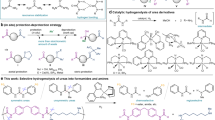
Abstract
The reactivity of carbonyl functional groups largely depends on the substituents on the carbon atom. Reversal of the commonly accepted order of reactivity of different carbonyl compounds requires novel synthetic approaches. Achieving selective reduction will enable the transformation of carbon resources such as plastic waste, carbon dioxide and biomass into valuable chemicals. In this Review, we explore the reduction of less reactive carbonyl groups in the presence of those typically considered more reactive. We discuss reductions, including the controlled reduction of ureas, amides and esters to aldehydes, as well as chemoselective reductions of carbonyl groups, including the reduction of ureas over carbamates, amides and esters; the reduction of amides over esters, ketones and aldehydes; and the reduction of ketones over aldehydes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dub, P. A. & Ikariya, T. Catalytic reductive transformations of carboxylic and carbonic acid derivatives using molecular hydrogen. ACS Catal. 2, 1718–1741 (2012).
Volkov, A., Tinnis, F., Slagbrand, T., Trillo, P. & Adolfsson, H. Chemoselective reduction of carboxamides. Chem. Soc. Rev. 45, 6685–6697 (2016).
Werkmeister, S., Junge, K. & Beller, M. Catalytic hydrogenation of carboxylic acid esters, amides, and nitriles with homogeneous catalysts. Org. Process Res. Dev. 18, 289–302 (2014).
Smith, A. M. & Whyman, R. Review of methods for the catalytic hydrogenation of carboxamides. Chem. Rev. 114, 5477–5510 (2014).
Cabrero-Antonino, J. R., Adam, R., Papa, V. & Beller, M. Homogeneous and heterogeneous catalytic reduction of amides and related compounds using molecular hydrogen. Nat. Commun. 11, 3893 (2020). This review covers the historical background of the hydrogenation of amide derivatives.
Corey, E. J. & Cheng, X.-M. The Logic of Chemical Synthesis (Wiley, 1989).
Senatore, R., Ielo, L., Monticelli, S., Castoldi, L. & Pace, V. Weinreb amides as privileged acylating agents for accessing α-substituted ketones. Synthesis 51, 2792–2808 (2019).
Wuts, P. G. M. Greene’s Protective Groups in Organic Synthesis 5th edn (Wiley, 2014).
Yahata, K. & Fujioka, H. In situ protection methodology in carbonyl chemistry. Chem. Pharm. Bull. 62, 1–11 (2014).
Young, I. S. & Baran, P. S. Protecting-group-free synthesis as an opportunity for invention. Nat. Chem. 1, 193–205 (2009).
Afagh, N. A. & Yudin, A. K. Chemoselectivity and the curious reactivity preferences of functional groups. Angew. Chem. Int. Ed. 49, 262–310 (2010).
Mahatthananchai, J., Dumas, A. M. & Bode, J. W. Catalytic selective synthesis. Angew. Chem. Int. Ed. 51, 10954–10990 (2012).
Trader, D. J. & Carlson, E. E. Chemoselective hydroxyl group transformation: an elusive target. Mol. Biosyst. 8, 2484–2493 (2012).
Kristensen, T. E. Chemoselective O-acylation of hydroxyamino acids and amino alcohols under acidic reaction conditions: history, scope and applications. Beilstein J. Org. Chem. 11, 446–468 (2015).
Shafir, A., Lichtor, P. A. & Buchwald, S. L. N- versus O-arylation of aminoalcohols: orthogonal selectivity in copper-based catalysts. J. Am. Chem. Soc. 129, 3490–3491 (2007).
Ohshima, T., Iwasaki, T., Maegawa, Y., Yoshiyama, A. & Mashima, K. Enzyme-like chemoselective acylation of alcohols in the presence of amines catalyzed by a tetranuclear zinc cluster. J. Am. Chem. Soc. 130, 2944–2945 (2008).
Strambeanu, I. I. & White, M. C. Catalyst-controlled C–O versus C–N allylic functionalization of terminal olefins. J. Am. Chem. Soc. 135, 12032–12037 (2013).
Uesugi, S., Li, Z., Yazaki, R. & Ohshima, T. Chemoselective catalytic conjugate addition of alcohols over amines. Angew. Chem. Int. Ed. 53, 1611–1615 (2014).
Fernandes, A. C. Reductive depolymerization as an efficient methodology for the conversion of plastic waste into value-added compounds. Green Chem. 23, 7330–7360 (2021).
Kumar, A. & Gao, C. Homogeneous (de)hydrogenative catalysis for circular chemistry—using waste as a resource. ChemCatChem 13, 1105–1134 (2021).
Liguori, F., Moreno-Marrodán, C. & Barbaro, P. Valorisation of plastic waste via metal-catalysed depolymerisation. Beilstein J. Org. Chem. 17, 589–621 (2021).
