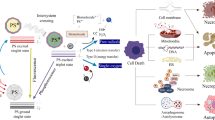
Abstract
Photodynamic therapy (PDT) — which combines light, oxygen and photosensitizers (PS) to generate reactive oxygen species — has emerged as an effective approach for targeted ablation of pathogenic cells with reduced risk of inducing resistance. Some organic PS are now being applied for PDT in the clinic or undergoing evaluation in clinical trials. A limitation of the first-generation organic PS was their potential off-target toxicity. This shortcoming prompted the design of constructs that can be activated by the presence of specific biomolecules — from small biomolecules to large enzymes — in the target cells. Here, we review advances in the design and synthesis of activatable organic PS and their contribution to PDT in the past decade. Important areas of research include novel synthetic methodologies to engineer smart PS with tuneable singlet oxygen generation, their integration into larger constructs such as bioconjugates, and finally, representative examples of their translational potential as antimicrobial and anticancer therapies.

Key points
-
Activatable organic photosensitizers (PS) are designed for targeted photodynamic therapy (PDT), reducing off-target toxicity by activating only at disease sites.
-
Chemical activation strategies include pH-responsive, redox-mediated, and enzyme-activatable PS, enhancing selectivity and efficacy in anticancer and antibacterial treatments.
-
Near-infrared (NIR) PS improve tissue penetration and targeting for clinical translatability, with modifications such as extended conjugation and heavy atom inclusion to increase the excitation wavelengths.
-
Successful biomedical applications demonstrate the potential of activatable PS in treating cancers and infectious diseases, with ongoing challenges in clinical adaptation.
-
Future directions focus on optimizing PS formulations for better biodistribution, developing NIR PS for deeper tissue penetration, and exploring sonodynamic activation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Robertson, C. A., Evans, D. H. & Abrahamse, H. Photodynamic therapy (PDT): a short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B Biol. 96, 1–8 (2009).
Wainwright, M. et al. Photoantimicrobials — are we afraid of the light? Lancet Infect. Dis. 17, e49–e55 (2017).
Matera, C. et al. Photoswitchable antimetabolite for targeted photoactivated chemotherapy. J. Am. Chem. Soc. 140, 15764–15773 (2018).
Durantini, A. M., Greene, L. E., Lincoln, R., Martínez, S. R. & Cosa, G. Reactive oxygen species mediated activation of a dormant singlet oxygen photosensitizer: from autocatalytic singlet oxygen amplification to chemicontrolled photodynamic therapy. J. Am. Chem. Soc. 138, 1215–1225 (2016).
Nguyen, V. N. et al. An emerging molecular design approach to heavy-atom-free photosensitizers for enhanced photodynamic therapy under hypoxia. J. Am. Chem. Soc. 141, 16243–16248 (2019).
Duan, X. et al. Photodynamic therapy mediated by nontoxic core–shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J. Am. Chem. Soc. 138, 16686–16695 (2016).
Mao, D. et al. Metal–organic‐framework‐assisted in vivo bacterial metabolic labeling and precise antibacterial therapy. Adv. Mater. 30, 1706831 (2018).
Jin, S. et al. Emerging new therapeutic antibody derivatives for cancer treatment. Signal Transduct. Target. Ther. 7, 39 (2022).
Maruani, A. et al. Site-selective multi-porphyrin attachment enables the formation of a next-generation antibody-based photodynamic therapeutic. Chem. Commun. 51, 15304–15307 (2015).
Subiros-Funosas, R. et al. Fluorogenic Trp(redBODIPY) cyclopeptide targeting keratin 1 for imaging of aggressive carcinomas. Chem. Sci. 11, 1368–1374 (2020).
Fernandez, A., Thompson, E. J., Pollard, J. W., Kitamura, T. & Vendrell, M. A fluorescent activatable AND-gate chemokine CCL2 enables in vivo detection of metastasis-associated macrophages. Angew. Chem. Int. Ed. 58, 16894–16898 (2019).
Wang, X., Luo, D. & Basilion, J. P. Photodynamic therapy: targeting cancer biomarkers for the treatment of cancers. Cancers 13, 2992 (2021).
Park, S. J. et al. Mechanistic elements and critical factors of cellular reprogramming revealed by stepwise global gene expression analyses. Stem Cell Res. 12, 730–741 (2014).
Zhao, C. et al. Searching for the optimal fluorophore to label antimicrobial peptides. ACS Comb. Sci. 18, 689–696 (2016).
Kaplaneris, N. et al. Chemodivergent manganese-catalyzed C–H activation: modular synthesis of fluorogenic probes. Nat. Commun. 12, 3389 (2021).
Barth, N. D. et al. A fluorogenic cyclic peptide for imaging and quantification of drug-induced apoptosis. Nat. Commun. 11, 4027 (2020).
