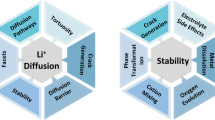
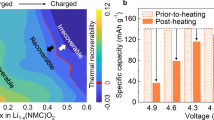
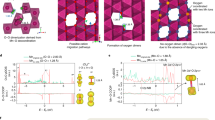
Abstract
The fact that ordered materials are rarely perfectly crystalline is widely acknowledged among materials scientists, but its impact is often overlooked or underestimated when studying how structure relates to properties. Various investigations demonstrate that intrinsic and extrinsic defects, and disorder generated by physicochemical reactions, are responsible for unexpectedly detrimental or beneficial functionalities. The task remains to modulate the disorder to produce desired properties in materials. As disorder is often correlated with local interactions, it is controllable. In this Review, we explore the structural disorder in cathode materials as a novel approach for improving their electrochemical performance. We revisit cathode materials for alkali-ion batteries and outline the origins and beneficial consequences of disorder. Focusing on layered, cubic rocksalt and other metal oxides, we discuss how disorder improves electrochemical properties of cathode materials and which interactions generate the disorder. We also present the potential pitfalls of disorder that must be considered. We conclude with perspectives for enhancing the electrochemical performance of cathode materials by using disorder.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Bragg, W. L. The structure of some crystals as indicated by their diffraction of X-rays. Proc. R. Soc. A 89, 248–277 (1913).
Rietveld, H. M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 2, 65–71 (1969).
Reynaud, M., Serrano-Sevillano, J. & Casas-Cabanas, M. Imperfect battery materials: a closer look at the role of defects in electrochemical performance. Chem. Mater. 35, 3345–3363 (2023). This paper categorizes various defects in battery materials.
Simonov, A. & Goodwin, A. L. Designing disorder into crystalline materials. Nat. Rev. Chem. 4, 657–673 (2020). This paper highlights the design principles to control the correlated disorder in a wide range of materials.
Yu, S., Qiu, C.-W., Chong, Y., Torquato, S. & Park, N. Engineered disorder in photonics. Nat. Rev. Mater. 6, 226–243 (2021).
Bennett, T. D., Cheetham, A. K., Fuchs, A. H. & Coudert, F.-X. Interplay between defects, disorder and flexibility in metal-organic frameworks. Nat. Chem. 9, 11–16 (2017).
He, Z., Taniyama, T., Kyômen, T. & Itoh, M. Field-induced order-disorder transition in the quasi-one-dimensional anisotropic antiferromagnet BaCo2V2O8. Phys. Rev. B 72, 172403 (2005).
Raja, A. et al. Dielectric disorder in two-dimensional materials. Nat. Nanotechnol. 14, 832–837 (2019).
Yu, Z. et al. Pressure effect on order–disorder ferroelectric transition in a hydrogen-bonded metal–organic framework. J. Phys. Chem. Lett. 11, 9566–9571 (2020).
Maier, J. Review — battery materials: why defect chemistry? J. Electrochem. Soc. 162, A2380–A2386 (2015).
Shan, X. et al. Structural water and disordered structure promote aqueous sodium-ion energy storage in sodium-birnessite. Nat. Commun. 10, 4975 (2019).
Gao, A. et al. In operando visualization of cation disorder unravels voltage decay in Ni‐rich cathodes. Small Methods 5, 2000730 (2021).
Ashton, T. E. et al. Stoichiometrically driven disorder and local diffusion in NMC cathodes. J. Mater. Chem. A 9, 10477–10486 (2021).
Zhu, H. et al. Bridging structural inhomogeneity to functionality: pair distribution function methods for functional materials development. Adv. Sci. 8, 2003534 (2021).
Huang, W. et al. Elastic lattice enabling reversible tetrahedral Li storage sites in a high‐capacity manganese oxide cathode. Adv. Mater. 34, 2202745 (2022).
Szymanski, N. J. et al. Modeling short-range order in disordered rocksalt cathodes by pair distribution function analysis. Chem. Mater. 35, 4922–4934 (2023).
Bragg, W. L. & Williams, E. J. The effect of thermal agitation on atomic arrangement in alloys. Proc. R. Soc. A 145, 699–730 (1934).
Bethe, H. A. Statistical theory of superlattices. Proc. R. Soc. A 150, 552–575 (1935).
Onsager, L. Crystal statistics. I. A two-dimensional model with an order-disorder transition. Phys. Rev. 65, 117–149 (1944).
Cowley, J. M. An approximate theory of order in alloys. Phys. Rev. 77, 669–675 (1950).
Welberry, T. R. & Butler, B. D. Diffuse X-ray scattering from disordered crystals. Chem. Rev. 95, 2369–2403 (1995).
Keen, D. A. & Goodwin, A. L. The crystallography of correlated disorder. Nature 521, 303–309 (2015). This paper first introduces the concept of correlated disorder of solid-state materials.
Moessner, R. & Ramirez, A. P. Geometrical frustration. Phys. Today 59, 24–29 (2006).