Wang, C. & El-Sepelgy, O. Reductive depolymerization of plastics catalyzed with transition metal complexes. Curr. Opin. Green Sustain. Chem. 32, 100547 (2021).
Lee, K., Jing, Y., Wang, Y. & Yan, N. A unified view on catalytic conversion of biomass and waste plastics. Nat. Rev. Chem. 6, 635–652 (2022).
Khopade, K. V., Chikkali, S. H. & Barsu, N. Metal-catalyzed plastic depolymerization. Cell Rep. Phys. Sci. 4, 101341 (2023).
Kumar, A., Daw, P. & Milstein, D. Homogeneous catalysis for sustainable energy: hydrogen and methanol economies, fuels from biomass, and related topics. Chem. Rev. 122, 385–441 (2022).
Sakakura, T., Choi, J.-C. & Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 107, 2365–2387 (2007).
Addis, D., Das, S., Junge, K. & Beller, M. Selective reduction of carboxylic acid derivatives by catalytic hydrosilylation. Angew. Chem. Int. Ed. 50, 6004–6011 (2011).
Balaraman, E., Ben-David, Y. & Milstein, D. Unprecedented catalytic hydrogenation of urea derivatives to amines and methanol. Angew. Chem. Int. Ed. 50, 11702–11705 (2011).
Miura, T., Held, I. E., Oishi, S., Naruto, M. & Saito, S. Catalytic hydrogenation of unactivated amides enabled by hydrogenation of catalyst precursor. Tetrahedron Lett. 54, 2674–2678 (2013).
Kothandaraman, J. et al. Efficient reversible hydrogen carrier system based on amine reforming of methanol. J. Am. Chem. Soc. 139, 2549–2552 (2017).
Miura, T., Naruto, M., Toda, K., Shimomura, T. & Saito, S. Multifaceted catalytic hydrogenation of amides via diverse activation of a sterically confined bipyridine–ruthenium framework. Sci. Rep. 7, 1586 (2017).
Xie, Y., Hu, P., Ben-David, Y. & Milstein, D. A reversible liquid organic hydrogen carrier system based on methanol-ethylenediamine and ethylene urea. Angew. Chem. Int. Ed. 58, 5105–5109 (2019).
Liu, X. et al. Hydrogenative depolymerization of silicon-modified polyureas. Chem. Commun. 58, 5415–5418 (2022).
Das, U. K., Kumar, A., Ben-David, Y., Iron, M. A. & Milstein, D. Manganese catalyzed hydrogenation of carbamates and urea derivatives. J. Am. Chem. Soc. 141, 12962–12966 (2019).
Liu, X. & Werner, T. Indirect reduction of CO2 and recycling of polymers by manganese-catalyzed transfer hydrogenation of amides, carbamates, urea derivatives, and polyurethanes. Chem. Sci. 12, 10590–10597 (2021).
Kumar, A. & Luk, J. Catalytic hydrogenation of urea derivatives and polyureas. Eur. J. Org. Chem. 2021, 4546–4550 (2021).
Zhou, W. et al. Depolymerization of technical-grade polyamide 66 and polyurethane materials through hydrogenation. ChemSusChem 14, 4176–4180 (2021).
Zubar, V., Haedler, A. T., Schütte, M., Hashmi, A. S. K. & Schaub, T. Hydrogenative depolymerization of polyurethanes catalyzed by a manganese pincer complex. ChemSusChem 15, e202101606 (2022).
Wei, Z., Li, H., Wang, Y. & Liu, Q. A tailored versatile and efficient NHC-based NNC-pincer manganese catalyst for hydrogenation of polar unsaturated compounds. Angew. Chem. Int. Ed. 62, e202301042 (2023).
Ding, J. et al. Direct synthesis of urea from carbon dioxide and ammonia. Nat. Commun. 14, 4586 (2023).
Tao, Z., Rooney, C. L., Liang, Y. & Wang, H. Accessing organonitrogen compounds via C−N coupling in electrocatalytic CO2 reduction. J. Am. Chem. Soc. 143, 19630–19642 (2021).
vom Stein, T. et al. Highly versatile catalytic hydrogenation of carboxylic and carbonic acid derivatives using a Ru-triphos complex: molecular control over selectivity and substrate scope. J. Am. Chem. Soc. 136, 13217–13225 (2014). This article demonstrates the hydrogenolysis of 1,3-diphenylurea to formanilide and aniline by a Ru catalyst.
Zhu, J. et al. Highly efficient ruthenium-catalyzed semi-hydrogenation of urea derivatives to formamides. Chem. Eur. J. 29, e202300106 (2023).
Zhu, J., Wang, Y., Yao, J. & Li, H. Switching the hydrogenation selectivity of urea derivatives via subtly tuning the amount and type of additive in the catalyst system. Chem. Sci. 15, 2089–2099 (2024).