Ghashghaei, O. et al. Multiple multicomponent reactions: unexplored substrates, selective processes, and versatile chemotypes in biomedicine. Chem. Eur. J. 24, 14513–14521 (2018).
Wu, X., Wang, R., Kwon, N., Ma, H. & Yoon, J. Activatable fluorescent probes for in situ imaging of enzymes. Chem. Soc. Rev. 51, 450–463 (2022).
Mendive-Tapia, L. & Vendrell, M. Activatable fluorophores for imaging immune cell function. Acc. Chem. Res. 55, 1183–1193 (2022).
Grimm, J. B. et al. Synthesis of a far-red photoactivatable silicon-containing rhodamine for super-resolution microscopy. Angew. Chem. Int. Ed. 55, 1723–1727 (2016).
Mochida, A., Ogata, F., Nagaya, T., Choyke, P. L. & Kobayashi, H. Activatable fluorescent probes in fluorescence-guided surgery: practical considerations. Bioorg. Med. Chem. 26, 925–930 (2018).
Park, W. et al. Advanced smart-photosensitizers for more effective cancer treatment. Biomater. Sci. 6, 79–90 (2018).
Pavlova, N. N., Zhu, J. & Thompson, C. B. The hallmarks of cancer metabolism: still emerging. Cell Metab. 34, 355–377 (2022).
Sharma, A. et al. Theranostic fluorescent probes. Chem. Rev. 124, 2699–2804 (2024).
Hu, Y. et al. Rationally designed monoamine oxidase A‐activatable AIE molecular photosensitizer for the specific imaging and cellular therapy of tumors. Aggregate 4, e256 (2023).
Hulikova, A., Harris, A. L., Vaughan-Jones, R. D. & Swietach, P. Regulation of intracellular pH in cancer cell lines under normoxia and hypoxia. J. Cell. Physiol. 228, 743–752 (2013).
Wang, C. et al. Twisted intramolecular charge transfer (TICT) and twists beyond TICT: from mechanisms to rational designs of bright and sensitive fluorophores. Chem. Soc. Rev. 50, 12656–12678 (2021).
Yamaoka, S., Okazaki, S. & Hirakawa, K. Activity control of pH-responsive photosensitizer bis(6-quinolinoxy)P(V)tetrakis(4-chlorophenyl)porphyrin through intramolecular electron transfer. Chem. Phys. Lett. 788, 139285 (2022).
Wang, S. et al. Molecular engineering to construct specific cancer cell lysosome targeting photosensitizer by adjusting the proton binding ability. Sens. Actuators B Chem. 371, 132546 (2022).
Siriwibool, S. et al. Near-infrared fluorescent pH responsive probe for targeted photodynamic cancer therapy. Sci. Rep. 10, 1283 (2020).
Tian, J. et al. A pH-activatable and aniline-substituted photosensitizer for near-infrared cancer theranostics. Chem. Sci. 6, 5969–5977 (2015).
Say, B. et al. Caging of BODIPY photosensitizers through hydrazone bond formation and their activation dynamics. ChemMedChem 18, e202300199 (2023).
Gallagher, W. M. et al. A potent nonporphyrin class of photodynamic therapeutic agent: cellular localisation, cytotoxic potential and influence of hypoxia. Br. J. Cancer 92, 1702–1710 (2005).
Kennedy, L., Sandhu, J. K., Harper, M. E. & Cuperlovic‐Culf, M. Role of glutathione in cancer: from mechanisms to therapies. Biomolecules 10, 1429 (2020).
Lee, M. H., Sessler, J. L. & Kim, J. S. Disulfide-based multifunctional conjugates for targeted theranostic drug delivery. Acc. Chem. Res. 48, 2935–2946 (2015).
Cao, J. J. et al. A glutathione-responsive photosensitizer with fluorescence resonance energy transfer characteristics for imaging-guided targeting photodynamic therapy. Eur. J. Med. Chem. 193, 112203 (2020).
Shi, W., Ng, D. K. P., Zhao, S. & Lo, P. A phthalocyanine‐based glutathione‐activated photosensitizer with a ferrocenyl boron dipyrromethene dark quencher for photodynamic therapy. ChemPhotoChem 3, 1004–1013 (2019).
Zhang, Y. H. et al. AIE based GSH activatable photosensitizer for imaging-guided photodynamic therapy. Chem. Commun. 56, 10317–10320 (2020).
Yang, G. et al. GSH-activatable NIR nanoplatform with mitochondria targeting for enhancing tumor-specific therapy. ACS Appl. Mater. Interfaces 11, 44961–44969 (2019).
Wang, R. et al. A glutathione activatable photosensitizer for combined photodynamic and gas therapy under red light irradiation. Adv. Healthc. Mater. 11, 2102017 (2022).