Snyder, J., Slusky, J. S., Cava, R. J. & Schiffer, P. How ‘spin ice’ freezes. Nature 413, 48–51 (2001).
Fennell, T. et al. Magnetic Coulomb phase in the spin ice Ho2Ti2O7. Science 326, 415–417 (2009).
Fabrèges, X. et al. Spin-lattice coupling, frustration, and magnetic order in multiferroic R MnO3. Phys. Rev. Lett. 103, 067204 (2009).
Wen, J.-J. et al. Disordered route to the Coulomb quantum spin liquid: random transverse fields on spin ice in Pr2Zr2O7. Phys. Rev. Lett. 118, 107206 (2017).
Salzmann, C. G., Radaelli, P. G., Slater, B. & Finney, J. L. The polymorphism of ice: five unresolved questions. Phys. Chem. Chem. Phys. 13, 18468–18480 (2011).
Playford, H. Y., Whale, T. F., Murray, B. J., Tucker, M. G. & Salzmann, C. G. Analysis of stacking disorder in ice I using pair distribution functions. J. Appl. Crystallogr. 51, 1211–1220 (2018).
Bramwell, S. T. & Gingras, M. J. P. Spin ice state in frustrated magnetic pyrochlore materials. Science 294, 1495–1501 (2001).
Düvel, A. et al. Is geometric frustration-induced disorder a recipe for high ionic conductivity? J. Am. Chem. Soc. 139, 5842–5848 (2017).
Di Stefano, D. et al. Superionic diffusion through frustrated energy landscape. Chem 5, 2450–2460 (2019).
Wang, S., Liu, Y. & Mo, Y. Frustration in super‐ionic conductors unraveled by the density of atomistic states. Angew. Chem. 135, e202215544 (2023).
Hsu, W.-L., Tsai, C.-W., Yeh, A.-C. & Yeh, J.-W. Clarifying the four core effects of high-entropy materials. Nat. Rev. Chem. 8, 471–485 (2024).
Aubry, S. & Hughes, D. A. Reductions in stacking fault widths in fcc crystals: semiempirical calculations. Phys. Rev. B 73, 224116 (2006).
Baruffi, C., Ghazisaeidi, M., Rodney, D. & Curtin, W. A. Equilibrium versus non-equilibrium stacking fault widths in NiCoCr. Scr. Mater. 235, 115536 (2023).
Coles, S. W. et al. Anion-polarisation-directed short-range-order in antiperovskite Li2FeSO. J. Mater. Chem. A 11, 13016–13026 (2023).
Uemura, N., Shirai, K., Eckert, H. & Kunstmann, J. Structure, nonstoichiometry, and geometrical frustration of α-tetragonal boron. Phys. Rev. B 93, 104101 (2016).
Goldschmidt, V. M. Die Gesetze der Krystallochemie. Naturwissenschaften 14, 477–485 (1926).
Pauling, L. The principles determining the structure of complex ionic crystals. J. Am. Chem. Soc. 51, 1010–1026 (1929).
George, J. et al. The limited predictive power of the Pauling rules. Angew. Chem. Int. Ed. 59, 7569–7575 (2020).
Delmas, C., Fouassier, C. & Hagenmuller, P. Structural classification and properties of the layered oxides. Physica B+C 99, 81–85 (1980).
Brunel, M., De Bergevin, F. & Gondrand, M. Determination theorique et domaines d’existence des differentes surstructures dans les composes A3+B1+X22− de type NaCl. J. Phys. Chem. Solids 33, 1927–1941 (1972).
Hauck, J. Short-range order and superstructures of ternary oxides AMO2, A2MO3 and A5MO6 of monovalent A and multivalent M metals related to the NaCl structure. Acta Crystallogr. A 36, 228–237 (1980).
Jacquet, Q. et al. Electrostatic interactions versus second order Jahn–Teller distortion as the source of structural diversity in Li3MO4 compounds (M = Ru, Nb, Sb and Ta). Chem. Mater. 30, 392–402 (2018). This paper compares the effect of electrostatic interactions and pseudo Jahn–Teller (second order Jahn–Teller) distortions on the stability of rocksalt-type structures.
Mather, G. C., Dussarrat, C., Etourneau, J. & West, A. R. A review of cation-ordered rock salt superstructure oxides. J. Mater. Chem. 10, 2219–2230 (2000).
Yang, R. et al. Intercalation in 2D materials and in-situ studies. Nat. Rev. Chem. 8, 410–432 (2024).
Clément, R. J., Lun, Z. & Ceder, G. Cation-disordered rocksalt transition metal oxides and oxyfluorides for high energy lithium-ion cathodes. Energy Environ. Sci. 13, 345–373 (2020).
Hewston, T. A. & Chamberland, B. L. A Survey of first-row ternary oxides LiMO2 (M = Sc-Cu). J. Phys. Chem. Solids 48, 97–108 (1987).