Iwasaki, T., Tsuge, K., Naito, N. & Nozaki, K. Chemoselectivity change in catalytic hydrogenolysis enabling urea-reduction to formamide/amine over more reactive carbonyl compounds. Nat. Commun. 14, 3279 (2023). This article demonstrates Ir-catalysed hydrogenolysis of ureas to formamides and amines even in the presence of ester, carbamate and amide, and its application to the chemical degradation of polyurea resins.
Khusnutdinova, J. R. & Milstein, D. Metal–ligand cooperation. Angew. Chem. Int. Ed. 54, 12236–12273 (2015).
Higashi, T., Kusumoto, S. & Nozaki, K. Cleavage of Si–H, B–H, and C–H bonds by metal–ligand cooperation. Chem. Rev. 119, 10393–10402 (2019).
Ignatyev, I. A., Thielemans, W. & Beke, B. V. Recycling of polymers: a review. ChemSusChem 7, 1579–1593 (2014).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Langsted, C. R. et al. Isocyanate-free synthesis of ureas and polyureas via ruthenium catalyzed dehydrogenation of amines and formamides. J. Appl. Polym. Sci. 139, 52088 (2022).
Larizza, A., Brancaccio, G. & Lettieri, G. The reaction of substituted ureas with lithium aluminum hydride. I. A new synthesis of three-substituted formamidines. J. Org. Chem. 29, 3697–3700 (1964).
Kikugawa, Y., Yamada, S.-I., Nagashima, H. & Kaji, K. The reaction of substituted ureas with sodium borohydride in pyridine. Tetrahedron Lett. 10, 699–702 (1969).
Pouessel, J., Jacquet, O. & Cantat, T. Pushing back the limits of hydrosilylation: unprecedented catalytic reduction of organic ureas to formamidines. ChemCatChem 5, 3552–3556 (2013).
Chardon, A., Morisset, E., Rouden, J. & Blanchet, J. Recent advances in amide reductions. Synthesis 50, 984–997 (2018).
Wojcik, B. & Adkins, H. Catalytic hydrogenation of amides to amines. J. Am. Chem. Soc. 56, 2419–2424 (1934).
Arnott, G. E. in Comprehensive Organic Synthesis 2nd edn, Vol. 8 (ed. Knochel, P.) 410–445 (Elsevier, 2014).
Nahm, S. & Weinreb, S. M. N-methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 22, 3815–3818 (1981).
Douat, C., Heitz, A., Martinez, J. & Fehrentz, J.-A. Synthesis of N-protected α-amino aldehydes from their morpholine amide derivatives. Tetrahedron Lett. 41, 37–40 (2000).
Comins, D. L. & Brown, J. D. Directed lithiation of tertiary β-amino benzamides. J. Org. Chem. 51, 3566–3572 (1986).
Ried, W. & Königstein, F. J. Eine neue Aldehyd-Synthese Die hydrogenolytische Spaltung von N-Acyl-pyrazolen [in German]. Angew. Chem. 70, 165 (1958).
Ramegowda, N. S. et al. A new synthesis of aldehydes from acids via reduction of N-acyl saccharins using sodium dihydro bis-(2-methoxy-ethoxyl)aluminate. Tetrahedron 29, 3985–3986 (1973).
Liu, H. et al. An efficient multigram synthesis of the potent histamine H3 antagonist GT-2331 and the reassessment of the absolute configuration. J. Org. Chem. 69, 192–194 (2004).
Nagao, Y., Kawabata, K., Seno, K. & Fujita, E. Utilisation of sulphur-containing leaving groups. Part 2. Monitored reduction of carboxylic acids into alcohols or aldehydes via 3-acyl-thiazolidine-2-thiones by sodium borohydride or di-isobutylaluminium hydride. J. Chem. Soc. Perkin Trans. 1, 2470–2473 (1980).
Izawa, T. & Mukaiyama, T. The partial reduction of carboxylic acids to aldehyde via 3-acylthiazolidine-2-thiones with diisobutylaluminum hydride. Chem. Lett. 6, 1443–1446 (1977).
Izawa, T. & Mukaiyama, T. The partial reduction of carboxylic acids to aldehydes via 3-acylthiazolidine-2-thiones with diisobutylaluminum hydride and with lithium tri-t-butoxyaluminum hydride. Bull. Chem. Soc. Jpn. 52, 555–558 (1979).
Brown, H. C. & Tsukamoto, A. The reaction of 1-acylaziridines with lithium aluminum hydride—a new aldehyde synthesis. J. Am. Chem. Soc. 83, 2016–2017 (1961).
Brown, H. C. & Tsukamoto, A. Selective reductions. I. The partial reduction of tertiary amides with lithium aluminum hydride. A new aldehyde synthesis via the 1-acylaziridines. J. Am. Chem. Soc. 83, 4549–4552 (1961).