Teng, K. X., Niu, L. Y., Kang, Y. F. & Yang, Q. Z. Rational design of a “dual lock-and-key” supramolecular photosensitizer based on aromatic nucleophilic substitution for specific and enhanced photodynamic therapy. Chem. Sci. 11, 9703–9711 (2020).
Kilic, E. et al. Activity-based photosensitizers with optimized triplet state characteristics toward cancer cell selective and image guided photodynamic therapy. ACS Appl. Bio Mater. 5, 2754–2767 (2022).
Savani, S. et al. Development of a cysteine responsive chlorinated hemicyanine for image-guided dual phototherapy. Bioorg. Chem. 122, 105725 (2022).
Rehman, T. et al. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 8, 4696–4707 (2020).
Zhang, Y., Xu, C., Sun, H., Ren, M. & Kong, F. A Cys-regulated fluorescent probe targeting cancer cells and their application in inflammation detection. J. Photochem. Photobiol. A Chem. 444, 114919 (2023).
Min, J. Y., Chun, K. S. & Kim, D. H. The versatile utility of cysteine as a target for cancer treatment. Front. Oncol. 12, 997919 (2023).
Nagarkar, S. S., Desai, A. V. & Ghosh, S. K. A nitro‐functionalized metal–organic framework as a reaction‐based fluorescence turn‐on probe for rapid and selective H2S detection. Chem. Eur. J. 21, 9994–9997 (2015).
Dirak, M. et al. Selective monitoring and treatment of neuroblastoma cells with hydrogen sulfide activatable phototheranostic agent. Dye. Pigment. 210, 111011 (2023).
Quan, Y. Y. et al. A multifunctional BODIPY based fluorescent probe for hydrogen sulfide detection and photodynamic anticancer therapy in HCT116 colon cancer cell. Dye. Pigment. 197, 109897 (2022).
Wang, R. et al. Activatable near-infrared emission-guided on-demand administration of photodynamic anticancer therapy with a theranostic nanoprobe. Chem. Sci. 10, 2785–2790 (2019).
Storz, P. Reactive oxygen species in tumor progression. Front. Biosci. 10, 1881–1896 (2005).
Wang, Z. W. et al. A H2O2-responsive boron dipyrromethene-based photosensitizer for imaging-guided photodynamic therapy. Molecules 24, 32 (2018).
Yuan, B., Wang, H., Xu, J. F. & Zhang, X. Activatable photosensitizer for smart photodynamic therapy triggered by reactive oxygen species in tumor cells. ACS Appl. Mater. Interfaces 12, 26982–26990 (2020).
Zeng, Q., Zhang, R., Zhang, T. & Xing, D. H2O2-responsive biodegradable nanomedicine for cancer-selective dual-modal imaging guided precise photodynamic therapy. Biomaterials 207, 39–48 (2019).
Heng, H. et al. Intrinsic mitochondrial reactive oxygen species (ROS) activate the in situ synthesis of trimethine cyanines in cancer cells. Angew. Chem. Int. Ed. 61, e202203444 (2022).
Chen, M. et al. An ultra-sensitive ESIPT fluorescent probe for distinguishing cancerous cells and diagnosing APAP-induced liver injury via HClO fluctuation. Sens. Actuators B Chem. 386, 133749 (2023).
Huang, T. et al. A hypochlorite-activated strategy for realizing fluorescence turn-on, type I and type II ROS-combined photodynamic tumor ablation. Biomaterials 297, 122108 (2023).
Dolmans, D., Fukumura, D. & Jain, R. Photodynamic therapy for cancer. Nat. Rev. Cancer 3, 380–387 (2003).
Pryor, W. A. & Squadrito, G. L. The chemistry of peroxynitrite: a product from the reaction of nitric oxide with superoxide. Am. J. Physiol. 268, L699–L722 (1995).
Zielonka, J. et al. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem. Res. Toxicol. 25, 1793–1799 (2012).
Zhai, W. et al. Universal scaffold for an activatable photosensitizer with completely inhibited photosensitivity. Angew. Chem. Int. Ed. 58, 16601–16609 (2019).
Tyagi, S., Bratu, D. P. & Kramer, F. R. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 16, 49–53 (1998).
Chen, J. et al. Protease-triggered photosensitizing beacon based on singlet oxygen quenching and activation. J. Am. Chem. Soc. 126, 11450–11451 (2004).
Li, M. et al. De novo design of phototheranostic sensitizers based on structure-inherent targeting for enhanced cancer ablation. J. Am. Chem. Soc. 140, 15820–15826 (2018).
Punganuru, S. R., Madala, H. R., Arutla, V. & Srivenugopal, K. S. Cancer-specific biomarker hNQO1-activatable fluorescent probe for imaging cancer cells in vitro and in vivo. Cancers 10, 470 (2018).
