Abstract
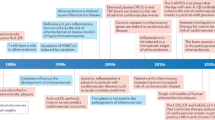
The presence of cholesterol crystals (CCs) in tissues was first described more than 100 years ago. CCs have a pathogenic role in various cardiovascular diseases, including myocardial infarction, aortic aneurysm and, most prominently, atherosclerosis. Although the underlying mechanisms and signalling pathways involved in CC formation are incompletely understood, numerous studies have highlighted the existence of CCs at various stages of atheroma progression. In this Review, we summarize the mechanisms underlying CC formation and the role of CCs in cardiovascular disease. In particular, we explore the established links between lipid metabolism across various cell types and the formation of CCs, with a focus on CC occurrence in the vasculature. We also discuss CC-induced inflammation as one of the pathogenic features of CCs in the atheroma. Finally, we summarize the therapeutic strategies aimed at reducing CC-mediated atherosclerotic burden, including approaches to inhibit CC formation in the vasculature or to mitigate the inflammatory response triggered by CCs. Addressing CC formation might emerge as a crucial component in our broader efforts to combat cardiovascular disease.
Key points
-
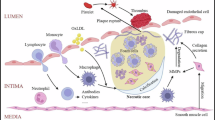
The presence of cholesterol crystals (CCs) in advanced atherosclerotic plaques was documented over a century ago and is associated with the accumulation of cholesterol from lipid-laden macrophages that die in the core of the atheroma.
-
CCs are produced by endothelial cells after only a short exposure to hyperlipidaemic conditions and are secreted to the basolateral side of the endothelium, resulting in the formation of a subendothelial space.
-
Although incompletely understood, the formation of CCs probably involves pathways that regulate lipid uptake, intracellular lysosome-mediated lipid metabolism, reverse cholesterol transport and autophagy.
-
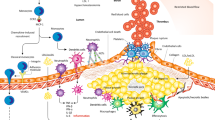
CCs can be taken up by pattern recognition receptors on various cell types, including macrophages, smooth muscle cells and endothelial cells, and cause various inflammatory responses, including NLRP3 inflammasome activation.
-
The in vivo imaging and detection of CCs need to be improved to identify vulnerable atherosclerotic plaques that are associated with an increased risk of future cardiovascular events.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Aschoff, L. Zur Morphologie der lipoiden Substanzen. Beitr. Pathol. Anat. Allg. Pathol. 47, 1–50 (1910).
Levene, C. I. The early lesions of atheroma in the coronary arteries. J. Pathol. Bacteriol. 72, 79–82 (1956).
Luo, Y. et al. Modeling of mechanical stress exerted by cholesterol crystallization on atherosclerotic plaques. PLoS ONE 11, e0155117 (2016).
Kiyak, J. H. Cholesterol crystals, smooth muscle cells and new data on the genesis of atherosclerosis. Pol. J. Pathol. 48, 49–55 (1997).
Varsano, N. et al. Two polymorphic cholesterol monohydrate crystal structures form in macrophage culture models of atherosclerosis. Proc. Natl Acad. Sci. USA 115, 7662–7669 (2018).
Vedre, A. et al. Physical factors that trigger cholesterol crystallization leading to plaque rupture. Atherosclerosis 203, 89–96 (2009).
Katz, S. S., Shipley, G. G. & Small, D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J. Clin. Invest. 58, 200–211 (1976).
Bogren, H. & Larsson, K. An X-ray-diffraction study of crystalline cholesterol in some pathological deposits in man. Biochim. Biophys. Acta 75, 65–69 (1963).
Park, M. H. et al. Non-linear optical imaging of atherosclerotic plaques in the context of SIV and HIV infection prominently detects crystalline cholesterol esters. PLoS ONE 16, e0251599 (2021).
Baumer, Y., McCurdy, S. G. & Boisvert, W. A. Formation and cellular impact of cholesterol crystals in health and disease. Adv. Biol. 5, e2100638 (2021).
Abela, G. S. Cholesterol crystals piercing the arterial plaque and intima trigger local and systemic inflammation. J. Clin. Lipidol. 4, 156–164 (2010).
Baumer, Y. et al. Hyperlipidemia-induced cholesterol crystal production by endothelial cells promotes atherogenesis. Nat. Commun. 8, 1129 (2017).
Venturelli, C., Jeannin, G., Sottini, L., Dallera, N. & Scolari, F. Cholesterol crystal embolism (atheroembolism). Heart Int. 2, 155 (2006).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Ioannou, G. N. et al. Cholesterol crystals in hepatocyte lipid droplets are strongly associated with human nonalcoholic steatohepatitis. Hepatol. Commun. 3, 776–791 (2019).
Koka, S. et al. Endothelial NLRP3 inflammasome activation and arterial neointima formation associated with acid sphingomyelinase during hypercholesterolemia. Redox Biol. 13, 336–344 (2017).
Rajamäki, K. et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS ONE 5, e11765 (2010).
Wang, R. et al. Activation of NLRP3 inflammasome promotes foam cell formation in vascular smooth muscle cells and atherogenesis via HMGB1. J. Am. Heart Assoc. 7, e008596 (2018).
Jones, G. T. in Diagnosis, Screening and Treatment of Abdominal, Thoracoabdominal and Thoracic Aortic Aneurysms Ch. 3 (ed. Grundmann, R. T.) (IntechOpen, 2011).
Abela, G. S. & Aziz, K. Cholesterol crystals rupture biological membranes and human plaques during acute cardiovascular events – a novel insight into plaque rupture by scanning electron microscopy. Scanning 28, 1–10 (2006).
Furukawa, E. et al. The impact of chronic kidney disease on cholesterol crystal embolism in autopsy cases [abstract]. Nephrol. Dial. Transplant. 34 (Suppl. 1), gfz106.FP072 (2019).
Haas, M., Spargo, B. H., Wit, E. J. & Meehan, S. M. Etiologies and outcome of acute renal insufficiency in older adults: a renal biopsy study of 259 cases. Am. J. Kidney Dis. 35, 433–447 (2000).