Weygand, F. & Eberhardt, G. Darstellung von Aldehyden durch Reduktion von Carbonsäure-N-methylaniliden mit Lithiumaluminiumhydrid [in German]. Angew. Chem. 64, 458 (1952).
Weygand, F., Eberhardt, G., Linden, H., Schäfer, F. & Eigen, I. Darstellung von Aldehyden aus Carbonsäuren [in German]. Angew. Chem. 65, 525–531 (1953).
Weygand, F. & Linden, H. Darstellung von Aldehyden aus Carbonsäuren [in German]. Angew. Chem. 66, 174–175 (1954).
Weygand, F. & Mitgau, R. Aromatische Aldehyde aus Kohlenwasserstoffon über die Carbonsäure-N-methylanilide. Aldehyde aus Carbonsäuren. VI. Mitteil [in German]. Chem. Ber. 88, 301–308 (1955).
Weygand, F. & Bestmann, H. J. Homologe Aldehyde aus Carbonsäuren [in German]. Chem. Ber. 92, 528–529 (1959).
Wittig, G. & Hornberger, P. Über die Reduktion ungesättigter Säureamide zu ungesättigten Aldehyden; ein Beitrag zum Aufbau von Polyenketten [in German]. Liebigs Ann. 577, 11–25 (1952).
Brown, H. C. & Tsukamoto, A. Lithium diethoxyaluminohydride as a selective reducing agent—the reduction of dimethylamides to aldehydes. J. Am. Chem. Soc. 81, 502–503 (1959).
Brown, H. C. & Tsukamoto, A. Selective reductions. V. The partial reduction of tertiary amides by lithium di- and triethoxyaluminohydrides—a new aldehyde synthesis via the dimethylamides. J. Am. Chem. Soc. 86, 1089–1095 (1964).
Woo, S.-M., Kim, M.-E. & An, D.-K. New synthetic method of aldehydes from tertiary amides by lithium diisobutylpiperidinohydroaluminate (LDBPA). Bull. Korean Chem. Soc. 27, 1913–1914 (2006).
Im, S. H., Shin, W. K., Jaladi, A. K. & An, D. K. Copper diisobutyl-t-butoxyaluminum hydride: a novel reagent for chemoselective reduction of tertiary amides over esters. Tetrahedron Lett. 59, 2335–2340 (2018).
Cha, J. S. et al. Selective reduction with lithium bis- or tris(dialkylamino)aluminum hydrides. III. Reduction of primary carboxamides to aldehydes by lithium tris(diethylamino)aluminum hydride. Tetrahedron Lett. 32, 6903–6904 (1991).
Muraki, M. & Mukaiyama, T. Aminoaluminum hydride as new reducing agents. III. Selective reduction of carboxamides to aldehydes. Chem. Lett. 4, 878 (1975).
Yoon, N. M., Ahn, J. H., An, D. K. & Shon, Y. S. Sodium diethylpiperidinohydroaluminate, a new selective reducing agent. J. Org. Chem. 58, 1941–1944 (1993).
Zakharkin, L. I., Maslin, D. N. & Gavrilenko, V. V. The preparation of aldehydes by reduction of dimethylamides of carboxylic acids with sodium aluminohydride. Tetrahedron 25, 5555–5569 (1969).
Bailey, C. L., Joh, A. Y., Hurley, Z. Q., Anderson, C. L. & Singaram, B. Controlled reduction of tertiary amides to the corresponding alcohols, aldehydes, or amines using dialkylboranes and aminoborohydride reagents. J. Org. Chem. 81, 3619–3628 (2016).
White, J. M., Tunoori, A. R. & Georg, G. I. A novel and expeditious reduction of tertiary amides to aldehydes using Cp2Zr(H)Cl. J. Am. Chem. Soc. 122, 11995–11996 (2000).
Spletstoser, J. T., White, J. M., Tunoori, A. R. & Georg, G. I. Mild and selective hydrozirconation of amides to aldehydes using Cp2Zr(H)Cl: scope and mechanistic insight. J. Am. Chem. Soc. 129, 3408–3419 (2007).
Zhao, Y. & Snieckus, V. A practical in situ generation of the Schwartz reagent. Reduction of tertiary amides to aldehydes and hydrozirconation. Org. Lett. 16, 390–393 (2014).
Ganem, B. & Franke, R. R. Paclitaxel from primary taxanes: a perspective on creative invention in organozirconium chemistry. J. Org. Chem. 72, 3981–3987 (2007).
Bower, S., Kreutzer, K. A. & Buchwald, S. L. A mild general procedure for the one-pot conversion of amides to aldehydes. Angew. Chem. Int. Ed. 35, 1515–1516 (1996).
Tinnis, F., Volkov, A., Slagbrand, T. & Adolfsson, H. Chemoselective reduction of tertiary amides under thermal control: formation of either aldehydes or amines. Angew. Chem. Int. Ed. 55, 4562–4566 (2016). This article describes the Mo-catalysed reduction of amides to aldehydes over aldehydes, ketones and esters using hydrosilane as reducing reagent.