Digby, E. M., Sadovski, O. & Beharry, A. A. An activatable photosensitizer targeting human NAD(P)H: quinone oxidoreductase 1. Chem. Eur. J. 26, 2713–2718 (2020).
Xue, E. Y., Kang, F., Zhou, Y. & Ng, D. K. P. Design and synthesis of a NAD(P)H:quinone oxidoreductase 1-activatable photosensitiser for controlled photodynamic therapy. Chem. Commun. 59, 7056–7059 (2023).
Lu, S. et al. PEGylated dimeric BODIPY photosensitizers as nanocarriers for combined chemotherapy and cathepsin B-activated photodynamic therapy in 3D tumor spheroids. ACS Appl. Bio Mater. 3, 3835–3845 (2020).
Wang, Q., Yu, L., Wong, R. C. H. & Lo, P. C. Construction of cathepsin B-responsive fluorescent probe and photosensitizer using a ferrocenyl boron dipyrromethene dark quencher. Eur. J. Med. Chem. 179, 828–836 (2019).
Liu, T. W. et al. Imaging of specific activation of photodynamic molecular beacons in breast cancer vertebral metastases. Bioconjug. Chem. 22, 1021–1030 (2011).
Almammadov, T. et al. Resorufin enters the photodynamic therapy arena: a monoamine oxidase activatable agent for selective cytotoxicity. ACS Med. Chem. Lett. 11, 2491–2496 (2020).
Lioret, V., Renault, K., Maury, O. & Romieu, A. Valkyrie probes: a novel class of enzyme-activatable photosensitizers based on sulfur- and seleno-rosamines with pyridinium unit. Chem. Asian J. 18, e202300756 (2023).
Li, Y. et al. Enzyme‐activatable near‐infrared photosensitizer with high enrichment in tumor cells based on a multi‐effect design. Angew. Chem. Int. Ed. 63, e202317773 (2024).
Verirsen, I., Uyar, B., Ozsamur, N. G., Demirok, N. & Erbas-Cakmak, S. Enzyme activatable photodynamic therapy agents targeting melanoma. Org. Biomol. Chem. 20, 8864–8868 (2022).
Liu, Y. et al. A tyrosinase-activated Pt(II) complex for melanoma photodynamic therapy and fluorescence imaging. Sens. Actuators B Chem. 374, 132836 (2023).
Chiba, M. et al. An activatable photosensitizer targeted to γ‐glutamyltranspeptidase. Angew. Chem. Int. Ed. 56, 10418–10422 (2017).
Shen, Z. & Tung, C. H. Selective photo-ablation of glioma cells using an enzyme activatable photosensitizer. Chem. Commun. 56, 13860–13863 (2020).
Li, J. et al. A fluorescence-activatable tumor-reporting probe for precise photodynamic therapy. J. Mater. Chem. B 9, 5829–5836 (2021).
Liu, F. et al. A multifunctional near-infrared fluorescent probe for in vitro and in vivo imaging of γ-glutamyltranspeptidase and photodynamic cancer therapy. Sens. Actuators B Chem. 363, 131838 (2022).
Zhou, X. et al. An APN-activated NIR photosensitizer for cancer photodynamic therapy and fluorescence imaging. Biomaterials 253, 120089 (2020).
Zhou, W. et al. AIE-active lysosome-targeted fluorescent organic nanoparticles for leucine aminopeptidase-activatable fluorescent imaging and precision photodynamic therapy potential. Dye. Pigment. 221, 111781 (2024).
Arslan, B. et al. A leucine aminopeptidase activatable photosensitizer for cancer cell selective photodynamic therapy action. Dye. Pigment. 195, 109735 (2021).
Piao, W. et al. Development of an azo-based photosensitizer activated under mild hypoxia for photodynamic therapy. J. Am. Chem. Soc. 139, 13713–13719 (2017).
Liu, W. et al. Azo-based hypoxia-responsive self-assembly near-infrared fluorescent nanoprobe for in vivo real-time bioimaging of tumors. ACS Appl. Bio Mater. 4, 2752–2758 (2021).
Tian, J. et al. Activatable type I photosensitizer with quenched photosensitization pre and post photodynamic therapy. Angew. Chem. Int. Ed. 62, e202307288 (2023).
Xiong, J. et al. Engineering a theranostic platform for synergistic hypoxia-responsive photodynamic therapy and chemotherapy. Matter 5, 1502–1519 (2022).
Wang, C. et al. Microenvironment-triggered dual-activation of a photosensitizer-fluorophore conjugate for tumor specific imaging and photodynamic therapy. Sci. Rep. 10, 12127 (2020).