Li, X., Bayliss, G. & Zhuang, S. Cholesterol crystal embolism and chronic kidney disease. Int. J. Mol. Sci. 18, 1120 (2017).
Shroff, H. & VanWagner, L. B. Cardiovascular disease in nonalcoholic steatohepatitis: screening and management. Curr. Hepatol. Rep. 19, 315–326 (2020).
Hammer, S. S. et al. Cholesterol crystal formation is a unifying pathogenic mechanism in the development of diabetic retinopathy. Diabetologia 66, 1705–1718 (2023).
Walton, K. W. & Dunkerley, D. J. Studies on the pathogenesis of corneal arcus formation II. Immunofluorescent studies on lipid deposition in the eye of the lipid-fed rabbit. J. Pathol. 114, 217–229 (1974).
Silva, G. B. et al. Cholesterol crystals and NLRP3 mediated inflammation in the uterine wall decidua in normal and preeclamptic pregnancies. Front. Immunol. 11, 564712 (2020).
Virchow, R. L. K. Cellular Pathology (John Churchill, 1860).
Marchand, F. Ueber atherosclerosis. Verhandlungen der Kongresse fuer Innere Medizin Vol. 21 (1904).
Ridker, P. M. How common is residual inflammatory risk? Circ. Res. 120, 617–619 (2017).
Aday, A. W. & Ridker, P. M. Targeting residual inflammatory risk: a shifting paradigm for atherosclerotic disease. Front. Cardiovasc. Med. 6, 16 (2019).
Ridker, P. M. et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 377, 1119–1131 (2017).
Nidorf, S. M., Eikelboom, J. W., Budgeon, C. A. & Thompson, P. L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 61, 404–410 (2013).
Tardif, J. C. et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N. Engl. J. Med. 381, 2497–2505 (2019).
Nidorf, S. M. et al. Colchicine in patients with chronic coronary disease. N. Engl. J. Med. 383, 1838–1847 (2020).
Baumer, Y. et al. Hyperlipidaemia and IFNγ/TNFα synergism are associated with cholesterol crystal formation in endothelial cells partly through modulation of lysosomal pH and cholesterol homeostasis. eBioMedicine 59, 102876 (2020).
Baumer, Y. et al. Chronic skin inflammation accelerates macrophage cholesterol crystal formation and atherosclerosis. JCI Insight 3, e97179 (2018).
Ho-Tin-Noé, B. et al. Cholesterol crystallization in human atherosclerosis is triggered in smooth muscle cells during the transition from fatty streak to fibroatheroma. J. Pathol. 241, 671–682 (2017).
Sheedy, F. J. et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 14, 812–820 (2013).
Tangirala, R. K. et al. Formation of cholesterol monohydrate crystals in macrophage-derived foam cells. J. Lipid Res. 35, 93–104 (1994).
Capua-Shenkar, J., Varsano, N., Kruth, H. S. & Addadi, L. in Cholesterol Crystals in Atherosclerosis and Other Related Diseases (eds Abela, G. S. & Nidorf, S. M.) 49–71 (Humana, 2023).
Nidorf, S. M. & Abela, G. S. in Cholesterol Crystals in Atherosclerosis and Other Related Diseases (eds Abela, G. S. & Nidorf, S. M.) 15–26 (Humana, 2023).
Konikoff, F. M., Chung, D. S., Donovan, J. M., Small, D. M. & Carey, M. C. Filamentous, helical, and tubular microstructures during cholesterol crystallization from bile. Evidence that cholesterol does not nucleate classic monohydrate plates. J. Clin. Invest. 90, 1155–1160 (1992).
Khaykovich, B. et al. Structure of cholesterol helical ribbons and self-assembling biological springs. Proc. Natl Acad. Sci. USA 104, 9656–9660 (2007).
Wilkens, J. A. & Krut, L. H. The effect of glucose on the crystallization of cholesterol. J. Atheroscler. Res. 5, 516–523 (1965).
Self-Medlin, Y., Byun, J., Jacob, R. F., Mizuno, Y. & Mason, R. P. Glucose promotes membrane cholesterol crystalline domain formation by lipid peroxidation. Biochim. Biophys. Acta 1788, 1398–1403 (2009).
Park, S., Sut, T. N., Ma, G. J., Parikh, A. N. & Cho, N. J. Crystallization of cholesterol in phospholipid membranes follows Ostwald’s rule of stages. J. Am. Chem. Soc. 142, 21872–21882 (2020).
Varsano, N., Fargion, I., Wolf, S. G., Leiserowitz, L. & Addadi, L. Formation of 3D cholesterol crystals from 2D nucleation sites in lipid bilayer membranes: implications for atherosclerosis. J. Am. Chem. Soc. 137, 1601–1607 (2015).
Tziakas, D. et al. Red blood cell distribution width: a strong prognostic marker in cardiovascular disease: is associated with cholesterol content of erythrocyte membrane. Clin. Hemorheol. Microcirc. 51, 243–254 (2012).
Tziakas, D. N. et al. Total cholesterol content of erythrocyte membranes is increased in patients with acute coronary syndrome: a new marker of clinical instability? J. Am. Coll. Cardiol. 49, 2081–2089 (2007).
Cantuti-Castelvetri, L. et al. Defective cholesterol clearance limits remyelination in the aged central nervous system. Science 359, 684–688 (2018).
Wang, X. et al. Endothelial cell-derived cholesterol crystals promote endothelial inflammation in early atherogenesis. Antioxid. Redox Signal. 41, 201–215 (2024).
Bainton, D. F. The discovery of lysosomes. J. Cell Biol. 91, 66s–76s (1981).
Capua-Shenkar, J. et al. Examining atherosclerotic lesions in three dimensions at the nanometer scale with cryo-FIB-SEM. Proc. Natl Acad. Sci. USA 119, e2205475119 (2022).
