, 4514–4528 (2015).
Frid, M. G., Kale, V. A. & Stenmark, K. R. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ. Res. 90, 1189–1196 (2002).
Cooley, B. C. et al. TGF-β signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci. Transl. Med. 6, 227ra234 (2014).
Kouzbari, K. et al. Oscillatory shear potentiates latent TGF-β1 activation more than steady shear as demonstrated by a novel force generator. Sci. Rep. 9, 6065 (2019).
Egorova, A. D. et al. Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ. Res. 108, 1093–1101 (2011).
Zhang, Y., Qiu, J., Wang, X., Zhang, Y. & Xia, M. AMP-activated protein kinase suppresses endothelial cell inflammation through phosphorylation of transcriptional coactivator p300. Arterioscler. Thromb. Vasc. Biol. 31, 2897–2908 (2011).
Cheng, C. K. et al. Activation of AMPK/miR-181b axis alleviates endothelial dysfunction and vascular inflammation in diabetic mice. Antioxidants https://doi.org/10.3390/antiox11061137 (2022).
Fisslthaler, B., Fleming, I., Keseru, B., Walsh, K. & Busse, R. Fluid shear stress and NO decrease the activity of the hydroxy-methylglutaryl coenzyme A reductase in endothelial cells via the AMP-activated protein kinase and FoxO1. Circ. Res. 100, e12–e21 (2007).
Evrard, S. et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 7, 11853 (2016).
Shao, Y. et al. Endothelial immunity trained by coronavirus infections, DAMP stimulations and regulated by anti-oxidant NRF2 may contribute to inflammations, myelopoiesis, COVID-19 cytokine storms and thromboembolism. Front. Immunol. 12, 653110 (2021).
Plein, A., Fantin, A., Denti, L., Pollard, J. W. & Ruhrberg, C. Erythro-myeloid progenitors contribute endothelial cells to blood vessels. Nature 562, 223–228 (2018).
Kumar, S. et al. Atorvastatin and blood flow regulate expression of distinctive sets of genes in mouse carotid artery endothelium. Curr. Top. Membr. 87, 97–130 (2021).
Sangwung, P. et al. KLF2 and KLF4 control endothelial identity and vascular integrity. JCI Insight 2, e91700 (2017).
Lee, G. H. et al. Betulinic acid induces eNOS expression via the AMPK-dependent KLF2 signaling pathway. J. Agric. Food Chem. 68, 14523–14530 (2020).
Davies, J. E. et al. Using Yoda-1 to mimic laminar flow in vitro: a tool to simplify drug testing. Biochem. Pharmacol. 168, 473–480 (2019).
Syeda, R. et al. Chemical activation of the mechanotransduction channel Piezo1. eLife https://doi.org/10.7554/eLife.07369 (2015).
Wang, Y. et al. A lever-like transduction pathway for long-distance chemical- and mechano-gating of the mechanosensitive Piezo1 channel. Nat. Commun. 9, 1300 (2018).
Wu, W. et al. Flow-dependent regulation of Krüppel-like factor 2 is mediated by microRNA-92a. Circulation 124, 633–641 (2011).
Kumar, S., Kim, C. W., Simmons, R. D. & Jo, H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler. Thromb. Vasc. Biol. 34, 2206–2216 (2014).
Meng, X., Yin, J., Yu, X. & Guo, Y. MicroRNA-205-5p promotes unstable atherosclerotic plaque formation in vivo. Cardiovasc. Drugs Ther. 34, 25–39 (2020).
Topper, J. N., Cai, J. X., Falb, D. & Gimbrone, M. A. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc. Natl Acad. Sci. USA 93, 10417–10422 (1996).
Dekker, R. J. et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100, 1689–1698 (2002).
Huddleson, J. P., Srinivasan, S., Ahmad, N. & Lingrel, J. B. Fluid shear stress induces endothelial KLF2 gene expression through a defined promoter region. Biol. Chem. 385, 723–729 (2004).
Hamik, A. et al. Kruppel-like factor 4 regulates endothelial inflammation. J. Biol. Chem. 282, 13769–13779 (2007).
