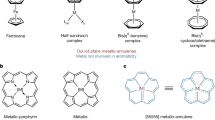
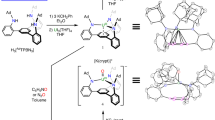
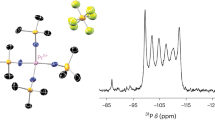
Abstract
Coordination chemistry is a tool to reveal the hidden nature of elements through controlled manipulation of their environment, and the benefits that this understanding has brought society are numerous. For a chemist, the actinide series represents an intriguing frontier wherein conventional chemical intuition yields to relativistic effects and atypical technical challenges influence the pace of progress. Much of the chemical understanding of transuranium elements was developed during and shortly after the Manhattan Project and was borne out of practical needs. Although theoretical interest in their fundamental bonding and behaviour has always existed, synthesis-led exploration was often not possible. Synthetic, analytical and computational advancements in the twenty-first century have changed this, and contemporary synthetic transuranium coordination chemistry has begun to reveal that their properties are more nuanced than previously appreciated. In this Review, we discuss the discovery of transuranium elements, their history and the logistical demands inherent to chemical advancement in the area, and present key progress in transuranium organometallic and selected metal–organic chemistry, with a focus on how the field has begun to mature.

Key points
-
The Review summarizes the history and current state of transuranium organometallic chemistry, with a focus on synthesis, characterization and reactivity up to early 2025.
-
The unique challenges in handling transuranium elements owing to their radioactivity, scarcity and high chemical toxicity are discussed from the perspective of synthetic chemistry.
-
Recent advancements in laboratory-scale instrumentation have enabled the characterization of transuranium complexes, doubling the number of crystallographically characterized compounds in the past decade.
-
The bonding and electronic structure of transuranium elements and ions are compared to those of lanthanide and earlier actinide analogues, emphasizing similarities and differences which manifest in their chemistry.
-
The Review outlines future directions for transuranium chemistry, including the exploration of new metal–carbon bonding motifs and the requirement for specialized ligand platforms to overcome oxidation–reduction limitations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Morss, L. R., Edelstein, N. M. & Fuger, J. The Chemistry of the Actinide and Transactinide Elements 4th edn (Springer, 2011).
Birnbaum, E. R., Fassbender, M. E., Ferrier, M. G., John, K. D. & Mastren, T. in Encyclopedia of Inorganic and Bioinorganic Chemistry (ed. Scott, R. A.) 1–21 (Wiley, 2018).
Martin, R. C., Knauer, J. B. & Balo, P. A. Production, distribution and applications of californium-252 neutron sources. Appl. Radiat. Isot. 53, 785–792 (2000).
Lange, R. G. & Carroll, W. P. Review of recent advances of radioisotope power systems. Energy Convers. Manag. 49, 393–401 (2008).
Khuyagbaatar, J. et al. 48Ca + 249Bk fusion reaction leading to element Z = 117: long-lived α-decaying 270Db and discovery of 266Lr. Phys. Rev. Lett. 112, 172501 (2014).
McMillan, E. & Abelson, P. H. Radioactive element 93. Phys. Rev. 57, 1185–1186 (1940).
Seaborg, G. T. in Actinides in Perspective (ed. Edelstein N. M.) 1–22 (Pergamon, 1982).
Seaborg, G. T. & Loveland, W. D. Elements Beyond Uranium (Wiley, 1991).
Seaborg, G. T. in Handbook on the Physics and Chemistry of Rare Earths Vol. 18 (eds Gschneidner, K. A. Jr. & Eyring, L.) 1–27 (Elsevier, 1994).
Balasubramanian, K. in Handbook on the Physics and Chemistry of Rare Earths Vol. 18 (eds Gschneidner, K. A. Jr. & Eyring, L.) 29–158 (Elsevier, 1994).
Seaborg, G. T. Overview of the actinide and lanthanide (the f) elements. Radiochim. Acta 61, 115–122 (1993).
Ghiorso, A., Sikkeland, T., Larsh, A. E. & Latimer, R. M. New element, lawrencium, atomic number 103. Phys. Rev. Lett. 6, 473–475 (1961).