Wu, E. J., Tepesch, P. D. & Ceder, G. Size and charge effects on the structural stability of LiMO2 (M = transition metal) compounds. Philos. Mag. B 77, 1039–1047 (1998).
Dutta, G., Manthiram, A., Goodenough, J. B. & Grenier, J.-C. Chemical synthesis and properties of Li1−δ−xNi1+δO2 and Li[Ni2]O4. J. Solid State Chem. 96, 123–131 (1992).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Lee, K.-S., Myung, S.-T., Amine, K., Yashiro, H. & Sun, Y.-K. Structural and electrochemical properties of layered Li[Ni1−2xCoxMnx]O2 (x = 0.1–0.3) positive electrode materials for Li-Ion batteries. J. Electrochem. Soc. 154, A971 (2007).
Lee, W. et al. New insight into Ni‐Rich layered structure for next‐generation li rechargeable batteries. Adv. Energy Mater. 8, 1701788 (2018).
Liang, C. et al. Unraveling the origin of instability in Ni-Rich LiNi1–2xCoxMnxO2 (NCM) cathode materials. J. Phys. Chem. C 120, 6383–6393 (2016).
Hahn, B. P., Long, J. W. & Rolison, D. R. Something from nothing: enhancing electrochemical charge storage with cation vacancies. Acc. Chem. Res. 46, 1181–1191 (2013).
Ruetschi, P. Cation‐vacancy model for MnO2. J. Electrochem. Soc. 131, 2737–2744 (1984).
Bouet, L., Tailhades, P., Rousset, A., Domenichini, B. & Gillot, B. Mixed valence states of iron and molybdenum ions in MoxFe3−xO4 magnetites and related cation deficient ferrites. Solid State Ion. 52, 285–286 (1992).
Bach, S. et al. Electrochemical proton insertion in Mn2.2Co0.27O4 from aqueous borate solution. Electrochim. Acta 45, 931–934 (1999).
Ménétrier, M., Shao-Horn, Y., Wattiaux, A., Fournès, L. & Delmas, C. Iron substitution in lithium-overstoichiometric “Li1.1CoO2”: combined 57Fe Mössbauer and 7Li NMR spectroscopies studies. Chem. Mater. 17, 4653–4659 (2005).
Li, W. et al. High substitution rate in TiO2 anatase nanoparticles with cationic vacancies for fast lithium storage. Chem. Mater. 27, 5014–5019 (2015).
Ma, J. et al. Layered lepidocrocite type structure isolated by revisiting the sol–gel chemistry of anatase TiO2: a new anode material for batteries. Chem. Mater. 29, 8313–8324 (2017).
Miyamoto, N. & Yamamoto, S. in Supra-Materials Nanoarchitectonics Ch. 7 (eds Ariga, K. & Aono, M.) 173–192 (Elsevier, 2017).
Nam, K. W. et al. The high performance of crystal water containing manganese birnessite cathodes for magnesium batteries. Nano Lett. 15, 4071–4079 (2015).
Ferrage, E. Investigation of the interlayer organization of water and ions in smectite from the combined use of diffraction experiments and molecular simulations: a review of methodology, applications, and perspectives. Clays Clay Miner. 64, 348–373 (2016).
Yang, J. et al. Size‐independent fast ion intercalation in two‐dimensional titania nanosheets for alkali‐metal‐ion batteries. Angew. Chem. Int. Ed. 58, 8740–8745 (2019).
Zheng, J. et al. Ni/Li disordering in layered transition metal oxide: electrochemical impact, origin, and control. Acc. Chem. Res. 52, 2201–2209 (2019).
Kanamori, J. Superexchange interaction and symmetry properties of electron orbitals. J. Phys. Chem. Solids 10, 87–98 (1959).
Goodenough, J. B. Magnetism and the Chemical Bond (R. E. Krieger, 1976).
Zheng, J. et al. Role of superexchange interaction on tuning of Ni/Li disordering in layered Li(NixMnyCoz)O2. J. Phys. Chem. Lett. 8, 5537–5542 (2017).
Bersuker, I. B. Jahn–Teller and pseudo-Jahn–Teller effects: from particular features to general tools in exploring molecular and solid state properties. Chem. Rev. 121, 1463–1512 (2021).
Freitag, R. & Conradie, J. Understanding the Jahn–Teller effect in octahedral transition-metal complexes: a molecular orbital view of the Mn(β-diketonato)3 complex. J. Chem. Educ. 90, 1692–1696 (2013).
Armstrong, A. R. & Bruce, P. G. Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 381, 499–500 (1996).
Manzi, J. et al. Monoclinic and orthorhombic NaMnO2 for secondary batteries: a comparative study. Energies 14, 1230 (2021).
Liu, T. et al. Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery. Nat. Commun. 10, 4721 (2019).
Asl, H. Y. & Manthiram, A. Reining in dissolved transition-metal ions. Science 369, 140–141 (2020).