Topper, J. N. & Gimbrone, M. A. Jr. Blood flow and vascular gene expression: fluid shear stress as a modulator of endothelial phenotype. Mol. Med. Today 5, 40–46 (1999).
Stöhr, R. et al. Loss of TIMP3 exacerbates atherosclerosis in ApoE null mice. Atherosclerosis 235, 438–443 (2014).
Son, D. J. et al. Interleukin-32α inhibits endothelial inflammation, vascular smooth muscle cell activation, and atherosclerosis by upregulating Timp3 and Reck through suppressing microRNA-205 biogenesis. Theranostics 7, 2186–2203 (2017).
Wang, Y. et al. ZBTB46 is a shear-sensitive transcription factor inhibiting endothelial cell proliferation via gene expression regulation of cell cycle proteins. Lab. Investig. https://doi.org/10.1038/s41374-018-0060-5 (2018).
Kim, C. W. et al. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler. Thromb. Vasc. Biol. https://doi.org/10.1161/ATVBAHA.112.300287 (2013).
Hosoya, T. et al. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J. Biol. Chem. 280, 27244–27250 (2005).
Shyy, Y. J., Hsieh, H. J., Usami, S. & Chien, S. Fluid shear stress induces a biphasic response of human monocyte chemotactic protein 1 gene expression in vascular endothelium. Proc. Natl Acad. Sci. USA 91, 4678–4682 (1994).
Miriyala, S. et al. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation 113, 2818–2825 (2006).
Vendrov, A. E., Madamanchi, N. R., Hakim, Z. S., Rojas, M. & Runge, M. S. Thrombin and NAD(P)H oxidase-mediated regulation of CD44 and BMP4-Id pathway in VSMC, restenosis, and atherosclerosis. Circ. Res. 98, 1254–1263 (2006).
Sorescu, G. P. et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J. Biol. Chem. 278, 31128–31135 (2003).
Son, J. W. et al. Serum BMP-4 levels in relation to arterial stiffness and carotid atherosclerosis in patients with type 2 diabetes. Biomark. Med. 5, 827–835 (2011).
Koga, M. et al. BMP4 is increased in the aortas of diabetic ApoE knockout mice and enhances uptake of oxidized low density lipoprotein into peritoneal macrophages. J. Inflamm. 10, 32 (2013).
Jank, M. et al. Platelet bone morphogenetic protein-4 mediates vascular inflammation and neointima formation after arterial injury. Cells https://doi.org/10.3390/cells10082027 (2021).
Csiszar, A., Labinskyy, N., Jo, H., Ballabh, P. & Ungvari, Z. Differential proinflammatory and prooxidant effects of bone morphogenetic protein-4 in coronary and pulmonary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 295, H569–H577 (2008).
Chang, K. et al. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 116, 1258–1266 (2007).
Nagel, T., Resnick, N., Atkinson, W. J., Dewey, C. F. Jr. & Gimbrone, M. A. Jr. Shear stress selectively upregulates intercellular adhesion molecule-1 expression in cultured human vascular endothelial cells. J. Clin. Investig. 94, 885–891 (1994).
Pamukcu, B., Lip, G. Y. & Shantsila, E. The nuclear factor–kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb. Res. 128, 117–123 (2011).
Barnes, P. J. & Karin, M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066–1071 (1997).
van der Heiden, K. et al. Role of nuclear factor κB in cardiovascular health and disease. Clin. Sci. 118, 593–605 (2010).
Hajra, L. et al. The NF-κB signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc. Natl Acad. Sci. USA 97, 9052–9057 (2000).
De Keulenaer, G. W. et al. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ. Res. 82, 1094–1101 (1998).
Goettsch, C. et al. Arterial flow reduces oxidative stress via an antioxidant response element and Oct-1 binding site within the NADPH oxidase 4 promoter in endothelial cells. Basic Res. Cardiol. 106, 551–561 (2011).
Gray, S. P. et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127, 1888–1902 (2013).
Jeon, H. & Boo, Y. C. Laminar shear stress enhances endothelial cell survival through a NADPH oxidase 2-dependent mechanism. Biochem. Biophys. Res. Commun. 430, 460–465 (2013).