Shio, H., Haley, N. J. & Fowler, S. Characterization of lipid-laden aortic cells from cholesterol-fed rabbits. III. Intracellular localization of cholesterol and cholesteryl ester. Lab. Invest. 41, 160–167 (1979).
Thelen, A. M. & Zoncu, R. Emerging roles for the lysosome in lipid metabolism. Trends Cell Biol. 27, 833–850 (2017).
Trinh, M. N. et al. Last step in the path of LDL cholesterol from lysosome to plasma membrane to ER is governed by phosphatidylserine. Proc. Natl Acad. Sci. USA 117, 18521–18529 (2020).
Heybrock, S. et al. Lysosomal integral membrane protein-2 (LIMP-2/SCARB2) is involved in lysosomal cholesterol export. Nat. Commun. 10, 3521 (2019).
Chu, B. B. et al. Cholesterol transport through lysosome-peroxisome membrane contacts. Cell 161, 291–306 (2015).
Höglinger, D. et al. NPC1 regulates ER contacts with endocytic organelles to mediate cholesterol egress. Nat. Commun. 10, 4276 (2019).
Platt, F. M., d’Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Dis. Prim. 4, 27 (2018).
Tylki-Szymańska, A. & Jurecka, A. Lysosomal acid lipase deficiency: wolman disease and cholesteryl ester storage disease. Pril 35, 99–106 (2014).
Wilson, D. P. et al. Lysosomal Acid Lipase Deficiency (MDText.com, 2000).
Baronio, F. et al. Diagnosis, treatment, and follow-up of a case of Wolman disease with hemophagocytic lymphohistiocytosis. Mol. Genet. Metab. Rep. 30, 100833 (2022).
Cox, B. E., Griffin, E. E., Ullery, J. C. & W. Gray, J. Effects of cellular cholesterol loading on macrophage foam cell lysosome acidifications. J. Lipid Res. 48, 1012–1021 (2007).
Jerome, W. G., Cox, B. E., Griffin, E. E. & Ullery, J. C. Lysosomal cholesterol accumulation inhibits subsequent hydrolysis of lipoprotein cholesteryl ester. Microsc. Microanal. 14, 138–149 (2008).
Munkacsi, A. B., Porto, A. F. & Sturley, S. L. Niemann–Pick type C disease proteins: orphan transporters or membrane rheostats? Fut. Lipidol. 2, 357–367 (2007).
Acuña, M. et al. Transgenic overexpression of Niemann–Pick C2 protein promotes cholesterol gallstone formation in mice. J. Hepatol. 64, 361–369 (2016).
van der Lienden, M. J. C. et al. GCase and LIMP2 abnormalities in the liver of Niemann Pick type C mice. Int. J. Mol. Sci. 22, 2532 (2021).
Cabrera-Reyes, F., Parra-Ruiz, C., Yuseff, M. I. & Zanlungo, S. Alterations in lysosome homeostasis in lipid-related disorders: impact on metabolic tissues and immune cells. Front. Cell Dev. Biol. 9, 790568 (2021).
Hasegawa, J., Maejima, I., Iwamoto, R. & Yoshimori, T. Selective autophagy: lysophagy. Methods 75, 128–132 (2015).
Papadopoulos, C., Kravic, B. & Meyer, H. Repair or lysophagy: dealing with damaged lysosomes. J. Mol. Biol. 432, 231–239 (2020).
Jia, J. et al. Galectin-3 coordinates a cellular system for lysosomal repair and removal. Dev. Cell 52, 69–87.e8 (2020).
Thazhathveettil, J., Kumawat, A. K., Demirel, I., Sirsjö, A. & Paramel, G. V. Vascular smooth muscle cells in response to cholesterol crystals modulates inflammatory cytokines release and promotes neutrophil extracellular trap formation. Mol. Med. 30, 42 (2024).
Baumer, Y. et al. Social determinants modulate NK cell activity via obesity, LDL, and DUSP1 signaling. Preprint at bioRxiv https://doi.org/10.1101/2023.09.12.556825 (2023).
Heckmann, B. L., Yang, X., Zhang, X. & Liu, J. The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Br. J. Pharmacol. 168, 163–171 (2013).
Baumer, Y., Mehta, N. N., Dey, A. K., Powell-Wiley, T. M. & Boisvert, W. A. Cholesterol crystals and atherosclerosis. Eur. Heart J. 41, 2236–2239 (2020).
Mullen, L. M., Chamberlain, G. & Sacre, S. Pattern recognition receptors as potential therapeutic targets in inflammatory rheumatic disease. Arthritis Res. Ther. 17, 122 (2015).
Freigang, S. et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur. J. Immunol. 41, 2040–2051 (2011).
van der Heijden, T. et al. NLRP3 inflammasome inhibition by MCC950 reduces atherosclerotic lesion development in apolipoprotein E-deficient mice – brief report. Arterioscler. Thromb. Vasc. Biol. 37, 1457–1461 (2017).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Nițulescu, I. M., Ciulei, G., Cozma, A., Procopciuc, L. M. & Orășan, O. H. From innate immunity to metabolic disorder: a review of the NLRP3 inflammasome in diabetes mellitus. J. Clin. Med. 12, 6022 (2023).
Shi, J., Guo, J., Li, Z., Xu, B. & Miyata, M. Importance of NLRP3 inflammasome in abdominal aortic aneurysms. J. Atheroscler. Thromb. 28, 454–466 (2021).
Toldo, S. & Abbate, A. The NLRP3 inflammasome in acute myocardial infarction. Nat. Rev. Cardiol. 15, 203–214 (2018).
Blevins, H. M., Xu, Y., Biby, S. & Zhang, S. The NLRP3 inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front. Aging Neurosci. 14, 879021 (2022).
Grebe, A., Hoss, F. & Latz, E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ. Res. 122, 1722–1740 (2018).