Yaghoobnejad Asl, H. & Manthiram, A. Proton-induced disproportionation of Jahn–Teller-active transition-metal ions in oxides due to electronically driven lattice instability. J. Am. Chem. Soc. 142, 21122–21130 (2020).
Bersuker, I. B. Pseudo Jahn-Teller origin of perovskite multiferroics, magnetic-ferroelectric crossover, and magnetoelectric effects: the d0−d10 problem. Phys. Rev. Lett. 108, 137202 (2012).
Urban, A., Abdellahi, A., Dacek, S., Artrith, N. & Ceder, G. Electronic-structure origin of cation disorder in transition-metal oxides. Phys. Rev. Lett. 119, 176402 (2017).
Zhang, J. et al. Regulating pseudo-Jahn–Teller effect and superstructure in layered cathode materials for reversible alkali-ion intercalation. J. Am. Chem. Soc. 144, 7929–7938 (2022).
Delmas, C., Carlier, D. & Guignard, M. The layered oxides in lithium and sodium‐ion batteries: a solid‐state chemistry approach. Adv. Energy Mater. 11, 2001201 (2021).
Reed, J. & Ceder, G. Charge, potential, and phase stability of layered Li(Ni0.5Mn0.5)O2. Electrochem. Solid-State Lett. 5, A145–A148 (2002).
Reed, J. & Ceder, G. Role of electronic structure in the susceptibility of metastable transition-metal oxide structures to transformation. Chem. Rev. 104, 4513–4534 (2004).
Ma, X., Kang, K., Ceder, G. & Meng, Y. S. Synthesis and electrochemical properties of layered LiNi2/3Sb1/3O2. J. Power Sources 173, 550–555 (2007).
Vinckevičiūtė, J., Radin, M. D., Faenza, N. V., Amatucci, G. G. & Van Der Ven, A. Fundamental insights about interlayer cation migration in Li-ion electrodes at high states of charge. J. Mater. Chem. A 7, 11996–12007 (2019).
Radin, M. D. et al. Narrowing the gap between theoretical and practical capacities in Li‐ion layered oxide cathode materials. Adv. Energy Mater. 7, 1602888 (2017).
Okubo, M. & Yamada, A. Molecular orbital principles of oxygen-redox battery electrodes. ACS Appl. Mater. Interfaces 9, 36463–36472 (2017).
Abakumov, A. M., Fedotov, S. S., Antipov, E. V. & Tarascon, J.-M. Solid state chemistry for developing better metal-ion batteries. Nat. Commun. 11, 4976 (2020).
Li, B. & Tarascon, J.-M. in Comprehensive Inorganic Chemistry III (eds Reedijk, J. & Poeppelmeier, K. R.) 6–45 (Elsevier, 2023).
Ben Yahia, M., Vergnet, J., Saubanère, M. & Doublet, M.-L. Unified picture of anionic redox in Li/Na-ion batteries. Nat. Mater. 18, 496–502 (2019).
Mizushima, K., Jones, P. C., Wiseman, P. J. & Goodenough, J. B. LixCoO2 (0 < x < −1): a new cathode material for batteries of high energy density. Mater. Res. Bull. 15, 783–789 (1980).
Rozier, P. & Tarascon, J. M. Review—Li-rich layered oxide cathodes for next-generation Li-ion batteries: chances and challenges. J. Electrochem. Soc. 162, A2490–A2499 (2015).
Molenda, J., Wilk, P. & Marzec, J. Structural, electrical and electrochemical properties of LiNiO2. Solid State Ion. 146, 73–79 (2002).
Huang, Z.-F., Meng, X., Wang, C.-Z., Sun, Y. & Chen, G. First-principles calculations on the Jahn–Teller distortion in layered LiMnO2. J. Power Sources 158, 1394–1400 (2006).
Yabuuchi, N. & Ohzuku, T. Novel lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries. J. Power Sources 119–121, 171–174 (2003).
Yoon, C. S. et al. Extracting maximum capacity from Ni-rich Li[Ni0.95Co0.025Mn0.025]O2 cathodes for high-energy-density lithium-ion batteries. J. Mater. Chem. A 6, 4126–4132 (2018).
Thackeray, M. M., Johnson, C. S., Vaughey, J. T., Li, N. & Hackney, S. A. Advances in manganese-oxide ‘composite’ electrodes for lithium-ion batteries. J. Mater. Chem. 15, 2257 (2005).
Strobel, P. & Lambert-Andron, B. Crystallographic and magnetic structure of Li2MnO3. J. Solid State Chem. 75, 90–98 (1988).
Yabuuchi, N. et al. High-capacity electrode materials for rechargeable lithium batteries: Li3NbO4 -based system with cation-disordered rocksalt structure. Proc. Natl Acad. Sci. USA 112, 7650–7655 (2015).
Kobayashi, T. et al. Nanosize cation‐disordered rocksalt oxides: Na2TiO3–NaMnO2 binary system. Small 16, 1902462 (2020).