Craige, S. M. et al. Endothelial NADPH oxidase 4 protects ApoE-/- mice from atherosclerotic lesions. Free Radic. Biol. Med. 89, 1–7 (2015).
Kim, J., Seo, M., Kim, S. K. & Bae, Y. S. Flagellin-induced NADPH oxidase 4 activation is involved in atherosclerosis. Sci. Rep. 6, 25437 (2016).
Langbein, H. et al. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 37, 1753–1761 (2016).
Galis, Z. S., Sukhova, G. K., Lark, M. W. & Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 94, 2493–2503 (1994).
Magid, R., Murphy, T. J. & Galis, Z. S. Expression of matrix metalloproteinase-9 in endothelial cells is differentially regulated by shear stress. Role of c-Myc. J. Biol. Chem. 278, 32994–32999 (2003).
Sho, E. et al. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp. Mol. Pathol. 73, 142–153 (2002).
Yun, S. et al. Transcription factor Sp1 phosphorylation induced by shear stress inhibits membrane type 1-matrix metalloproteinase expression in endothelium. J. Biol. Chem. 277, 34808–34814 (2002).
Volger, O. L. et al. Distinctive expression of chemokines and transforming growth factor-β signaling in human arterial endothelium during atherosclerosis. Am. J. Pathol. 171, 326–337 (2007).
Akimoto, S., Mitsumata, M., Sasaguri, T. & Yoshida, Y. Laminar shear stress inhibits vascular endothelial cell proliferation by inducing cyclin-dependent kinase inhibitor p21Sdi1/Cip1/Waf1. Circ. Res. 86, 185–190 (2000).
Kadohama, T., Nishimura, K., Hoshino, Y., Sasajima, T. & Sumpio, B. E. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J. Cell Physiol. 212, 244–251 (2007).
Bao, X., Lu, C. & Frangos, J. A. Mechanism of temporal gradients in shear-induced ERK1/2 activation and proliferation in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 281, H22–H29 (2001).
Jo, H. et al. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. Gi2- and Gβ/γ-dependent signaling pathways. J. Biol. Chem. 272, 1395–1401 (1997).
Moura, R. et al. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in ApoE−/− mice. Circ. Res. 103, 1181–1189 (2008).
Hu, S. et al. Vascular semaphorin 7A upregulation by disturbed flow promotes atherosclerosis through endothelial β1 integrin. Arterioscler. Thromb. Vasc. Biol. 38, 335–343 (2018).
Henn, D. et al. MicroRNA-regulated pathways of flow-stimulated angiogenesis and vascular remodeling in vivo. J. Transl. Med. 17, 22 (2019).
Green, J. P. et al. Atheroprone flow activates inflammation via endothelial ATP-dependent P2X7-p38 signalling. Cardiovasc. Res. 114, 324–335 (2018).
Glaser, S. F. et al. The histone demethylase JMJD2B regulates endothelial-to-mesenchymal transition. Proc. Natl Acad. Sci. USA 117, 4180–4187 (2020).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Björck, H. M. et al. Characterization of shear-sensitive genes in the normal rat aorta identifies Hand2 as a major flow-responsive transcription factor. PLoS ONE 7, e52227 (2012).
Yeh, C.-F. et al. Targeting mechanosensitive endothelial TXNDC5 to stabilize eNOS and reduce atherosclerosis in vivo. Sci. Adv. 8, eabl8096 (2022).
Fang, Y., Shi, C., Manduchi, E., Civelek, M. & Davies, P. F. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc. Natl Acad. Sci. USA 107, 13450–13455 (2010).
Qin, X. et al. MicroRNA-19a mediates the suppressive effect of laminar flow on cyclin D1 expression in human umbilical vein endothelial cells. Proc. Natl Acad. Sci. USA 107, 3240–3244 (2010).
Chen, H., Li, X., Liu, S., Gu, L. & Zhou, X. MircroRNA-19a promotes vascular inflammation and foam cell formation by targeting HBP-1 in atherogenesis. Sci. Rep. 7, 12089 (2017).
Wang, K. C. et al. Role of microRNA-23b in flow-regulation of Rb phosphorylation and endothelial cell growth. Proc. Natl Acad. Sci. USA 107, 3234–3239 (2010).