Socha, M. W., Malinowski, B., Puk, O., Dubiel, M. & Wiciński, M. The NLRP3 inflammasome role in the pathogenesis of pregnancy induced hypertension and preeclampsia. Cells 9, 1642 (2020).
Ioannou, G. N. et al. Cholesterol crystallization within hepatocyte lipid droplets and its role in murine NASH. J. Lipid Res. 58, 1067–1079 (2017).
Abela, G. S. et al. Cholesterol crystals induce mechanical trauma, inflammation, and neo-vascularization in solid cancers as in atherosclerosis. Am. Heart J. 35, 100317 (2023).
Khair, M., Khair, M., Vangaveti, V. N. & Malabu, U. H. The role of the NLRP3 inflammasome in atherosclerotic disease: systematic review and meta-analysis. J. Cardiol. 84, 14–21 (2024).
Que, X., Zheng, S., Song, Q., Pei, H. & Zhang, P. Fantastic voyage: the journey of NLRP3 inflammasome activation. Genes. Dis. 11, 819–829 (2024).
Ren, G.-M. et al. Pharmacological targeting of NLRP3 deubiquitination for treatment of NLRP3-associated inflammatory diseases. Sci. Immunol. 6, eabe2933 (2021).
Yalcinkaya, M. et al. Cholesterol trafficking to the ER leads to activation of CaMKII/JNK/NLRP3 and promotes atherosclerosis. J. Lipid Res. 65, 100534 (2024).
Niyonzima, N. et al. Mitochondrial C5aR1 activity in macrophages controls IL-1β production underlying sterile inflammation. Sci. Immunol. 6, eabf2489 (2021).
West, E. E. & Kemper, C. Complosome – the intracellular complement system. Nat. Rev. Nephrol. 19, 426–439 (2023).
Samstad, E. O. et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J. Immunol. 192, 2837–2845 (2014).
Kiyotake, R. et al. Human Mincle binds to cholesterol crystals and triggers innate immune responses. J. Biol. Chem. 290, 25322–25332 (2015).
Tan, R.-Z. et al. Macrophages mediate psoriasis via Mincle-dependent mechanism in mice. Cell Death Discov. 9, 140 (2023).
Jin, Z. et al. SIRT6 inhibits cholesterol crystal-induced vascular endothelial dysfunction via Nrf2 activation. Exp. Cell Res. 387, 111744 (2020).
Mani, A. M., Chattopadhyay, R., Singh, N. K. & Rao, G. N. Cholesterol crystals increase vascular permeability by inactivating SHP2 and disrupting adherens junctions. Free. Radic. Biol. Med. 123, 72–84 (2018).
McConathy, W. J., Koren, E. & Stiers, D. L. Cholesterol crystal uptake and metabolism by P388D1 macrophages. Atherosclerosis 77, 221–225 (1989).
Warnatsch, A., Ioannou, M., Wang, Q. & Papayannopoulos, V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 349, 316–320 (2015).
Zheng, Z. et al. Protective effect of SIRT6 on cholesterol crystal-induced endothelial dysfunction via regulating ACE2 expression. Exp. Cell Res. 402, 112526 (2021).
Nymo, S., Niyonzima, N., Espevik, T. & Mollnes, T. E. Cholesterol crystal-induced endothelial cell activation is complement-dependent and mediated by TNF. Immunobiology 219, 786–792 (2014).
Bennett, M. R., Sinha, S. & Owens, G. K. Vascular smooth muscle cells in atherosclerosis. Circ. Res. 118, 692–702 (2016).
Grootaert, M. O. J. & Bennett, M. R. Vascular smooth muscle cells in atherosclerosis: time for a re-assessment. Cardiovasc. Res. 117, 2326–2339 (2021).
Döring, Y., Libby, P. & Soehnlein, O. Neutrophil extracellular traps participate in cardiovascular diseases. Circ. Res. 126, 1228–1241 (2020).
Botts, S. R., Fish, J. E. & Howe, K. L. Dysfunctional vascular endothelium as a driver of atherosclerosis: emerging insights into pathogenesis and treatment. Front. Pharmacol. 12, 787541 (2021).
Zhang, Y. et al. Coronary endothelial dysfunction induced by nucleotide oligomerization domain-like receptor protein with pyrin domain containing 3 inflammasome activation during hypercholesterolemia: beyond inflammation. Antioxid. Redox Signal. 22, 1084–1096 (2015).
Yalcinkaya, M. et al. Cholesterol accumulation in macrophages drives NETosis in atherosclerotic plaques via IL-1β secretion. Cardiovasc. Res. 119, 969–981 (2023).
Waldiceu, A. et al. IL-33 induces neutrophil migration in rheumatoid arthritis and is a target of anti-TNF therapy. Ann. Rheum. Dis. 69, 1697–1703 (2010).
Baumer, Y. et al. Ultramorphological analysis of plaque advancement and cholesterol crystal formation in Ldlr knockout mouse atherosclerosis. Atherosclerosis 287, 100–111 (2019).
Guyton, J. R. & Klemp, K. F. The lipid-rich core region of human atherosclerotic fibrous plaques. Prevalence of small lipid droplets and vesicles by electron microscopy. Am. J. Pathol. 134, 705–717 (1989).
Guyton, J. R. & Klemp, K. F. Development of the atherosclerotic core region. Chemical and ultrastructural analysis of microdissected atherosclerotic lesions from human aorta. Arterioscler. Thromb. 14, 1305–1314 (1994).
Parker, F. An electron microscopic study of experimental atherosclerosis. Am. J. Pathol. 36, 19–53 (1960).
Cahill, P. A. & Redmond, E. M. Vascular endothelium – gatekeeper of vessel health. Atherosclerosis 248, 97–109 (2016).
Gimbrone, M. A. Jr. & Garcia-Cardena, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 22, 9–15 (2013).
Gimbrone, M. A. Jr. & Garcia-Cardena, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 118, 620–636 (2016).
Boren, J. et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 41, 2313–2330 (2020).