Chan, G. H., Ong, D. Y., Yen, Z. & Chiba, S. Reduction of N,N-dimethylcarboxamides to aldehydes by sodium hydride–iodide composite. Helv. Chim. Acta 101, e1800049 (2018).
Xiao, P. et al. Chemoselective reduction of sterically demanding N,N-diisopropylamides to aldehydes. J. Org. Chem. 83, 1687–1700 (2018).
Gausas, L., Jackstell, R., Skrydstrup, T. & Beller, M. Toward a practical catalyst for convenient deaminative hydrogenation of amides under mild conditions. ACS Sustain. Chem. Eng. 10, 16046–16054 (2022).
Arnott, G. E. in Comprehensive Organic Synthesis 2nd edn, Vol. 8 (ed. Knochel, P.) 368–409 (Elsevier, 2014).
Ohta, T., Kamiya, M., Nobutomo, M., Kusui, K. & Furukawa, I. Reduction of carboxylic acid derivatives using diphenylsilanein the presence of a Rh–PPh3 complex. Bull. Chem. Soc. Jpn. 78, 1856–1861 (2005).
Cheng, C. & Brookhart, M. Iridium-catalyzed reduction of secondary amides to secondary amines and imines by diethylsilane. J. Am. Chem. Soc. 134, 11304–11307 (2012). This article describes the reduction of secondary amides to imines using an Ir catalyst and hydrosilane as reducing reagent.
Schedler, D. J. A., Godfrey, A. G. & Ganem, B. Reductive deoxygenation by Cp2ZrHCl: selective formation of imines via zirconation/hydrozirconation of amides. Tetrahedron Lett. 34, 5035–5038 (1993).
Schedler, D. J. A., Li, J. & Ganem, B. Reduction of secondary carboxamides to imines. J. Org. Chem. 61, 4115–4119 (1996).
Donnelly, L. J., Berthet, J.-C. & Cantat, T. Selective reduction of secondary amides to imines catalysed by Schwartz’s reagent. Angew. Chem. Int. Ed. 61, e202206170 (2022).
Kehner, R. A., Zhang, G. & Bayeh-Romero, L. Mild divergent semireductive transformations of secondary and tertiary amides via zirconocene hydride catalysis. J. Am. Chem. Soc. 145, 4921–4927 (2023).
Motoyama, Y., Aoki, M., Takaoka, N., Aoto, R. & Nagashima, H. Highly efficient synthesis of aldenamines from carboxamides by iridium-catalyzed silane-reduction/dehydration under mild conditions. Chem. Commun. 45, 1574–1576 (2009).
Matheau-Raven, D. et al. Catalytic reductive functionalization of tertiary amides using Vaska’s complex: synthesis of complex tertiary amine building blocks and natural products. ACS Catal. 10, 8880–8897 (2020).
Pelletier, G., Bechara, W. S. & Charette, A. B. Controlled and chemoselective reduction of secondary amides. J. Am. Chem. Soc. 132, 12817–12819 (2010).
Yuan, M.-L., Xie, J.-H. & Zhou, Q.-L. Boron Lewis acid promoted ruthenium-catalyzed hydrogenation of amides: an efficient approach to secondary amines. ChemCatChem 8, 3036–3040 (2016). This article describes the fact that the combined use of a catalytic amount of a Ru complex, a boron Lewis acid and a Brønsted acid enables the selective reduction of amide to amine over ester.
Pan, Y. et al. Ru-catalyzed deoxygenative transfer hydrogenation of amides to amines with formic acid/triethylamine. Adv. Synth. Catal. 361, 3800–3806 (2019).
Ravn, A. K. & Rezayee, N. M. The investigation of a switchable iridium catalyst for the hydrogenation of amides: a case study of C−O versus C−N bond scission. ACS Catal. 12, 11927–11933 (2022). This article demonstrates the selective hydrogenation of formamides to N-methylated amines in the presence of esters by a cationic Ir complex.
Sitte, N. A., Bursch, M., Grimme, S. & Paradies, J. Frustrated Lewis pair catalyzed hydrogenation of amides: halides as active Lewis base in the metal-free hydrogen activation. J. Am. Chem. Soc. 141, 159–162 (2019).
Stephan, D. W. Frustrated Lewis pairs: from concept to catalysis. Acc. Chem. Res. 48, 306–316 (2015).
Köring, L., Sitte, N. A., Bursch, M., Grimme, S. & Paradies, J. Hydrogenation of secondary amides using phosphane oxide and frustrated Lewis pair catalysis. Chem. Eur. J. 27, 14179–14183 (2021).
Kadyrov, R. Hydrogenolysis of amide acetals and iminium esters. ChemCatChem 10, 170–172 (2018).