Goldwhite, H. The Manhattan Project. J. Fluor. Chem. 33, 109–132 (1986).
Clark, D. L., Geeson, D. A. & Hanrahan, R. J. Jr. Plutonium Handbook 2nd edn (American Nuclear Society, 2019).
Edelstein, N. M., Fuger, J., Katz, J. J. & Morss, L. R. in The Chemistry of the Actinide and Transactinide Elements (eds Morss, L. R., Edelstein, N. M. & Fuger, J.) 1753–1835 (Springer, 2011).
Schlesinger, H. I. et al. New developments in the chemistry of diborane and the borohydrides. I. General summary. J. Am. Chem. Soc. 75, 186–190 (1953).
Schlesinger, H. I. & Brown, H. C. Uranium(IV) borohydride. J. Am. Chem. Soc. 75, 219–221 (1953).
Liddle, S. T. The renaissance of non-aqueous uranium chemistry. Angew. Chem. Int. Ed. 54, 8604–8641 (2015).
Scott, B. L. Actinide research quarterly (Los Alamos National Laboratory, 2015).
White, F. D., Dan, D. & Albrecht‐Schmitt, T. E. Contemporary chemistry of berkelium and californium. Chem. Eur. J. 25, 10251–10261 (2019).
White, F. D. & Marsh, M. L. in Handbook on the Physics and Chemistry of Rare Earths Vol. 55 (eds Bünzli, J.-C. G. & Pecharsky, V. K.) 123–158 (Elsevier2019).
Gilson, S. E. & Burns, P. C. The crystal and coordination chemistry of neptunium in all its oxidation states: an expanded structural hierarchy of neptunium compounds. Coord. Chem. Rev. 445, 213994 (2021).
Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. The Cambridge Structural Database. Acta Crystallogr. B 72, 171–179 (2016).
Kaltsoyannis, N. Transuranic computational chemistry. Chem. Eur. J. 24, 2815–2825 (2018).
Burns, C. J., Clark, D. L. & Sattelberger, A. P. in Encyclopedia of Inorganic Chemistry (eds King, R. B. et al.) (Wiley, 2006).
La Pierre, H. S. & Meyer, K. in Progress in Inorganic Chemistry Vol. 58 (ed Karlin, K. D.) 303–416 (Wiley, 2014).
Liddle, S. T. Progress in nonaqueous molecular uranium chemistry: where to next? Inorg. Chem. 63, 9366–9384 (2024).
Liddle, S. T., Mills, D. P. & Natrajan, L. S. The Lanthanides and Actinides (World Scientific, 2022).
Gibson, J. K. & Haire, R. G. Berkelium and californium organometallic ions. Radiochim. Acta 89, 363–369 (2001).
Gibson, J. K. & Haire, R. G. Activation of pentamethylcyclopentadiene by Bk+, Cf+, and Es+ ions in the gas phase: probing electronic structures of transcurium actinides. Organometallics 24, 119–126 (2005).
Gibson, J. K. & Haire, R. G. Gas-phase californium ion chemistry. Int. J. Mass Spectrom. 203, 127–142 (2000).
Tague, T. J. Jr., Andrews, L. & Hunt, R. D. Matrix infrared spectra of the products of uranium-atom reactions with carbon monoxide and carbon dioxide. J. Phys. Chem. 97, 10920–10924 (1993).
Zhou, M., Andrews, L., Li, J. & Bursten, B. E. Reaction of laser-ablated uranium atoms with CO: infrared spectra of the CUO, CUO–, OUCCO, (η2-C2)UO2, and U(CO)x (x = 1−6) molecules in solid neon. J. Am. Chem. Soc. 121, 9712–9721 (1999).
Gibson, J. K. et al. Gas-phase reactions of hydrocarbons with An+ and AnO+ (An = Th, Pa, U, Np, Pu, Am, Cm): the active role of 5f electrons in organoprotactinium chemistry. Organometallics 26, 3947–3956 (2007).
Carter, K. P. et al. Structural and spectroscopic characterization of an einsteinium complex. Nature 590, 85–88 (2021).