Guyton, J. R. & Klemp, K. F. Transitional features in human atherosclerosis. Intimal thickening, cholesterol clefts, and cell loss in human aortic fatty streaks. Am. J. Pathol. 143, 1444–1457 (1993).
Gerrity, R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am. J. Pathol. 103, 191–200 (1981).
Kruth, H. S. Macrophage foam cells and atherosclerosis. Front. Biosci. 6, D429–D455 (2001).
Baumer, Y., Ortiz-Whittingham, L. R., Baez, A. S., Powell-Wiley, T. M. & Boisvert, W. A. in Cholesterol Crystals in Atherosclerosis and Other Related Diseases (eds Abela, G. S. & Nidorf, S. M.) 127–142 (Humana, 2023).
Ross, R. Atherosclerosis – an inflammatory disease. N. Engl. J. Med. 340, 115–126 (1999).
Gelfand, J. M. et al. Risk of myocardial infarction in patients with psoriasis. JAMA 296, 1735–1741 (2006).
Duan, Y. et al. Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics. Signal. Transduct. Target. Ther. 7, 265 (2022).
Bodzioch, M. et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nat. Genet. 22, 347–351 (1999).
Brooks-Wilson, A. et al. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22, 336–345 (1999).
Rust, S. et al. Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1. Nat. Genet. 22, 352–355 (1999).
Goodman, Z., Terracciano, L. & Wee, A. in MacSween’s Pathology of the Liver 6th edn Ch. 14 (eds Burt, A. D., Portmann, B. C. & Ferrell, L. D.) 761–851 (Churchill Livingstone, 2011).
Amiri, M. et al. Circulating lipoprotein(a) and all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis. Eur. J. Epidemiol. 38, 485–499 (2023).
Kamstrup, P. R., Tybjaerg-Hansen, A. & Nordestgaard, B. G. Lipoprotein(a) and risk of myocardial infarction – genetic epidemiologic evidence of causality. Scand. J. Clin. Lab. Invest. 71, 87–93 (2011).
Seed, M. et al. Relation of serum lipoprotein(a) concentration and apolipoprotein(a) phenotype to coronary heart disease in patients with familial hypercholesterolemia. N. Engl. J. Med. 322, 1494–1499 (1990).
Bennet, A. et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch. Intern. Med. 168, 598–608 (2008).
Clarke, R. et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 361, 2518–2528 (2009).
The Emerging Risk Factors Collaboration Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA 302, 412–423 (2009).
Saleheen, D. et al. Apolipoprotein(a) isoform size, lipoprotein(a) concentration, and coronary artery disease: a mendelian randomisation analysis. Lancet Diabetes Endocrinol. 5, 524–533 (2017).
Kaiser, Y. et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J. Am. Coll. Cardiol. 79, 223–233 (2022).
Langsted, A. & Nordestgaard, B. G. Lipoprotein(a): is it more, less or equal to LDL as a causal factor for cardiovascular disease and mortality? Curr. Opin. Lipidol. 31, 125–131 (2020).
Xue, C. et al. The relationships between cholesterol crystals, NLRP3 inflammasome, and coronary atherosclerotic plaque vulnerability in acute coronary syndrome: an optical coherence tomography study. Front. Cardiovasc. Med. 9, 905363 (2022).
Abela, G. S. et al. Effect of cholesterol crystals on plaques and intima in arteries of patients with acute coronary and cerebrovascular syndromes. Am. J. Cardiol. 103, 959–968 (2009).
Al-Handawi, M. B. et al. Mechanical and crystallographic analysis of cholesterol crystals puncturing biological membranes. Chemistry 24, 11493–11497 (2018).
Frink, R. J. Parallel cholesterol crystals: a sign of impending plaque rupture? J. Invasive Cardiol. 22, 406–411 (2010).
Nidorf, S. M., Fiolet, A. & Abela, G. S. Viewing atherosclerosis through a crystal lens: how the evolving structure of cholesterol crystals in atherosclerotic plaque alters its stability. J. Clin. Lipidol. 14, 619–630 (2020).
El-Khatib, L. A. et al. Cholesterol induced heart valve inflammation and injury: efficacy of cholesterol lowering treatment. Open. Heart 7, e001274 (2020).
Niepmann, S. T. et al. Dissolving cholesterol crystals reduces aortic valve stenosis development in mice [abstract ehaa946]. Eur. Heart J. 41 (Suppl. 2), 3719 (2020).
Boumegouas, M. et al. Cholesterol crystal induced inflammation and mechanical cardiac valve injury: implications for transcatheter aortic-valve replacement. Interv. Cardiol. 13, 53–57 (2021).
Al‐Kassou, B. et al. Cholesterol crystal dissolution rate of serum predicts outcomes in patients with aortic stenosis undergoing transcatheter aortic valve replacement. J. Am. Heart Assoc. 13, e031997 (2024).
Filipovic, M. G. & Luedi, M. M. Cardiovascular biomarkers: current status and future directions. Cells 12, 2647 (2023).
Rempakos, A., Prescott, B., Mitchell, G. F., Vasan, R. S. & Xanthakis, V. Association of life’s essential 8 with cardiovascular disease and mortality: the Framingham Heart Study. J. Am. Heart Assoc. 12, e030764 (2023).
Lieberg, J. et al. Mortality after elective and ruptured abdominal aortic aneurysm surgical repair: 12-year single-center experience of Estonia. Scand. J. Surg. 107, 152–157 (2018).
Meyrier, A. Cholesterol crystal embolism: diagnosis and treatment. Kidney Int. 69, 1308–1312 (2006).
Shi, C., Mammadova-Bach, E., Li, C., Liu, D. & Anders, H.-J. Pathophysiology and targeted treatment of cholesterol crystal embolism and the related thrombotic angiopathy. FASEB J. 37, e23179 (2023).
Lazareth, H. & Karras, A. Cholesterol crystal embolization after transcatheter aortic-valve replacement. N. Engl. J. Med. 381, 655 (2019).