Tam, L. K. B. et al. Dual cathepsin B and glutathione-activated dimeric and trimeric phthalocyanine-based photodynamic molecular beacons for targeted photodynamic therapy. J. Med. Chem. 64, 17455–17467 (2021).
Tam, L. K. B., He, L., Ng, D. K. P., Cheung, P. C. K. & Lo, P. A tumor‐targeting dual‐stimuli‐activatable photodynamic molecular beacon for precise photodynamic therapy. Chem. Eur. J. 28, e202201652 (2022).
Tam, L. K. B. et al. Enzyme-responsive double-locked photodynamic molecular beacon for targeted photodynamic anticancer therapy. J. Am. Chem. Soc. 145, 7361–7375 (2023).
Sun, J. et al. GSH and H2O2 co‐activatable mitochondria‐targeted photodynamic therapy under normoxia and hypoxia. Angew. Chem. Int. Ed. 59, 12122–12128 (2020).
Benson, S. et al. Photoactivatable metabolic warheads enable precise and safe ablation of target cells in vivo. Nat. Commun. 12, 2369 (2021).
Almammadov, T. et al. Locked and loaded: β-galactosidase activated photodynamic therapy agent enables selective imaging and targeted treatment of glioblastoma multiforme cancer cells. ACS Appl. Bio Mater. 5, 4284–4293 (2022).
Xiong, J., Cheung, Y. K., Fong, W. P., Wong, C. T. T. & Ng, D. K. P. Selective photodynamic eradication of senescent cells with a β-galactosidase-activated photosensitiser. Chem. Commun. 59, 3471–3474 (2023).
Chiba, M. et al. Activatable photosensitizer for targeted ablation of lacZ-positive cells with single-cell resolution. ACS Cent. Sci. 5, 1676–1681 (2019).
Yue, X. et al. Three birds one stone: an enzyme-activatable theragnostic agent for fluorescence diagnosis, photodynamic and inhibitor therapies. Chem. Commun. 59, 4676–4679 (2023).
Yuan, Y. et al. Specific light‐up bioprobe with aggregation‐induced emission and activatable photoactivity for the targeted and image‐guided photodynamic ablation of cancer cells. Angew. Chem. Int. Ed. 54, 1780–1786 (2015).
Chu, J. C. H., Fong, W. P., Wong, C. T. T. & Ng, D. K. P. Facile synthesis of cyclic peptide-phthalocyanine conjugates for epidermal growth factor receptor-targeted photodynamic therapy. J. Med. Chem. 64, 2064–2076 (2021).
Kim, J., Won, Y., Goh, S. H. & Choi, Y. A redox-responsive theranostic agent for target-specific fluorescence imaging and photodynamic therapy of EGFR-overexpressing triple-negative breast cancers. J. Mater. Chem. B 4, 6787–6790 (2016).
Chu, J. C. H., Wong, C. T. T. & Ng, D. K. P. Toward precise antitumoral photodynamic therapy using a dual receptor-mediated bioorthogonal activation approach. Angew. Chem. Int. Ed. 62, e202214473 (2023).
Xiong, J., Chu, J. C. H., Fong, W. P., Wong, C. T. T. & Ng, D. K. P. Specific activation of photosensitizer with extrinsic enzyme for precisive photodynamic therapy. J. Am. Chem. Soc. 144, 10647–10658 (2022).
Xiong, J., Xue, E. Y., Wu, Q., Lo, P. C. & Ng, D. K. P. A tetrazine-responsive isonitrile-caged photosensitiser for site-specific photodynamic therapy. J. Control. Release 353, 663–674 (2023).
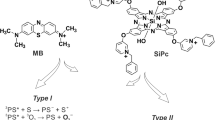
Maharjan, P. S. & Bhattarai, H. K. Singlet oxygen, photodynamic therapy, and mechanisms of cancer cell death. J. Oncol. 2022, 7211485 (2022).
Benson, S. et al. Environmentally sensitive photosensitizers enable targeted photodynamic ablation of Gram-positive antibiotic resistant bacteria. Theranostics 13, 3814–3825 (2023).
Yang, C. et al. Traceable cancer cell photoablation with a new mitochondria-responsive and -activatable red-emissive photosensitizer. Chem. Commun. 55, 3801–3804 (2019).
Lincoln, R., Van Kessel, A. T. M., Zhang, W. & Cosa, G. A dormant BODIPY-acrolein singlet oxygen photosensitizer intracellularly activated upon adduct formation with cysteine residues. Photochem. Photobiol. Sci. 18, 2003–2011 (2019).
Linden, G. & Vázquez, O. Bioorthogonal turn‐on BODIPY‐peptide photosensitizers for tailored photodynamic therapy. Chem. Eur. J. 26, 10014–10023 (2020).