Kadyrov, R. Reduction of amides to amines under mild conditions via catalytic hydrogenation of amide acetals and imidates. Adv. Synth. Catal. 361, 185–191 (2019).
Barbe, G. & Charette, A. B. Highly chemoselective metal-free reduction of tertiary amides. J. Am. Chem. Soc. 130, 18–19 (2008).
Kuwano, R., Takahashi, M. & Ito, Y. Reduction of amides to amines via catalytic hydrosilylation by a rhodium complex. Tetrahedron Lett. 39, 1017–1020 (1998).
Gerona-Navarro, G. et al. Simple access to novel azetidine-containing conformationally restricted amino acids by chemoselective reduction of β-lactams. Tetrahedron Lett. 45, 2193–2196 (2004).
Das, S. et al. Selective rhodium-catalyzed reduction of tertiary amides in amino acid esters and peptides. Angew. Chem. Int. Ed. 54, 12389–12393 (2015).
Das, S., Li, Y., Lu, L.-Q., Junge, K. & Beller, M. A general and selective rhodium-catalyzed reduction of amides, N-acyl amino esters, and dipeptides using phenylsilane. Chem. Eur. J. 22, 7050–7053 (2016).
Sunada, Y., Kawakami, H., Imaoka, T., Motoyama, Y. & Nagashima, H. Hydrosilane reduction of tertiary carboxamides by iron carbonyl catalysts. Angew. Chem. Int. Ed. 48, 9511–9514 (2009).
Dombray, T., Helleu, C., Darcel, C. & Sortais, J.-B. Cobalt carbonyl-based catalyst for hydrosilylation of carboxamides. Adv. Synth. Catal. 355, 3358–3362 (2013).
Sasakuma, H., Motoyama, Y. & Nagashima, H. Functional group-selective poisoning of molecular catalysts: a ruthenium cluster-catalysed highly amide-selective silane reduction that does not affect ketones or esters. Chem. Commun. 43, 4916–4918 (2007).
Han, F., Lu, G.-S., Wu, D.-P. & Huang, P.-Q. Iridium and B(C6F5)3 co-catalyzed chemoselective deoxygenative reduction of tertiary amides: application to the efficient synthesis and late-stage modification of pharmaceuticals. Sci. China Chem. 66, 1094–1100 (2023).
Simmons, B. J., Hoffmann, M., Hwang, J., Jackl, M. K. & Garg, N. K. Nickel-catalyzed reduction of secondary and tertiary amides. Org. Lett. 19, 1910–1913 (2017).
Pandey, P. & Bera, J. K. Hydrosilylative reduction of primary amides to primary amines catalyzed by a terminal [Ni–OH] complex. Chem. Commun. 57, 9204–9207 (2021).
Vinayagam, V., Kumar, T. V. H., Nune, R., Karre, S. K. & Sadhukhan, S. K. Visible-light-promoted dual photoredox/nickel-catalyzed chemoselective reduction of secondary and tertiary amides with hydrosilanes in the presence of an ester. J. Org. Chem. 88, 2122–2131 (2023).
Hanada, S., Tsutsumi, E., Motoyama, Y. & Nagashima, H. Practical access to amines by platinum-catalyzed reduction of carboxamides with hydrosilanes: synergy of dual Si–H groups leads to high efficiency and selectivity. J. Am. Chem. Soc. 131, 15032–15040 (2009).
Das, S., Join, B., Junge, K. & Beller, M. A general and selective copper-catalyzed reduction of secondary amides. Chem. Commun. 48, 2683–2685 (2012).
Mikami, Y. et al. Highly efficient gold nanoparticle catalyzed deoxygenation of amides, sulfoxides, and pyridine N-oxides. Chem. Eur. J. 17, 1768–1772 (2011).
Das, S., Addis, D., Zhou, S., Junge, K. & Beller, M. Zinc-catalyzed reduction of amides: unprecedented selectivity and functional group tolerance. J. Am. Chem. Soc. 132, 1770–1771 (2010).
Hammerstad, T. A., Hegde, P. V., Wang, K. J. & Aldrich, C. C. Chemoselective reduction of tertiary amides by 1,3-diphenyldisiloxane (DPDS). Synthesis 54, 2205–2212 (2022).
Das, S., Addis, D., Junge, K. & Beller, M. Zinc-catalyzed chemoselective reduction of tertiary and secondary amides to amines. Chem. Eur. J. 17, 12186–12192 (2011).
Kornet, M. J., Thio, P. A. & Tan, S. I. The borane reduction of amido esters. J. Org. Chem. 33, 3637–3639 (1968).
Li, Y. et al. Selective reduction of amides to amines by boronic acid catalyzed hydrosilylation. Angew. Chem. Int. Ed. 52, 11577–11580 (2013).