Duell, P. B. et al. Nonalcoholic fatty liver disease and cardiovascular risk: a scientific statement from the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 42, e168–e185 (2022).
Ioannou, G. N., Haigh, W. G., Thorning, D. & Savard, C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J. Lipid Res. 54, 1326–1334 (2013).
Wu, P. et al. Preeclampsia and future cardiovascular health. Circ. Cardiovasc. Qual. Outcomes 10, e003497 (2017).
Hod, T., Cerdeira, A. S. & Karumanchi, S. A. Molecular mechanisms of preeclampsia. Cold Spring Harb. Perspect. Med. 5, a023473 (2015).
Salafia, C. et al. Pregnancy-induced hypertension: the potential role of cholesterol crystals in the maternal decidua. J. Clin. Lipidol. 16, e75 (2022).
Matyori, A., Brown, C. P., Ali, A. & Sherbeny, F. Statins utilization trends and expenditures in the U.S. before and after the implementation of the 2013 ACC/AHA guidelines. Saudi Pharm. J. 31, 795–800 (2023).
Ioannou, G. N. et al. Cholesterol-lowering drugs cause dissolution of cholesterol crystals and disperse Kupffer cell crown-like structures during resolution of NASH. J. Lipid Res. 56, 277–285 (2015).
Inia, J. A. et al. Atorvastatin attenuates diet-induced non-alcoholic steatohepatitis in APOE*3-Leiden mice by reducing hepatic inflammation. Int. J. Mol. Sci. 24, 7818 (2023).
Boland, A. J. et al. Simvastatin suppresses interleukin Iβ release in human peripheral blood mononuclear cells stimulated with cholesterol crystals. J. Cardiovasc. Pharmacol. Ther. 23, 509–517 (2018).
Abela, G. S. et al. Effect of statins on cholesterol crystallization and atherosclerotic plaque stabilization. Am. J. Cardiol. 107, 1710–1717 (2011).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364, 127–135 (2011).
Matsuo, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 148, 197–203 (2022).
Ballantyne, C. M. & Nambi, V. HDL therapeutics – time for a curtain call or time to reconceptualize? N. Engl. J. Med. 390, 1622–1623 (2024).
Niyonzima, N. et al. Reconstituted high-density lipoprotein attenuates cholesterol crystal-induced inflammatory responses by reducing complement activation. J. Immunol. 195, 257–264 (2015).
Thacker, S. G. et al. High-density lipoprotein reduces inflammation from cholesterol crystals by inhibiting inflammasome activation. Immunology 149, 306–319 (2016).
Luo, Y. et al. Phospholipid nanoparticles: therapeutic potentials against atherosclerosis via reducing cholesterol crystals and inhibiting inflammation. eBioMedicine 74, 103725 (2021).
Zhang, L. Cyclodextrin related drug delivery system to promote atherosclerosis regression. Pharmazie 75, 619–625 (2020).
Mahjoubin-Tehran, M., Kovanen, P. T., Xu, S., Jamialahmadi, T. & Sahebkar, A. Cyclodextrins: potential therapeutics against atherosclerosis. Pharmacol. Ther. 214, 107620 (2020).
Pilely, K. et al. Alpha-cyclodextrin inhibits cholesterol crystal-induced complement-mediated inflammation: a potential new compound for treatment of atherosclerosis. Atherosclerosis 283, 35–42 (2019).
Zimmer, S. et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 8, 333ra350 (2016).
Bakke, S. S. et al. Cyclodextrin reduces cholesterol crystal-induced inflammation by modulating complement activation. J. Immunol. 199, 2910–2920 (2017).
Lopez, A. M. et al. Systemic administration of 2-hydroxypropyl-β-cyclodextrin to symptomatic Npc1-deficient mice slows cholesterol sequestration in the major organs and improves liver function. Clin. Exp. Pharmacol. Physiol. 41, 780–787 (2014).
Wagner, E. M., Jen, K. L., Artiss, J. D. & Remaley, A. T. Dietary alpha-cyclodextrin lowers low-density lipoprotein cholesterol and alters plasma fatty acid profile in low-density lipoprotein receptor knockout mice on a high-fat diet. Metabolism 57, 1046–1051 (2008).
Sakurai, T. et al. Dietary α-cyclodextrin reduces atherosclerosis and modifies gut flora in apolipoprotein E-deficient mice. Mol. Nutr. Food Res. 61, https://doi.org/10.1002/mnfr.201600804 (2017).
Zhu, T. et al. Beneficial effects of three dietary cyclodextrins on preventing fat accumulation and remodeling gut microbiota in mice fed a high-fat diet. Foods 11, 1118 (2022).
Amar, M. J. A. et al. Randomized double blind clinical trial on the effect of oral α-cyclodextrin on serum lipids. Lipids Health Dis. 15, 115 (2016).
Tiwari, G., Tiwari, R. & Rai, A. K. Cyclodextrins in delivery systems: applications. J. Pharm. Bioallied Sci. 2, 72–79 (2010).
Houben, T. et al. Pro-inflammatory implications of 2-hydroxypropyl-β-cyclodextrin treatment. Front. Immunol. 12, 716357 (2021).
Zhu, L. et al. Biomimetic nanoparticles to enhance the reverse cholesterol transport for selectively inhibiting development into foam cell in atherosclerosis. J. Nanobiotechnol 21, 307 (2023).
Itoh, M. et al. Lysosomal cholesterol overload in macrophages promotes liver fibrosis in a mouse model of NASH. J. Exp. Med. 220, e20220681 (2023).
Appelqvist, H. et al. Sensitivity to lysosome-dependent cell death is directly regulated by lysosomal cholesterol content. PLoS ONE 7, e50262 (2012).
Cao, M., Luo, X., Wu, K. & He, X. Targeting lysosomes in human disease: from basic research to clinical applications. Signal. Transduct. Target. Ther. 6, 379 (2021).