Ortu, F., Formanuik, A., Innes, J. R. & Mills, D. P. New vistas in the molecular chemistry of thorium: low oxidation state complexes. Dalton Trans. 45, 7537–7549 (2016).
Jones, M. B. & Gaunt, A. J. Recent developments in synthesis and structural chemistry of nonaqueous actinide complexes. Chem. Rev. 113, 1137–1198 (2013).
Gaunt, A. J. & Neu, M. P. Recent developments in nonaqueous plutonium coordination chemistry. C. R. Chim. 13, 821–831 (2010).
Riedhammer, J., Halter, D. P. & Meyer, K. Nonaqueous electrochemistry of uranium complexes: a guide to structure–reactivity tuning. Chem. Rev. 123, 7761–7781 (2023).
Ephritikhine, M. Recent advances in organoactinide chemistry as exemplified by cyclopentadienyl compounds. Organometallics 32, 2464–2488 (2013).
Walter, O. Actinide organometallic complexes with π-ligands. Chem. Eur. J. 25, 2927–2934 (2019).
Cryer, J. D. & Liddle, S. T. in Comprehensive Organometallic Chemistry IV 4th edn (eds Parkin, G., Meyer, K. & O’Hare, D.) 460–501 (Elsevier, 2022).
Arnold, P. L., Dutkiewicz, M. S. & Walter, O. Organometallic neptunium chemistry. Chem. Rev. 117, 11460–11475 (2017).
Farnaby, J. H., Chowdhury, T., Horsewill, S. J., Wilson, B. & Jaroschik, F. Lanthanides and actinides: annual survey of their organometallic chemistry covering the year 2019. Coord. Chem. Rev. 437, 213830 (2021).
Marks, T. J. & Ernst, R. D. in Comprehensive Organometallic Chemistry (eds Wilkinson, G., Stone, F. G. A. & Abel, E. W.) 173–270 (Pergamon, 1982).
Nugent, L. J. Standard electrode potentials and enthalpies of formation of some lanthanide and actinide aquo-ions. J. Inorg. Nucl. Chem. 37, 1767–1770 (1975).
Nugent, L. J., Baybarz, R. D., Burnett, J. L. & Ryan, J. L. Electron-transfer and f → d absorption bands of some lanthanide and actinide complexes and the standard (III–IV) oxidation potentials for each member of the lanthanide and actinide series. J. Inorg. Nucl. Chem. 33, 2503–2530 (1971).
Cotton, S. Lanthanide and Actinide Chemistry 2nd edn (Wiley, 2024).
Gompa, T. P., Ramanathan, A., Rice, N. T. & La Pierre, H. S. The chemical and physical properties of tetravalent lanthanides: Pr, Nd, Tb, and Dy. Dalton Trans. 49, 15945–15987 (2020).
Palumbo, C. T., Zivkovic, I., Scopelliti, R. & Mazzanti, M. Molecular complex of Tb in the +4 oxidation state. J. Am. Chem. Soc. 141, 9827–9831 (2019).
Xue, T. et al. Tetravalent terbium chelates: stability enhancement and property tuning. Precis. Chem. 1, 583–591 (2023).
Xue, T., Ding, Y.-S. & Zheng, Z. A tetravalent praseodymium complex with field-induced slow magnetic relaxation. Dalton Trans. 53, 5779–5783 (2024).
Piro, N. A., Robinson, J. R., Walsh, P. J. & Schelter, E. J. The electrochemical behavior of cerium(III/IV) complexes: thermodynamics, kinetics and applications in synthesis. Coord. Chem. Rev. 260, 21–36 (2014).
Rice, N. T. et al. Design, isolation, and spectroscopic analysis of a tetravalent terbium complex. J. Am. Chem. Soc. 141, 13222–13233 (2019).
Boggiano, A. C. et al. A four-coordinate Pr4+ imidophosphorane complex. Angew. Chem. Int. Ed. 63, e202409789 (2024).
Woen, D. H. & Evans, W. J. in Handbook on the Physics and Chemistry of Rare Earths Vol. 50 (eds Bünzli, J.-C. G. & Pecharsky, V. K.) 337–394 (Elsevier, 2016).