Koketsu, T. et al. Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2. Nat. Mater. 16, 1142–1148 (2017).
Boyd, S. et al. Effects of interlayer confinement and hydration on capacitive charge storage in birnessite. Nat. Mater. 20, 1689–1694 (2021).
Stiles, J. W., McClure, E. T., Bashian, N. H., Tappan, B. A. & Melot, B. C. Reversible intercalation of Li ions in an earth-abundant phyllosilicate clay. Inorg. Chem. 61, 5757–5761 (2022).
Wu, V. C. et al. Unlocking new redox activity in alluaudite cathodes through compositional design. Chem. Mater. 34, 4088–4103 (2022).
Van Der Ven, A., Bhattacharya, J. & Belak, A. A. Understanding Li diffusion in Li-intercalation compounds. Acc. Chem. Res. 46, 1216–1225 (2013).
Abdellahi, A., Urban, A., Dacek, S. & Ceder, G. The effect of cation disorder on the average Li intercalation voltage of transition-metal oxides. Chem. Mater. 28, 3659–3665 (2016).
Abdellahi, A., Urban, A., Dacek, S. & Ceder, G. Understanding the effect of cation disorder on the voltage profile of lithium transition-metal oxides. Chem. Mater. 28, 5373–5383 (2016). This paper clarifies the causal relationship between cation disorder and the voltage profile based on Li–vacancy interactions.
Kitchaev, D. A. et al. Design principles for high transition metal capacity in disordered rocksalt Li-ion cathodes. Energy Environ. Sci. 11, 2159–2171 (2018).
Lee, G., Lau, V. W., Yang, W. & Kang, Y. Utilizing oxygen redox in layered cathode materials from multiscale perspective. Adv. Energy Mater. 11, 2003227 (2021).
Lin, T., Seaby, T., Huang, X. & Wang, L. On the disparity in reporting Li-rich layered oxide cathode materials. Chem. Commun. 59, 2888–2902 (2023).
Zhang, M. et al. Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Nat. Rev. Mater. 7, 522–540 (2022).
Sathiya, M. et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 12, 827–835 (2013).
Seo, D.-H. et al. The structural and chemical origin of the oxygen redox activity in layered and cation-disordered Li-excess cathode materials. Nat. Chem. 8, 692–697 (2016).
Perez, A. J. et al. Approaching the limits of cationic and anionic electrochemical activity with the Li-rich layered rocksalt Li3IrO4. Nat. Energy 2, 954–962 (2017).
Song, J. et al. A high‐performance Li–Mn–O Li‐rich cathode material with rhombohedral symmetry via intralayer Li/Mn disordering. Adv. Mater. 32, 2000190 (2020).
Ning, F. et al. Inhibition of oxygen dimerization by local symmetry tuning in Li-rich layered oxides for improved stability. Nat. Commun. 11, 4973 (2020). This paper demonstrates that intralayer Li/Ru mixing suppresses oxygen dimerization and subsequent oxygen loss.
McColl, K. et al. Transition metal migration and O2 formation underpin voltage hysteresis in oxygen-redox disordered rocksalt cathodes. Nat. Commun. 13, 5275 (2022). This paper elucidates that TM migration is necessary for the formation of O2 trapped in the bulk.
Cao, X. et al. Stabilizing anionic redox chemistry in a Mn‐based layered oxide cathode constructed by Li‐deficient pristine state. Adv. Mater. 33, 2004280 (2021).
Yabuuchi, N. et al. A new electrode material for rechargeable sodium batteries: P2-type Na2/3[Mg0.28Mn0.72]O2 with anomalously high reversible capacity. J. Mater. Chem. A 2, 16851–16855 (2014).
Maitra, U. et al. Oxygen redox chemistry without excess alkali-metal ions in Na2/3[Mg0.28Mn0.72]O2. Nat. Chem. 10, 288–295 (2018).
Dai, K. et al. High reversibility of lattice oxygen redox quantified by direct bulk probes of both anionic and cationic redox reactions. Joule 3, 518–541 (2019).
Wang, Y. et al. Ultralow‐strain Zn‐substituted layered oxide cathode with suppressed P2–O2 transition for stable sodium ion storage. Adv. Funct. Mater. 30, 1910327 (2020).
Zheng, W. et al. Oxygen redox activity with small voltage hysteresis in Na0.67Cu0.28Mn0.72O2 for sodium-ion batteries. Energy Storage Mater. 28, 300–306 (2020).
Song, B. et al. Understanding the low-voltage hysteresis of anionic redox in Na2Mn3O7. Chem. Mater. 31, 3756–3765 (2019).
Gent, W. E. et al. Coupling between oxygen redox and cation migration explains unusual electrochemistry in lithium-rich layered oxides. Nat. Commun. 8, 2091 (2017).
Lai, Y. et al. Ion‐migration mechanism: an overall understanding of anionic redox activity in metal oxide cathodes of Li/Na‐ion batteries. Adv. Mater. 34, 2206039 (2022).