Geisslinger, F., Müller, M., Vollmar, A. M. & Bartel, K. Targeting lysosomes in cancer as promising strategy to overcome chemoresistance – a mini review. Front. Oncol. 10, 1156 (2020).
Marques, A. R. A., Ramos, C., Machado-Oliveira, G. & Vieira, O. V. Lysosome (dys)function in atherosclerosis– a big weight on the shoulders of a small organelle. Front. Cell Dev. Biol. 9, 658995 (2021).
Razani, B. et al. Autophagy links inflammasomes to atherosclerotic progression. Cell Metab. 15, 534–544 (2012).
Sergin, I. et al. Exploiting macrophage autophagy–lysosomal biogenesis as a therapy for atherosclerosis. Nat. Commun. 8, 15750 (2017).
Schott, M. B., Rozeveld, C. N., Weller, S. G. & McNiven, M. A. Lipophagy at a glance. J. Cell Sci. 135, jcs259402 (2022).
Lu, H., Sun, J., Hamblin, M. H., Chen, Y. E. & Fan, Y. Transcription factor EB regulates cardiovascular homeostasis. eBioMedicine 63, 103207 (2021).
Li, X. et al. Hypericin-mediated sonodynamic therapy induces autophagy and decreases lipids in THP-1 macrophage by promoting ROS-dependent nuclear translocation of TFEB. Cell Death Dis. 7, e2527 (2016).
Theofani, E. et al. TFEB signaling attenuates NLRP3-driven inflammatory responses in severe asthma. Allergy 77, 2131–2146 (2022).
Wang, K. et al. TFEB SUMOylation in macrophages accelerates atherosclerosis by promoting the formation of foam cells through inhibiting lysosomal activity. Cell Mol. Life Sci. 80, 358 (2023).
Kruth, H. S., Skarlatos, S. I., Lilly, K., Chang, J. & Ifrim, I. Sequestration of acetylated LDL and cholesterol crystals by human monocyte-derived macrophages. J. Cell Biol. 129, 133–145 (1995).
Juhl, A. D. et al. Direct observation of uptake and dissolution of cholesterol crystals by macrophages using combined fluorescence and x-ray microscopy. Microsc. Microanal. 29, 1158–1159 (2023).
He, J. et al. Anchoring β-CD on simvastatin-loaded rHDL for selective cholesterol crystals dissolution and enhanced anti-inflammatory effects in macrophage/foam cells. Eur. J. Pharm. Biopharm. 174, 144–154 (2022).
Olsen, M. B. et al. Targeting the inflammasome in cardiovascular disease. JACC Basic. Transl. Sci. 7, 84–98 (2022).
Libby, P. Targeting inflammatory pathways in cardiovascular disease: the inflammasome, interleukin-1, interleukin-6 and beyond. Cells 10, 951 (2021).
Toldo, S. et al. Targeting the NLRP3 inflammasome in cardiovascular diseases. Pharmacol. Ther. 236, 108053 (2022).
Potere, N. et al. Inflammasome signaling, thromboinflammation, and venous thromboembolism. JACC Basic. Transl. Sci. 8, 1245–1261 (2023).
Ren, P. et al. Targeting the NLRP3 inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J. Am. Heart Assoc. 9, e014044 (2020).
Ramos-Tovar, E. & Muriel, P. NLRP3 inflammasome in hepatic diseases: a pharmacological target. Biochem. Pharmacol. 217, 115861 (2023).
Huang, G., Zhang, Y., Zhang, Y. & Ma, Y. Chronic kidney disease and NLRP3 inflammasome: pathogenesis, development and targeted therapeutic strategies. Biochem. Biophys. Rep. 33, 101417 (2023).
Hu, Y. et al. Cholesterol crystals induce inflammatory cytokines expression in a human retinal pigment epithelium cell line by activating the NF-κB pathway. Discov. Med. 18, 7–14 (2014).
Lillo, S. & Saleh, M. Inflammasomes in cancer progression and anti-tumor immunity. Front. Cell Dev. Biol. 10, 839041 (2022).
Missiroli, S. et al. Targeting the NLRP3 inflammasome as a new therapeutic option for overcoming cancer. Cancers 13, 2297 (2021).
Chen, S. et al. Sex-specific effects of the Nlrp3 inflammasome on atherogenesis in LDL receptor-deficient mice. JACC Basic. Transl. Sci. 5, 582–598 (2020).
Nelson, K., Fuster, V. & Ridker, P. M. Low-dose colchicine for secondary prevention of coronary artery disease: JACC review topic of the week. J. Am. Coll. Cardiol. 82, 648–660 (2023).
Nidorf, S. M., Ben-Chetrit, E. & Ridker, P. M. Low-dose colchicine for atherosclerosis: long-term safety. Eur. Heart J. 45, 1596–1601 (2024).
Akrami, M. et al. Effects of colchicine on major adverse cardiac events in next 6-month period after acute coronary syndrome occurrence; a randomized placebo-control trial. BMC Cardiovasc. Disord. 21, 583 (2021).
Al Wssawi, A. F. A. et al. Colchicine reduces major adverse cardiovascular events in patients undergoing percutaneous coronary intervention: a meta-analysis of randomized controlled trials [abstract]. J. Am. Coll. Cardiol. 83, 919 (2024).
Misawa, T. et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 14, 454–460 (2013).
Papatriantafyllou, M. Inflammasomes: microtubules pull mitochondria to NLRP3. Nat. Rev. Immunol. 13, 306 (2013).
Schwarz, N. et al. Colchicine exerts anti-atherosclerotic and -plaque-stabilizing effects targeting foam cell formation. FASEB J. 37, e22846 (2023).
Abideen, Z. U., Pathak, D. R., Sabanci, R., Manu, M. & Abela, G. S. The effect of colchicine on cholesterol crystal formation, expansion and morphology: a potential mechanism in atherosclerosis. Front. Cardiovasc. Med. 11, 1345521 (2024).