MacDonald, M. R., Ziller, J. W. & Evans, W. J. Synthesis of a crystalline molecular complex of Y2+, [(18-crown-6)K][(C5H4SiMe3)3Y]. J. Am. Chem. Soc. 133, 15914–15917 (2011).
MacDonald, M. R., Bates, J. E., Ziller, J. W., Furche, F. & Evans, W. J. Completing the series of +2 ions for the lanthanide elements: synthesis of molecular complexes of Pr2+, Gd2+, Tb2+, and Lu2+. J. Am. Chem. Soc. 135, 9857–9868 (2013).
Fieser, M. E. et al. Structural, spectroscopic, and theoretical comparison of traditional vs recently discovered Ln2+ ions in the [K(2.2.2-cryptand)][(C5H4SiMe3)3Ln] complexes: the variable nature of Dy2+ and Nd2+. J. Am. Chem. Soc. 137, 369–382 (2015).
Crabtree, R. H. The Organometallic Chemistry of the Transition Metals 6th edn (Wiley, 2014).
Kiselev, Y. M. et al. On existence and properties of plutonium(VIII) derivatives. Radiochim. Acta 102, 227–237 (2014).
Sullivan, J. C. et al. Pulse radiolysis studies of americum(III) and curium(III) ions in perchlorate media. The preparation of Am II, Am IV, Cm II and Cm IV. Inorg. Nucl. Chem. Lett. 12, 599–601 (1976).
Runde, W. H. & Mincher, B. J. Higher oxidation states of americium: preparation, characterization and use for separations. Chem. Rev. 111, 5723–5741 (2011).
Cary, S. K. et al. Emergence of californium as the second transitional element in the actinide series. Nat. Commun. 6, 6827 (2015).
Neidig, M. L., Clark, D. L. & Martin, R. L. Covalency in f-element complexes. Coord. Chem. Rev. 257, 394–406 (2013).
Walensky, J. R., Martin, R. L., Ziller, J. W. & Evans, W. J. Importance of energy level matching for bonding in Th3+-Am3+ actinide metallocene amidinates, (C5Me5)2[iPrNC(Me)NiPr]An. Inorg. Chem. 49, 10007–10012 (2010).
Kaltsoyannis, N. Does covalency increase or decrease across the actinide series? Implications for minor actinide partitioning. Inorg. Chem. 52, 3407–3413 (2013).
Tassell, M. J. & Kaltsoyannis, N. Covalency in AnCp4 (An = Th-Cm): a comparison of molecular orbital, natural population and atoms-in-molecules analyses. Dalton Trans. 39, 6719–6725 (2010).
Kirker, I. & Kaltsoyannis, N. Does covalency really increase across the 5f series? A comparison of molecular orbital, natural population, spin and electron density analyses of AnCp3 (An = Th-Cm; Cp = η5-C5H5). Dalton Trans. 40, 124–131 (2011).
Su, J. et al. Energy-degeneracy-driven covalency in actinide bonding. J. Am. Chem. Soc. 140, 17977–17984 (2018).
Chandrasekar, A. & Ghanty, T. K. Uncovering heavy actinide covalency: implications for minor actinide partitioning. Inorg. Chem. 58, 3744–3753 (2019).
Polinski, M. J. et al. Unusual structure, bonding and properties in a californium borate. Nat. Chem. 6, 387–392 (2014).
Silver, M. A. et al. Characterization of berkelium(III) dipicolinate and borate compounds in solution and the solid state. Science 353, aaf3762 (2016).
Sperling, J. M. et al. Compression of curium pyrrolidine-dithiocarbamate enhances covalency. Nature 583, 396–399 (2020).
Kerridge, A. Quantification of f-element covalency through analysis of the electron density: insights from simulation. Chem. Commun. 53, 6685–6695 (2017).
Kelley, M. P. et al. On the origin of covalent bonding in heavy actinides. J. Am. Chem. Soc. 139, 9901–9908 (2017).