Li, J. et al. Activatable dual ROS-producing probe for dual organelle-engaged photodynamic therapy. ACS Appl. Bio Mater. 4, 4618–4628 (2021).
Yi, Z. et al. Mitochondria-targeting type-I photodrug: harnessing caspase-3 activity for pyroptotic oncotherapy. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.4c01929 (2024).
Tu, L. et al. Augmenting cancer therapy with a supramolecular immunogenic cell death inducer: a lysosome-targeted NIR-light-activated ruthenium(II) metallacycle. J. Am. Chem. Soc. https://doi.org/10.1021/jacs.3c13224 (2024).
Zeng, S. et al. An ER‐targeted, viscosity‐sensitive hemicyanine dye for the diagnosis of nonalcoholic fatty liver and photodynamic cancer therapy by activating pyroptosis pathway. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202316487 (2024).
Linden, G. et al. Conditional singlet oxygen generation through a bioorthogonal DNA-targeted tetrazine reaction. Angew. Chem. Int. Ed. 58, 12868–12873 (2019).
Li, J. et al. A coumarin-based fluorescent probe for NIR imaging-guided photodynamic therapy against S. aureus-induced infection in mouse models. J. Mater. Chem. B 10, 1427–1433 (2022).
Zhao, E. et al. Light-enhanced bacterial killing and wash-free imaging based on AIE fluorogen. ACS Appl. Mater. Interfaces 7, 7180–7188 (2015).
Xiao, P. et al. Precise molecular engineering of type I photosensitizers with near-infrared aggregation-induced emission for image-guided photodynamic killing of multidrug-resistant bacteria. Adv. Sci. 9, 2104079 (2022).
Wu, X. et al. Reactivity differences enable ROS for selective ablation of bacteria. Angew. Chem. Int. Ed. 61, e202200808 (2022).
Digby, E. M., Ma, T., Zipfel, W. R., Milstein, J. N. & Beharry, A. A. highly potent photoinactivation of bacteria using a water-soluble, cell-permeable, DNA-binding photosensitizer. ACS Infect. Dis. 7, 3052–3061 (2021).
Cantelli, A. et al. Concanavalin A-rose bengal bioconjugate for targeted Gram-negative antimicrobial photodynamic therapy. J. Photochem. Photobiol. B Biol. 206, 111852 (2020).
Zhao, Y. et al. Glycomimetic-conjugated photosensitizer for specific pseudomonas aeruginosa recognition and targeted photodynamic therapy. Bioconjug. Chem. 29, 3222–3230 (2018).
Jiang, F., Cai, C., Wang, X. & Han, S. A dual biomarker-targeting probe enables signal-on surface labeling of Staphylococcus aureus. Bioorg. Med. Chem. Lett. 93, 129428 (2023).
Dutta, A. K. et al. Trehalose conjugation enhances toxicity of photosensitizers against mycobacteria. ACS Cent. Sci. 5, 644–650 (2019).
Hu, F. et al. Visualization and in situ ablation of intracellular bacterial pathogens through metabolic labeling. Angew. Chem. Int. Ed. 59, 9288–9292 (2020).
Yu, E. Y. et al. One-step light-up metabolic probes for in situ discrimination and killing of intracellular bacteria. Mater. Chem. Front. 6, 450–458 (2022).
Ucuncu, M. et al. Polymyxin-based photosensitizer for the potent and selective killing of Gram-negative bacteria. Chem. Commun. 56, 3757–3760 (2020).
Mills, B., Kiang, A., Mohanan, S. M. P. C., Bradley, M. & Klausen, M. Riboflavin-vancomycin conjugate enables simultaneous antibiotic photo-release and photodynamic killing against resistant Gram-positive pathogens. JACS Au 3, 3014–3023 (2023).
Scott, J. I., Deng, Q. & Vendrell, M. Near-infrared fluorescent probes for the detection of cancer-associated proteases. ACS Chem. Biol. 16, 1304–1317 (2021).
Mellanby, R. J. et al. Tricarbocyanine N-triazoles: the scaffold-of-choice for long-term near-infrared imaging of immune cells in vivo. Chem. Sci. 9, 7261–7270 (2018).
Turan, I. S., Cakmak, F. P., Yildirim, D. C., Cetin‐Atalay, R. & Akkaya, E. U. Near‐IR absorbing BODIPY derivatives as glutathione‐activated photosensitizers for selective photodynamic action. Chem. Eur. J. 20, 16088–16092 (2014).
Xiong, H., Zhou, K., Yan, Y., Miller, J. B. & Siegwart, D. J. Tumor-activated water-soluble photosensitizers for near-infrared photodynamic cancer therapy. ACS Appl. Mater. Interfaces 10, 16335–16343 (2018).