Fry, L. et al. Effect of aspirin on cholesterol crystallization: a potential mechanism for plaque stabilization. Am. Heart J. 13, 100083 (2022).
Powell-Wiley, T. M. et al. Social determinants of cardiovascular disease. Circ. Res. 130, 782–799 (2022).
Farmer, N. et al. Neighborhood environment associates with trimethylamine-N-oxide (TMAO) as a cardiovascular risk marker. Int. J. Env. Res. Public. Health 18, 4296 (2021).
Ortiz-Whittingham, L. R. et al. Associations between neighborhood socioeconomic deprivation, IFNγ, and high-density lipoprotein particle size: data from the Washington, D.C. Cardiovascular Health and Nneeds Assessment. Psychoneuroendocrinology 157, 106346 (2023).
Malkowska, P. & Sawczuk, M. Cytokines as biomarkers for evaluating physical exercise in trained and non-trained individuals: a narrative review. Int. J. Mol. Sci. 24, 11156 (2023).
Albarrati, A. M. et al. Effectiveness of low to moderate physical exercise training on the level of low-density lipoproteins: a systematic review. Biomed. Res. Int. 2018, 5982980 (2018).
Nikara, S., Ahmadi, E. & Nia, A. A. Effects of different preparation techniques on the microstructural features of biological materials for scanning electron microscopy. J. Agric. Food Res. 2, 100036 (2020).
Hsu, L.-Y. & Nordman, C. Phase transition and crystal structure of the 37 C form of cholesterol. Science 220, 604–606 (1983).
Kumar, S. & Burns, S. Cracking cholesterol from a phase transition at body temperatures. Mater. Sci. Eng. C. 3, 153–158 (1995).
Addadi, L., Geva, M. & Kruth, H. S. Structural information about organized cholesterol domains from specific antibody recognition. Biochim. Biophys. Acta 1610, 208–216 (2003).
Perl-Treves, D., Kessler, N., Izhaky, D. & Addadi, L. Monoclonal antibody recognition of cholesterol monohydrate crystal faces. Chem. Biol. 3, 567–577 (1996).
Li, C., Deng, C., Shi, B. & Zhao, R. Thin-cap fibroatheroma in acute coronary syndrome: implication for intravascular imaging assessment. Int. J. Cardiol. 405, 131965 (2024).
Fabris, E. et al. Thin-cap fibroatheroma rather than any lipid plaques increases the risk of cardiovascular events in diabetic patients: insights from the COMBINE OCT-FFR trial. Circ. Cardiovasc. Interv. 15, e011728 (2022).
Kubo, T. Optical coherence tomography in vulnerable plaque and acute coronary syndrome. Interv. Cardiol. Clin. 12, 203–214 (2023).
Fujiyoshi, K. et al. Incidence, factors, and clinical significance of cholesterol crystals in coronary plaque: an optical coherence tomography study. Atherosclerosis 283, 79–84 (2019).
Nelles, G. et al. Cholesterol crystals at the culprit lesion in patients with acute coronary syndrome are associated with worse cardiovascular outcomes at two years follow up – results from the translational OPTICO-ACS study program (R2). Int. J. Cardiol. 399, 131665 (2023).
Tian, J. et al. Distinct morphological features of ruptured culprit plaque for acute coronary events compared to those with silent rupture and thin-cap fibroatheroma: a combined optical coherence tomography and intravascular ultrasound study. J. Am. Coll. Cardiol. 63, 2209–2216 (2014).
Saiin, K. et al. Association of coronary plaque morphology with inflammatory biomarkers and target lesion revascularization in patients with chronic coronary syndrome: an optical coherence tomography study. Am. J. Cardiovasc. Dis. 13, 309–319 (2023).
Kinoshita, D. et al. High-risk plaques on coronary computed tomography angiography: correlation with optical coherence tomography. JACC Cardiovasc. Imaging 17, 382–391 (2024).
Seegers, L. M. et al. Sex differences in coronary atherosclerotic phenotype and healing pattern on optical coherence tomography imaging. Circ. Cardiovasc. Imaging 16, e015227 (2023).
Wang, C. et al. Pancoronary plaque characteristics and clinical outcomes in acute coronary syndrome patients with cancer history. Atherosclerosis 378, 117118 (2023).
Yamaguchi, M. et al. Clinical significance of the presence of puff-chandelier ruptures detected by nonobstructive aortic angioscopy. Catheter. Cardiovasc. Interv. 96, 784–792 (2020).
Komatsu, S. et al. Different characteristics and interleukin-6 ratios of scattering-type aortic plaques. Cureus 16, e52949 (2024).
Nishimiya, K., Poduval, R. K. & Tearney, G. J. OCT emerging technologies: coronary micro-optical coherence tomography. Interv. Cardiol. Clin. 12, 237–244 (2023).
Baumer, Y. et al. CD98 regulates vascular smooth muscle cell proliferation in atherosclerosis. Atherosclerosis 256, 105–114 (2017).
Author information
Authors and Affiliations
Contributions
The authors contributed substantially to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Cardiology thanks George S. Abela, Eicke Latz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baumer, Y., Irei, J. & Boisvert, W.A. Cholesterol crystals in the pathogenesis of atherosclerosis. Nat Rev Cardiol 22, 315–332 (2025). https://doi.org/10.1038/s41569-024-01100-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41569-024-01100-3
This article is cited by
-
HDL in Abdominal Aortic Aneurysm: Mechanistic Insight and Therapeutic Potential
Current Atherosclerosis Reports (2026)
-
APOE4 promotes nigral tau hyperphosphorylation through cholesterol in atherosclerosis
Cell Death Discovery (2025)
-
State-of-the-art wearable sensors for cardiovascular health: a review
npj Cardiovascular Health (2025)
-
Targeting cholesterol-driven pyroptosis: a promising strategy for the prevention and treatment of atherosclerosis
Molecular Biology Reports (2025)
-
Phospholipid metabolism in innate immunity and inflammation: from basic to clinic
Immunity & Inflammation (2025)