Goodwin, C. A. P. et al. Isolation and characterization of a californium metallocene. Nature 599, 421–424 (2021).
Strittmatter, R. J. & Bursten, B. E. Bonding in tris(η5-cyclopentadienyl) actinide complexes. 5. A comparison of the bonding in neptunium, plutonium, and transplutonium compounds with that in lanthanide compounds and a transition-metal analogue. J. Am. Chem. Soc. 113, 552–559 (1991).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Baumgärtner, F., Fischer, E. O., Kanellakopulos, B. & Laubereau, P. Triscyclopentadienylplutonium. Angew. Chem. Int. Ed. 4, 878 (1965).
Baumgärtner, F., Fischer, E. O., Kanellakopulos, B. & Laubereau, P. Tri(cyclopentadienyl)americium(III). Angew. Chem. Int. Ed. 5, 134–135 (1966).
Laubereau, P. G. & Burns, J. H. Tricyclopentadienyl-curium. Inorg. Nucl. Chem. Lett. 6, 59–63 (1970).
Laubereau, P. G. & Burns, J. H. Microchemical preparation of tricyclopentadienyl compounds of berkelium, californium, and some lanthanide elements. Inorg. Chem. 9, 1091–1095 (1970).
Apostolidis, C., Dutkiewicz, M. S., Kovács, A. & Walter, O. Solid-state structure of tris-cyclopentadienide uranium(III) and plutonium(III). Chem. Eur. J. 24, 2841–2844 (2018).
Dutkiewicz, M. S., Apostolidis, C., Walter, O. & Arnold, P. L. Reduction chemistry of neptunium cyclopentadienide complexes: from structure to understanding. Chem. Sci. 8, 2553–2561 (2017).
Maier, R., Kanellakopulos, B., Apostolidis, C., Meyer, D. & Rebizant, J. Molecular structure and charge distribution in organometallics of the 4f and 5f elements — V: crystal and molecular structure of tetrakis(η5-cyclopentadienyl)-thorium(IV) and the temperature dependence of its electrical dipole moment. J. Alloy. Compd. 190, 269–271 (1993).
Burns, J. H. The molecular and crystal structure of tetracyclopentadienyluranium(IV). J. Organomet. Chem. 69, 225–233 (1974).
Baumgärtner, F., Fischer, E. O., Kanellakopulos, B. & Laubereau, P. Tetrakis(cyclopentadienyl)protactinium(IV). Angew. Chem. Int. Ed. 8, 202–202 (1969).
Baumgärtner, F., Fischer, E. O., Kanellakopulos, B. & Laubereau, P. Tetrakis(cyclopentadienyl)neptunium(IV). Angew. Chem. Int. Ed. 7, 634–634 (1968).
Grignard, F. A. V. Sur quelques nouvelles combinaisons organométalliques du magnésium et leur application à des synthèses d’alcools et d’hydrocarbures. C. R. Hebd. Séances Acad. Sci. 130, 1322–1323 (1900).
Mond, L., Langer, C. & Quincke, F. Action of carbon monoxide on nickel. J. Chem. Soc. Trans. 57, 749–753 (1890).
Muetterties, E. L., Bleeke, J. R., Wucherer, E. J. & Albright, T. Structural, stereochemical, and electronic features of arene-metal complexes. Chem. Rev. 82, 499–525 (1982).
Long, N. J. Metallocenes: An Introduction to Sandwich Complexes (Blackwell Science, 1998).
Streitwieser, A. & Mueller-Westerhoff, U. Bis(cyclooctatetraenyl)uranium (uranocene). A new class of sandwich complexes that utilize atomic f orbitals. J. Am. Chem. Soc. 90, 7364–7364 (1968).
Streitwieser, A. et al. Preparation and properties of uranocene, di-π-cyclooctatetraeneuranium(IV). J. Am. Chem. Soc. 95, 8644–8649 (1973).
Lukens, W. W. et al. The roles of 4f- and 5f-orbitals in bonding: a magnetochemical, crystal field, density functional theory, and multi-reference wavefunction study. Dalton Trans. 45, 11508–11521 (2016).