Li, N. et al. Unraveling the cationic and anionic redox reactions in a conventional layered oxide cathode. ACS Energy Lett. 4, 2836–2842 (2019).
Lebens-Higgins, Z. W. et al. Revisiting the charge compensation mechanisms in LiNi0.8Co0.2−yAlyO2 systems. Mater. Horiz. 6, 2112–2123 (2019).
Lee, G. et al. Reversible anionic redox activities in conventional LiNi1/3Co1/3Mn1/3O2 cathodes. Angew. Chem. Int. Ed. 59, 8681–8688 (2020).
Zhuo, Z. et al. Cycling mechanism of Li2MnO3: Li–CO2 batteries and commonality on oxygen redox in cathode materials. Joule 5, 975–997 (2021).
Lee, H. J., Shin, J. & Choi, J. W. Intercalated water and organic molecules for electrode materials of rechargeable batteries. Adv. Mater. 30, 1705851 (2018).
Mitchell, J. B., Chagnot, M. & Augustyn, V. Hydrous transition metal oxides for electrochemical energy and environmental applications. Annu. Rev. Mater. Res. 53, 1–23 (2023).
Yang, J. H., Kim, H. & Ceder, G. Insights into layered oxide cathodes for rechargeable batteries. Molecules 26, 3173 (2021).
Zhao, F. et al. Synergistically controlled mechanism of sodium birnessite with a larger interlayer distance for fast ion intercalation toward sodium-ion batteries. J. Phys. Chem. C 124, 28431–28436 (2020).
Kundu, D., Adams, B. D., Duffort, V., Vajargah, S. H. & Nazar, L. F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 1, 16119 (2016).
Charles, D. S. et al. Structural water engaged disordered vanadium oxide nanosheets for high capacity aqueous potassium-ion storage. Nat. Commun. 8, 15520 (2017).
Nam, K. W., Kim, H., Choi, J. H. & Choi, J. W. Crystal water for high performance layered manganese oxide cathodes in aqueous rechargeable zinc batteries. Energy Environ. Sci. 12, 1999–2009 (2019).
Levi, E., Gofer, Y. & Aurbach, D. On the way to rechargeable Mg batteries: the challenge of new cathode materials. Chem. Mater. 22, 860–868 (2010).
Lukatskaya, M. R. et al. Ultra-high-rate pseudocapacitive energy storage in two-dimensional transition metal carbides. Nat. Energy 2, 17105 (2017).
Zhang, K. et al. Pseudocapacitive behavior and ultrafast kinetics from solvated ion cointercalation into MoS2 for its alkali ion storage. ACS Appl. Energy Mater. 2, 3726–3735 (2019).
Park, J., Kim, S. J., Lim, K., Cho, J. & Kang, K. Reconfiguring sodium intercalation process of TiS2 electrode for sodium-ion batteries by a partial solvent cointercalation. ACS Energy Lett. 7, 3718–3726 (2022).
Reimers, J. N., Rossen, E., Jones, C. D. & Dahn, J. R. Structure and electrochemistry of LixFeyNi1-yO2. Solid State Ion. 61, 335–344 (1993).
Lee, J. et al. Unlocking the potential of cation-disordered oxides for rechargeable lithium batteries. Science 343, 519–522 (2014). This paper explores possible local Li diffusion and long-range percolation paths in rocksalt materials.
Ruetschi, P. & Giovanoli, R. Cation vacancies in MnO2 and their influence on electrochemical reactivity. J. Electrochem. Soc. 135, 2663–2669 (1988).
Powell, M. J. Site percolation in randomly packed spheres. Phys. Rev. B 20, 4194–4198 (1979).
Reimers, J. N. & Dahn, J. R. Electrochemical and in situ x‐ray diffraction studies of lithium intercalation in LixCoO2. J. Electrochem. Soc. 139, 2091–2097 (1992).
Shao-Horn, Y., Levasseur, S., Weill, F. & Delmas, C. Probing lithium and vacancy ordering in O3 layered LixCoO2 ( x ≈ 0.5 ) : an electron diffraction study. J. Electrochem. Soc. 150, A366–A373 (2003).
Marianetti, C. A., Kotliar, G. & Ceder, G. A first-order Mott transition in LixCoO2. Nat. Mater. 3, 627–631 (2004).
Wolverton, C. & Zunger, A. First-principles prediction of vacancy order-disorder and intercalation battery voltages in LixCoO2. Phys. Rev. Lett. 81, 606–609 (1998).
Van Der Ven, A., Aydinol, M. K., Ceder, G., Kresse, G. & Hafner, J. First-principles investigation of phase stability in LixCoO2. Phys. Rev. B 58, 2975–2987 (1998).
Lee, W. et al. Advances in the cathode materials for lithium rechargeable batteries. Angew. Chem. Int. Ed. 59, 2578–2605 (2020).