Mukherjee, D., Shirase, S., Mashima, K. & Okuda, J. Chemoselective reduction of tertiary amides to amines catalyzed by triphenylborane. Angew. Chem. Int. Ed. 55, 13326–13329 (2016).
Lucas, K. M. et al. Versatile, mild, and selective reduction of various carbonyl groups using an electron-deficient boron catalyst. Org. Biomol. Chem. 14, 5774–5778 (2016).
Chardon, A. et al. Borinic acid catalysed reduction of tertiary amides with hydrosilanes: a mild and chemoselective synthesis of amines. Chem. Eur. J. 23, 2005–2009 (2017).
Peruzzi, M. T., Mei, Q. Q., Lee, S. J. & Gagné, M. R. Chemoselective amide reductions by heteroleptic fluoroaryl boron Lewis acids. Chem. Commun. 54, 5855–5858 (2018).
Ni, J., Oguro, T., Sawazaki, T., Sohma, Y. & Kanai, M. Hydroxy group directed catalytic hydrosilylation of amides. Org. Lett. 20, 7371–7374 (2018).
Huang, P.-Q., Lang, Q.-W. & Wang, Y.-R. Mild metal-free hydrosilylation of secondary amides to amines. J. Org. Chem. 81, 4235–4243 (2016).
Fukuyama, T., Lin, S.-C. & Li, L. Facile reduction of ethyl thiol esters to aldehydes: application to a total synthesis of (+)-neothramycin A methyl ether. J. Am. Chem. Soc. 112, 7050–7051 (1990).
Nakanishi, J., Tatamidani, H., Fukumoto, Y. & Chatani, N. A new synthesis of aldehydes by the palladium-catalyzed reaction of 2-pyridinyl esters with hydrosilanes. Synlett 2006, 869–872 (2006).
Parks, D. J. & Piers, W. E. Tris(pentafluorophenyl)boron-catalyzed hydrosilation of aromatic aldehydes, ketones, and esters. J. Am. Chem. Soc. 118, 9440–9441 (1996). This article demonstrates the conversion of esters to aldehydes by boron-catalysed hydrosilylation.
Parks, D. J., Blackwell, J. M. & Piers, W. E. Studies on the mechanism of B(C6F5)3-catalyzed hydrosilation of carbonyl functions. J. Org. Chem. 65, 3090–3098 (2000).
Igarashi, M., Mizuno, R. & Fuchikami, T. Ruthenium complex catalyzed hydrosilylation of esters: a facile transformation of esters to alkyl silyl acetals and aldehydes. Tetrahedron Lett. 42, 2149–2151 (2001).
Cheng, C. & Brookhart, M. Efficient reduction of esters to aldehydes through iridium-catalyzed hydrosilylation. Angew. Chem. Int. Ed. 51, 9422–9424 (2012).
Li, H. et al. Selective reduction of esters to aldehydes under the catalysis of well-defined NHC–iron complexes. Angew. Chem. Int. Ed. 52, 8045–8049 (2013).
Xu, P., Liu, S. & Huang, Z. Desymmetric partial reduction of malonic esters. J. Am. Chem. Soc. 144, 6918–6927 (2022).
Zakharkin, L. I. & Khorlina, I. M. Reduction of esters of carboxylic acids into aldehydes with diisobutylaluminium hydride. Tetrahedron Lett. 3, 619–620 (1962).
Zakharkin, L. I., Gavrilenko, V. V., Maslin, D. N. & Khorlina, I. M. The preparation of aldehydes by reduction of esters of carboxylic acids with sodium aluminium hydride. Tetrahedron Lett. 4, 2087–2090 (1963).
Weissman, P. M. & Brown, H. C. Selective reductions. VI. The reaction of lithium tri-t-butoxyaluminohydride with phenolic esters. A new aldehyde synthesis. J. Org. Chem. 31, 283–287 (1966).
Muraki, M. & Mukaiyama, T. Aminoaluminum hydride as new reducing agents. II. Selective reduction of esters of carboxylic acids to aldehydes. Chem. Lett. 4, 215–218 (1975).
Kanazawa, R. & Tokoroyama, T. Modified sodium bis[2-methoxyethoxy]aluminum hydride reagents for the partial reduction of lactones and esters. Synthesis 1976, 526–527 (1976).
Abe, T. et al. Large scale synthesis of N-benzyl-4-formylpiperidine through partial reduction of esters using aluminum hydride reagents modified with pyrrolidine. Tetrahedron 57, 2701–2710 (2001).
Song, J. I. & An, D. K. New method for synthesis of aldehydes from esters by sodium diisobutyl-t-butoxyaluminum hydride. Chem. Lett. 36, 886–887 (2007).
Otsuka, T., Ishii, A., Dub, P. A. & Ikariya, T. Practical selective hydrogenation of α-fluorinated esters with bifunctional pincer-type ruthenium(II) catalysts leading to fluorinated alcohols or fluoral hemiacetals. J. Am. Chem. Soc. 135, 9600–9603 (2013).