Iaconetti, C. et al. Down-regulation of miR-23b induces phenotypic switching of vascular smooth muscle cells in vitro and in vivo. Cardiovasc. Res. 107, 522–533 (2015).
Melo, S. A. & Kalluri, R. Angiogenesis is controlled by miR-27b associated with endothelial tip cells. Blood 119, 2439–2440 (2012).
Demolli, S. et al. Shear stress-regulated miR-27b controls pericyte recruitment by repressing SEMA6A and SEMA6D. Cardiovasc. Res. 113, 681–691 (2017).
Suzuki, H. I. et al. Regulation of TGF-β-mediated endothelial-mesenchymal transition by microRNA-27. J. Biochem. 161, 417–420 (2017).
Zeng, X., Huang, C., Senavirathna, L., Wang, P. & Liu, L. miR-27b inhibits fibroblast activation via targeting TGFβ signaling pathway. BMC Cell Biol. 18, 9 (2017).
Chen, K. et al. MicroRNA-101 mediates the suppressive effect of laminar shear stress on mTOR expression in vascular endothelial cells. Biochem. Biophys. Res. Commun. 427, 138–142 (2012).
Kim, J. H. et al. Hypoxia-responsive microRNA-101 promotes angiogenesis via heme oxygenase-1/vascular endothelial growth factor axis by targeting cullin 3. Antioxid. Redox Signal. 21, 2469–2482 (2014).
Zhang, N. et al. MicroRNA-101 overexpression by IL-6 and TNF-α inhibits cholesterol efflux by suppressing ATP-binding cassette transporter A1 expression. Exp. Cell Res. 336, 33–42 (2015).
Cordes, K. R. et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710 (2009).
Kohlstedt, K. et al. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ. Res. 112, 1150–1158 (2013).
Sala, F. et al. MiR-143/145 deficiency attenuates the progression of atherosclerosis in Ldlr−/− mice. Thromb. Haemost. 112, 796–802 (2014).
Climent, M. et al. TGFβ triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ. Res. 116, 1753–1764 (2015).
Zernecke, A. et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2, ra81 (2009).
Zhou, J. et al. Regulation of vascular smooth muscle cell turnover by endothelial cell-secreted microRNA-126: role of shear stress. Circ. Res. 113, 40–51 (2013).
Schober, A. et al. MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat. Med. 20, 368–376 (2014).
Wang, Y. et al. MicroRNA-126 attenuates palmitate-induced apoptosis by targeting TRAF7 in HUVECs. Mol. Cell. Biochem. 399, 123–130 (2015).
Tang, S. T., Wang, F., Shao, M., Wang, Y. & Zhu, H. Q. MicroRNA-126 suppresses inflammation in endothelial cells under hyperglycemic condition by targeting HMGB1. Vasc. Pharmacol. 88, 48–55 (2017).
Cerutti, C. et al. MiR-126 and miR-126* regulate shear-resistant firm leukocyte adhesion to human brain endothelium. Sci. Rep. 7, 45284 (2017).
Tang, F. & Yang, T. L. MicroRNA-126 alleviates endothelial cells injury in atherosclerosis by restoring autophagic flux via inhibiting of PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 495, 1482–1489 (2018).
Bonauer, A. et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 324, 1710–1713 (2009).
Fang, Y. & Davies, P. F. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler. Thromb. Vasc. Biol. 32, 979–987 (2012).
Loyer, X. et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ. Res. 114, 434–443 (2014).
Ni, C. W., Qiu, H. & Jo, H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 300, H1762–H1769 (2011).
Afonyushkin, T., Oskolkova, O. V. & Bochkov, V. N. Permissive role of miR-663 in induction of VEGF and activation of the ATF4 branch of unfolded protein response in endothelial cells by oxidized phospholipids. Atherosclerosis 225, 50–55 (2012).
Kheirolomoom, A. et al. Multifunctional nanoparticles facilitate molecular targeting and miRNA delivery to inhibit atherosclerosis in ApoE−/− mice. ACS Nano 9, 8885–8897 (2015).
Cheng, Y. & Zhang, C. MicroRNA-21 in cardiovascular disease. J. Cardiovasc. Transl. Res. 3, 251–255 (2010).