Wang, B. et al. A S-substituted Nile blue-derived bifunctional near-infrared fluorescent probe for in vivo carboxylesterase imaging-guided photodynamic therapy of hepatocellular carcinoma. J. Mater. Chem. B 11, 7623–7628 (2023).
Lv, W. et al. Development of a red absorbing Se-rhodamine photosensitizer and its application for bio-orthogonally activatable photodynamic therapy. Chem. Commun. 55, 7037–7040 (2019).
Li, X. et al. A lipid droplet-targeted multifunctional AIE-active fluorescent probe for hydrogen peroxide detection and imaging-guided photodynamic therapy. Sens. Actuators B Chem. 375, 132892 (2023).
Zheng, J. et al. A nitroreductase-activatable near-infrared theranostic photosensitizer for photodynamic therapy under mild hypoxia. Chem. Commun. 56, 5819–5822 (2020).
Yang, Y. et al. A glutathione activatable pro-drug-photosensitizer for combined chemotherapy and photodynamic therapy. Chin. Chem. Lett. 33, 4583–4586 (2022).
Cheng, Z. et al. Enzyme‐activatable near‐infrared hemicyanines as modular scaffolds for in vivo photodynamic therapy. Angew. Chem. Int. Ed. 63, e202404587 (2024).
Ebaston, T. M., Nakonechny, F., Talalai, E., Gellerman, G. & Patsenker, L. Iodinated xanthene-cyanine NIR dyes as potential photosensitizers for antimicrobial photodynamic therapy. Dye. Pigment. 184, 108854 (2021).
Xu, F. et al. Hypoxia-activated NIR photosensitizer anchoring in the mitochondria for photodynamic therapy. Chem. Sci. 10, 10586–10594 (2019).
Zhang, Y. et al. Hemicyanine-based type I photosensitizers for antihypoxic activatable photodynamic therapy. ACS Mater. Lett. 5, 3058–3067 (2023).
Liu, H. W. et al. A mitochondrial-targeted prodrug for NIR imaging guided and synergetic NIR photodynamic-chemo cancer therapy. Chem. Sci. 8, 7689–7695 (2017).
Li, Z., Feng, Q., Hou, J. & Shen, J. NQO-1 activatable NIR photosensitizer for visualization and selective killing of breast cancer cells. Bioorg. Chem. 143, 107021 (2024).
Seah, D., Cheng, Z. & Vendrell, M. Fluorescent probes for imaging in humans: where are we now? ACS Nano 17, 19478–19490 (2023).
Peng, Q. et al. 5-Aminolevulinic acid-based photodynamic therapy. Cancer 79, 2282–2308 (1997).
Vermandel, M. et al. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: a preliminary analysis of the INDYGO clinical trial. J. Neurooncol. 152, 501–514 (2021).
Akimoto, J., Fukami, S., Nagai, K. & Kohno, M. First clinical report of the intraoperative macro- and micro-photodiagnosis and photodynamic therapy using talaporfin sodium for a patient with disseminated lumbar medulloblastoma. J. Clin. Med. 12, 432 (2023).
Bao, L. L. et al. In vitro and in vivo evaluation of a pyropheophorbide-a derivative as a potential photosensitizer for age-related macular degeneration. Biomed. Pharmacother. 88, 1220–1226 (2017).
Martins, S. H. L. et al. Effect of surgical periodontal treatment associated to antimicrobial photodynamic therapy on chronic periodontitis: a randomized controlled clinical trial. J. Clin. Periodontol. 44, 717–728 (2017).
Yano, T. & Wang, K. K. Photodynamic therapy for gastrointestinal cancer. Photochem. Photobiol. 96, 517–523 (2020).
Luo, Y. et al. Fibroblast activation protein α activatable theranostic pro-photosensitizer for accurate tumor imaging and highly-specific photodynamic therapy. Theranostics 12, 3610–3627 (2022).
Huang, L. et al. Ultralow-power near infrared lamp light operable targeted organic nanoparticle photodynamic therapy. J. Am. Chem. Soc. 138, 14586–14591 (2016).
Ghosh, S., Carter, K. A. & Lovell, J. F. Liposomal formulations of photosensitizers. Biomaterials 218, 119341 (2019).
Cho, H. J. et al. Injectable single-component peptide depot: autonomously rechargeable tumor photosensitization for repeated photodynamic therapy. ACS Nano 14, 15793–15805 (2020).
Teh, D. B. L. et al. A Flexi‐PEGDA upconversion implant for wireless brain photodynamic therapy. Adv. Mater. 32, e2001459 (2020).
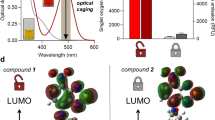
Nestoros, E. et al. Tuning singlet oxygen generation with caged organic photosensitizers. Nat. Commun. https://doi.org/10.1038/s41467-024-51872-y (2024).