Luche, J.-L. & Gemal, A. L. Lanthanoids in organic synthesis. 5. Selective reductions of ketones in the presence of aldehydes. J. Am. Chem. Soc. 101, 5848–5849 (1979).
Paradisi, M. P., Zecchini, G. P. & Ortar, G. A convenient one-pot procedure for the selective reduction of ketones in the presence of aldehydes. Tetrahedron Lett. 21, 5085–5088 (1980).
Bastug, G., Dierick, S., Lebreux, F. & Markó, I. E. Highly chemoselective reduction of carbonyl groups in the presence of aldehydes. Org. Lett. 14, 1306–1309 (2012).
Fujioka, H. et al. Reversing the reactivity of carbonyl functions with phosphonium salts: enantioselective total synthesis of (+)-centrolobine. Angew. Chem. Int. Ed. 50, 12232–12235 (2011).
Yahata, K., Minami, M., Yoshikawa, Y., Watanabe, K. & Fujioka, H. Methodology for in situ protection of aldehydes and ketones using trimethylsilyl trifluoromethanesulfonate and phosphines: selective alkylation and reduction of ketones, esters, amides, and nitriles. Chem. Pharm. Bull. 61, 1298–1307 (2013).
Fujioka, H., Morita, K., Murai, K. & Arisawa, M. Selective reduction and dihydroxylation of α,β-unsaturated esters in the presence of enals: one-pot synthesis of a 2,5-disubstituted tetrahydrofuran. Heterocycles 103, 311–321 (2021).
Maruoka, K., Saito, S., Concepcion, A. B. & Yamamoto, H. Chemo-selective functionalization of more hindered aldehyde carbonyls with the methylaluminum bis(2,6-diphenylphenoxide)/alkyllithium system. J. Am. Chem. Soc. 115, 1183–1184 (1993).
Takahashi, Y., Mitsudome, T., Mizugaki, T., Jitsukawa, K. & Kaneda, K. Highly atom-efficient and chemoselective reduction of ketones in the presence of aldehydes using heterogeneous catalysts. Green Chem. 15, 2695–2698 (2013).
Deutsch, C. & Krause, N. CuH-catalyzed reactions. Chem. Rev. 108, 2916–2927 (2008).
Fujihara, T., Semba, K., Terao, J. & Tsuji, Y. Copper-catalyzed hydrosilylation with a bowl-shaped phosphane ligand: preferential reduction of a bulky ketone in the presence of an aldehyde. Angew. Chem. Int. Ed. 49, 1472–1476 (2010). This article demonstrates a unique ligand effect of a bowl-shaped phosphine on the selective reduction of sterically bulky ketones over aldehydes.
Call, A., Casadevall, C., Acuña-Parés, F., Casitas, A. & Lloret-Fillol, J. Dual cobalt–copper light-driven catalytic reduction of aldehydes and aromatic ketones in aqueous media. Chem. Sci. 8, 4739–4749 (2017). This article describes the selective photoinduced reduction of ketones over aldehydes using a Co and Cu binary catalyst, water as hydride and proton source, and a tertiary amine as terminal reductant.
Roth, H. G., Romero, N. A. & Nicewicz, D. A. Experimental and calculated electrochemical potentials of common organic molecules for applications to single-electron redox chemistry. Synlett 27, 714–723 (2016).
Fokin, I., Kuessner, K.-T. & Siewert, I. Transition metal complex catalyzed photo- and electrochemical (de)hydrogenations involving C=O and C=N bonds. Synthesis 54, 295–314 (2022).
Acknowledgements
This work was supported by the Grant-in-Aid for Scientific Research(B) (Nos. JP23H01955 and JP23K26648) from the Japan Society for the Promotion of Science; the Grant-in-Aid for Transformative Research Areas (A) JP21A204 in Digitalization-driven Transformative Organic Synthesis (Digi-TOS) (Nos. JP22H05340 and JP24H01061) from the Ministry of Education, Culture, Sports, Science and Technology; JST ERATO (No. JPMJER2103) from the Japan Science and Technology Agency; Sumitomo Foundation; and Fujimori Science and Technology Foundation.
Author information
Authors and Affiliations
Contributions
T.I. contributed to writing and editing this manuscript. K.N. contributed to reviewing and editing this manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Jianliang Xiao, Rosa Adam Ortiz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hydrogenation
-
Reaction involving the addition of hydrogen gas (H2) to multiple bonds.
- Hydrogenolysis
-
Bond cleavage reaction via addition of hydrogen gas (H2) to a carbon–heteroatom single bond.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iwasaki, T., Nozaki, K. Counterintuitive chemoselectivity in the reduction of carbonyl compounds. Nat Rev Chem 8, 518–534 (2024). https://doi.org/10.1038/s41570-024-00608-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41570-024-00608-z