Weber, M., Baker, M. B., Moore, J. P. & Searles, C. D. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem. Biophys. Res. Commun. 393, 643–648 (2010).
Buscaglia, L. E. B. & Li, Y. Apoptosis and the target genes of microRNA-21. Chin. J. Cancer 30, 371–380 (2011).
Zhou, J. et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-α in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc. Natl Acad. Sci. USA 108, 10355–10360 (2011).
McDonald, R. A. et al. miRNA-21 is dysregulated in response to vein grafting in multiple models and genetic ablation in mice attenuates neointima formation. Eur. Heart J. 34, 1636–1643 (2013).
Li, S. et al. MicroRNA-21 negatively regulates Treg cells through a TGF-β1/Smad-independent pathway in patients with coronary heart disease. Cell. Physiol. Biochem. 37, 866–878 (2015).
Sun, H. X. et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension 60, 1407–1414 (2012).
Weber, M., Kim, S., Patterson, N., Rooney, K. & Searles, C. D. MiRNA-155 targets myosin light chain kinase and modulates actin cytoskeleton organization in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 306, H1192–H1203 (2014).
He, S., Yang, L., Li, D. & Li, M. Kruppel-like factor 2-mediated suppression of microRNA-155 reduces the proinflammatory activation of macrophages. PLoS ONE 10, e0139060 (2015).
Zhang, H. et al. Genistein protects against ox-LDL-induced inflammation through microRNA-155/SOCS1-mediated repression of NF-κB signaling pathway in HUVECs. Inflammation 40, 1450–1459 (2017).
Nazari-Jahantigh, M. et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J. Clin. Invest. 122, 4190–4202 (2012).
Michalik, K. M. et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 114, 1389–1397 (2014).
Wang, C., Qu, Y., Suo, R. & Zhu, Y. Long non-coding RNA MALAT1 regulates angiogenesis following oxygen-glucose deprivation/reoxygenation. J. Cell. Mol. Med. https://doi.org/10.1111/jcmm.14204 (2019).
Leisegang, M. S. et al. Long noncoding RNA MANTIS facilitates endothelial angiogenic function. Circulation 136, 65–79 (2017).
Huang, T. S. et al. LINC00341 exerts an anti-inflammatory effect on endothelial cells by repressing VCAM1. Physiol. Genomics 49, 339–345 (2017).
Josipovic, I. et al. Long noncoding RNA LISPR1 is required for S1P signaling and endothelial cell function. J. Mol. Cell. Cardiol. 116, 57–68 (2018).
Man, H. S. J. et al. Angiogenic patterning by STEEL, an endothelial-enriched long noncoding RNA. Proc. Natl Acad. Sci. USA 115, 2401–2406 (2018).
Acknowledgements
I.A.T. is supported by NIH grant 5F31HL149285-03. K.I.B. is supported by NIH grants 5T32HL007745 and F32HL167625. H.J. is supported by NIH grants HL119798, HL139757 and HL151358. H.J. is also supported by the Wallace H. Coulter Distinguished Faculty Professorship.
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
H.J. is the founder of Flokines Pharma. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks Shu Chien and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamargo, I.A., Baek, K.I., Kim, Y. et al. Flow-induced reprogramming of endothelial cells in atherosclerosis. Nat Rev Cardiol 20, 738–753 (2023). https://doi.org/10.1038/s41569-023-00883-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-023-00883-1
This article is cited by
-
Targeting mechanosensitive cannabinoid receptor 1 with isoflavone prodrugs attenuates atherosclerotic endothelial dysfunction
Journal of Biomedical Science (2026)
-
Mechanomedicine
Nature Reviews Bioengineering (2026)
-
Mapping the distribution of radial artery atherosclerosis by optical coherence tomography
BMC Medical Imaging (2025)
-
A dual-targeting bio-liposomes nanodrug repair endothelial cell dysfunction and restore macrophage cholesterol flow homeostasis to treat early atherosclerosis
Journal of Nanobiotechnology (2025)
-
Endothelial IGFBP6 suppresses vascular inflammation and atherosclerosis
Nature Cardiovascular Research (2025)