Zou, L. et al. Revealing cycling rate-dependent structure evolution in Ni-rich layered cathode materials. ACS Energy Lett. 3, 2433–2440 (2018).
Zhang, H. et al. Layered oxide cathodes for Li-ion batteries: oxygen loss and vacancy evolution. Chem. Mater. 31, 7790–7798 (2019).
Zhao, R. et al. The origin of heavy element doping to relieve the lattice thermal vibration of layered materials for high energy density Li ion cathodes. J. Mater. Chem. A 8, 12424–12435 (2020).
Oh, P. et al. New ion substitution method to enhance electrochemical reversibility of co‐rich layered materials for Li‐ion batteries. Adv. Energy Mater. 13, 2202237 (2023).
Chen, J. et al. Structure/interface coupling effect for high‐voltage LiCoO2 cathodes. Adv. Mater. 34, 2204845 (2022).
Zhao, W. et al. Optimized Al doping improves both interphase stability and bulk structural integrity of Ni-Rich NMC cathode materials. ACS Appl. Energy Mater. 3, 3369–3377 (2020).
Nayak, P. K. et al. Al doping for mitigating the capacity fading and voltage decay of layered Li and Mn-rich cathodes for Li-ion batteries. Adv. Energy Mater. 6, 1502398 (2016).
Eum, D. et al. Voltage decay and redox asymmetry mitigation by reversible cation migration in lithium-rich layered oxide electrodes. Nat. Mater. 19, 419–427 (2020).
Huang, J. et al. Inhibiting collective cation migration in Li-rich cathode materials as a strategy to mitigate voltage hysteresis. Nat. Mater. 22, 353–361 (2023). This paper proposes cation-disordered structures to enhance reversibility, effectively constraining the collective effects of cation migration.
Zhai, X.-Z. et al. Layered birnessite cathode with a displacement/intercalation mechanism for high-performance aqueous zinc-ion batteries. Nanomicro Lett. 12, 56 (2020).
Nam, K. W. et al. Critical role of crystal water for a layered cathode material in sodium ion batteries. Chem. Mater. 27, 3721–3725 (2015).
Jo, M. R. et al. Triggered reversible phase transformation between layered and spinel structure in manganese-based layered compounds. Nat. Commun. 10, 3385 (2019).
House, R. A. et al. Superstructure control of first-cycle voltage hysteresis in oxygen-redox cathodes. Nature 577, 502–508 (2020).
Zhang, J. et al. Tuning oxygen redox chemistry in li‐rich Mn‐based layered oxide cathodes by modulating cation arrangement. Adv. Mater. 31, 1901808 (2019).
Yamamoto, K. et al. Charge compensation mechanism of lithium-excess metal oxides with different covalent and ionic characters revealed by operando soft and hard X-ray absorption spectroscopy. Chem. Mater. 32, 139–147 (2020). This paper establishes the effect of bond character on the stability of oxygen redox.
Myeong, S. et al. Understanding voltage decay in lithium-excess layered cathode materials through oxygen-centred structural arrangement. Nat. Commun. 9, 3285 (2018).
Wang, Q. et al. Unlocking anionic redox activity in O3-type sodium 3d layered oxides via Li substitution. Nat. Mater. 20, 353–361 (2021).
Zhao, C. et al. Rational design of layered oxide materials for sodium-ion batteries. Science 370, 708–711 (2020).
Ma, J. et al. Ordered and disordered polymorphs of Na(Ni2/3Sb1/3)O2: honeycomb-ordered cathodes for Na-ion batteries. Chem. Mater. 27, 2387–2399 (2015).
Susanto, D. et al. Anionic redox activity as a key factor in the performance degradation of NaFeO2 cathodes for sodium ion batteries. Chem. Mater. 31, 3644–3651 (2019).
Yu, Y. et al. Triggering reversible anion redox chemistry in O3-type cathodes by tuning Na/Mn anti-site defects. Energy Environ. Sci. 16, 584–597 (2023).
Kang, K. & Ceder, G. Factors that affect Li mobility in layered lithium transition metal oxides. Phys. Rev. B 74, 094105 (2006).
Yu, H. et al. Study of the lithium/nickel ions exchange in the layered LiNi0.42Mn0.42Co0.16O2 cathode material for lithium ion batteries: experimental and first-principles calculations. Energy Environ. Sci. 7, 1068 (2014).
Sun, G. et al. The effect of cation mixing controlled by thermal treatment duration on the electrochemical stability of lithium transition-metal oxides. Phys. Chem. Chem. Phys. 19, 29886–29894 (2017).
Xiao, Y. et al. Insight into the origin of lithium/nickel ions exchange in layered Li(NixMnyCoz)O2 cathode materials. Nano Energy 49, 77–85 (2018). This paper investigates the advantageous effects of Ni2+/Li+ mixing to alleviate magnetic frustration, thereby enhancing structural stability.
Ménétrier, M. et al. NMR evidence of LiF coating rather than fluorine substitution in Li(Ni0.425Mn0.425Co0.15)O2. J. Solid State Chem. 181, 3303–3307 (2008).