Lochenie, C. et al. Photosensitizer-amplified antimicrobial materials for broad-spectrum ablation of resistant pathogens in ocular infections. Adv. Mater. 36, e2404107 (2024).
Ma, C. W. et al. Rapid broad spectrum detection of carbapenemases with a dual fluorogenic-colorimetric probe. J. Am. Chem. Soc. 143, 6886–6894 (2021).
Cosco, E. D. et al. Shortwave infrared polymethine fluorophores matched to excitation lasers enable non-invasive, multicolour in vivo imaging in real time. Nat. Chem. 12, 1123–1130 (2020).
Cosco, E. D. et al. Bright chromenylium polymethine dyes enable fast, four-color in vivo imaging with shortwave infrared detection. J. Am. Chem. Soc. 143, 6836–6846 (2021).
McHale, A. P., Callan, J. F., Nomikou, N., Fowley, C. & Callan, B. Sonodynamic therapy: concept, mechanism and application to cancer treatment. Adv. Exp. Med. Biol. 880, 429–450 (2016).
Acknowledgements
The authors acknowledge funding from the Medical Research Council (MR/R01566X/1), the Engineering and Physical Sciences Research Council (EP/W015706/1, to M.V.), an ERC Consolidator Grant (DYNAFLUORS, 771443, to M.V.), the National Research Foundation of Korea (CRI project no. 2018R1A3B1052702, to J.S.K.), the Prestigious DBT-Ramalingaswami Fellowship (BT/RLF/Re-entry/59/2018, to A.S.) and SERB (CRG/2021/0022476, to A.S.). The authors acknowledge BioRender.com for the assistance with figure creation.
Author information
Authors and Affiliations
Contributions
E.N. and A.S. contributed equally to this work and were responsible for researching data for the article. E.N. and M.V. discussed the content and wrote the article with contributions from A.S., E.K. and J.S.K.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Activatable fluorophores
-
Fluorescent molecules whose emission varies in response to biological or chemical stimuli.
- Bioorthogonal reactions
-
Chemical reactions that occur inside living systems without interfering with native biochemical processes, often used for labelling and tracking biomolecules.
- Boron dipyrromethenes
-
(BODIPY). A class of fluorescent dyes with their high photostability and brightness, and used in various imaging applications.
- Cyclic RGD peptides
-
Short peptides containing the amino acid sequence arginine–glycine–aspartic acid, which target integrins on cell membranes.
- Dual-lock activation strategies
-
Methods that require two distinct triggers to activate a photosensitizer or drug, enhancing specificity and reducing off-target effects.
- Enzyme-activatable PS
-
Photosensitizers that are activated by specific enzymes, allowing for targeted photodynamic therapy in areas wherein those enzymes are present.
- Glutathione
-
(GSH). A tripeptide that acts as an antioxidant in cells, often used as a trigger for activating photosensitizers in anticancer therapy owing to its high levels in cancer cells.
- Intramolecular charge transfer
-
(ICT). A process used in designing fluorescent probes and photosensitizers wherein a charge is transferred from an electron-rich donor moiety to an electron-poor acceptor moiety.
- Near-infrared
-
(NIR). A region of the electromagnetic spectrum with wavelengths just beyond visible light and enhanced penetration in living tissues.
- Photoinduced electron transfer
-
(PeT). A mechanism through which an electron is transferred between two chemical groups of photosensitizers and fluorophores, and used to quench or activate singlet oxygen generation or fluorescence emission, respectively.
- Photosensitizers
-
(PS). Molecules that produce reactive oxygen species when exposed to light and used in photodynamic therapy to kill targeted cells.
- Reactive oxygen species
-
(ROS). Chemically reactive molecules containing oxygen, which can cause cell damage and are used in photodynamic therapy to kill cancer cells or pathogens.
- Singlet oxygen
-
A highly reactive form of oxygen generated by photosensitizers during photodynamic therapy, responsible for inducing cell death.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nestoros, E., Sharma, A., Kim, E. et al. Smart molecular designs and applications of activatable organic photosensitizers. Nat Rev Chem 9, 46–60 (2025). https://doi.org/10.1038/s41570-024-00662-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41570-024-00662-7
This article is cited by
-
Emerging non-antibiotic strategies for implant-associated biofilm infections by reprogramming the dysregulated immune microenvironment
npj Biofilms and Microbiomes (2026)
-
Design of second near-infrared fluorescent molecules for disease phototheranostics
Science China Chemistry (2026)
-
Bacterial microenvironment-responsive Fe-Ce6 nanoparticles accelerate infected wound healing via in situ generation of nanozyme and photodynamic antibacterial activity
Science China Materials (2025)