Richards, W. D., Dacek, S. T., Kitchaev, D. A. & Ceder, G. Fluorination of lithium‐excess transition metal oxide cathode materials. Adv. Energy Mater. 8, 1701533 (2018).
Chen, R. et al. Li+ intercalation in isostructural Li2VO3 and Li2VO2F with O2− and mixed O2−/F− anions. Phys. Chem. Chem. Phys. 17, 17288–17295 (2015).
Takeda, N. et al. Reversible Li storage for nanosize cation/anion-disordered rocksalt-type oxyfluorides: LiMoO2 – x LiF (0≤x≤2) binary system. J. Power Sources 367, 122–129 (2017).
Lee, J. et al. Mitigating oxygen loss to improve the cycling performance of high capacity cation-disordered cathode materials. Nat. Commun. 8, 981 (2017).
Lun, Z. et al. Improved cycling performance of Li‐excess cation‐disordered cathode materials upon fluorine substitution. Adv. Energy Mater. 9, 1802959 (2019).
Clément, R. J., Kitchaev, D. & Lee, J. & Ceder, G. Short-range order and unusual modes of nickel redox in a fluorine-substituted disordered rocksalt oxide lithium-ion cathode. Chem. Mater. 30, 6945–6956 (2018).
Jiao, S. et al. The mechanism of fluorine doping for the enhanced lithium storage behavior in cation‐disordered cathode oxide. Adv. Energy Mater. 13, 2301636 (2023).
Lun, Z. et al. Design principles for high-capacity Mn-based cation-disordered rocksalt cathodes. Chem 6, 153–168 (2020).
Sun, Y. et al. Expandable Li percolation network: the effects of site distortion in cation-disordered rock-salt cathode material. J. Am. Chem. Soc. 145, 11717–11726 (2023).
Urban, A., Lee, J. & Ceder, G. The configurational space of rocksalt-type oxides for high-capacity lithium battery electrodes. Adv. Energy Mater. 4, 1400478 (2014).
Ji, H. et al. Hidden structural and chemical order controls lithium transport in cation-disordered oxides for rechargeable batteries. Nat. Commun. 10, 592 (2019).
Lun, Z. et al. Cation-disordered rocksalt-type high-entropy cathodes for Li-ion batteries. Nat. Mater. 20, 214–221 (2021).
Squires, A. G. & Scanlon, D. O. Understanding the limits to short-range order suppression in many-component disordered rock salt lithium-ion cathode materials. J. Mater. Chem. A 11, 13765–13773 (2023).
Wang, Y. et al. Unraveling the nature and role of layered cation ordering in cation-disordered rock-salt cathodes. J. Am. Chem. Soc. 144, 19838–19848 (2022).
Yang, J. H. & Ceder, G. Activated internetwork pathways in partially‐disordered spinel cathode materials with ultrahigh rate performance. Adv. Energy Mater. 13, 2202955 (2023).
Rutt, A. et al. Novel structural motif to promote Mg-ion mobility: investigating ABO4 zircons as magnesium intercalation cathodes. ACS Appl. Mater. Interfaces 15, 34983–34991 (2023).
Flores, E., Mozhzhukhina, N., Aschauer, U. & Berg, E. J. Operando monitoring the insulator–metal transition of LiCoO2. ACS Appl. Mater. Interfaces 13, 22540–22548 (2021).
Chen, Q. et al. Zircon structure as a prototype host for fast monovalent and divalent ionic conduction. Chem. Mater. 35, 6313–6322 (2023).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government Ministry of Science and ICT (MSIT) (NRF-2022R1A2B5B03001781) and the Nano & Material Technology Development Program through the NRF funded by the MSIT (RS-2023-00282389). Y.-M.K acknowledges the partial financial support from LG Energy Solution.
Author information
Authors and Affiliations
Contributions
S.K. and S.L. contributed equally. All authors contributed to the discussion of the content and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, S., Lee, S., Lee, H. et al. Manipulating disorder within cathodes of alkali-ion batteries. Nat Rev Chem 8, 587–604 (2024). https://doi.org/10.1038/s41570-024-00622-1
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41570-024-00622-1
This article is cited by
-
Closed-shell elements Li & Sn substituted P2-type layered cathode materials for wide-voltage sodium-ion batteries
Science China Materials (2026)
-
Inhibiting and rejuvenating dead lithium in battery materials
Nature Reviews Chemistry (2025)
-
Vacancy-ordered perovskite superlattice in cerium titanate negative electrode for enhanced lithium-ion storage
Nature Communications (2025)
-
Tuning anionic bands and lattice stability by short-range disorder at nanoscale for ultrastable Co-free Li-rich cathode
Science China Chemistry (2025)
-
Noninvasive rejuvenation strategy of nickel-rich layered positive electrode for Li-ion battery through magneto-electrochemical synergistic activation
Nature Communications (2024)