Cloke, F. G. N. & Tsoureas, N. in Comprehensive Organometallic Chemistry IV 4th edn (eds Parkin, G., Meyer, K. & O’hare, D.) 405–459 (Elsevier, 2022).
Wilkinson, G. & Birmingham, J. M. Cyclopentadienyl compounds of Sc, Y, La, Ce and some lanthanide elements. J. Am. Chem. Soc. 76, 6210 (1954).
Reynolds, L. T. & Wilkinson, G. π-Cyclopentadienyl compounds of uranium-IV and thorium-IV. J. Inorg. Nucl. Chem. 2, 246–253 (1956).
Karraker, D. G. & Stone, J. A. Mössbauer and magnetic susceptibility studies of uranium(III), uranium(IV), neptunium(IV) compounds with the cyclopentadiene ion. Inorg. Chem. 11, 1742–1746 (1972).
Calderazzo, F. in Encyclopedia of Inorganic Chemistry (2006).
Nolan, S. P. & Marks, T. J. Spectroscopic detection of organolanthanide dihydrogen and olefin complexes. J. Am. Chem. Soc. 111, 8538–8540 (1989).
Sheline, R. K. & Slater, J. L. Spectral evidence for lanthanoid and actinoid carbonyl compounds. Angew. Chem. Int. Ed. 14, 309–313 (1975).
Slater, J. L., DeVore, T. C. & Calder, V. Detection of neodymium and ytterbium carbonyls using matrix isolation. Inorg. Chem. 12, 1918–1921 (1973).
Selg, P., Brintzinger, H. H., Schultz, M. & Andersen, R. A. Solution infrared spectroscopic studies on equilibrium reactions of CO with the decamethylmetallocenes Cp*2MII, where MII = Mg, Ca, Sr, Ba, Sm, Eu, Yb. Organometallics 21, 3100–3107 (2002).
del Mar Conejo, M. et al. Carbon monoxide and isocyanide complexes of trivalent uranium metallocenes. Chem. Eur. J. 5, 3000–3009 (1999).
Evans, W. J., Kozimor, S. A., Nyce, G. W. & Ziller, J. W. Comparative reactivity of sterically crowded nf3 (C5Me5)3Nd and (C5Me5)3U complexes with CO: formation of a nonclassical carbonium ion versus an f element metal carbonyl complex. J. Am. Chem. Soc. 125, 13831–13835 (2003).
Ward, R. J., Rosal, I. D., Kelley, S. P., Maron, L. & Walensky, J. R. Isolation of C1 through C4 derivatives from CO using heteroleptic uranium(III) metallocene aryloxide complexes. Chem. Sci. 14, 2024–2032 (2023).
Maron, L., Eisenstein, O. & Andersen, R. A. The bond between CO and Cp′3U in Cp′3U(CO) involves back-bonding from the Cp′3U ligand-based orbitals of π-symmetry, where Cp′ represents a substituted cyclopentadienyl ligand. Organometallics 28, 3629–3635 (2009).
Ortu, F. Rare earth starting materials and methodologies for synthetic chemistry. Chem. Rev. 122, 6040–6116 (2022).
Asprey, L. B., Keenan, T. K. & Kruse, F. H. Preparation and crystal data for lanthanide and actinide triiodides. Inorg. Chem. 3, 1137–1141 (1964).
Enriquez, A. E., Matonic, J. H., Scott, B. L. & Neu, M. P. Preparation and structures of homoleptic Pu(III) and U(III) acetonitrile salts. Chem. Commun. https://doi.org/10.1039/B303558A (2003).
Avens, L. R. et al. A convenient entry into trivalent actinide chemistry: synthesis and characterization of AnI3(THF)4 and An[N(SiMe3)2]3 (An = U, Np, Pu). Inorg. Chem. 33, 2248–2256 (1994).
Copping, R. et al. A versatile precursor for non-aqueous neptunyl(V) chemistry. Chem. Commun. 47, 5497–5499 (2011).
Reilly, S. D., Brown, J. L., Scott, B. L. & Gaunt, A. J. Synthesis and characterization of NpCl4(DME)2 and PuCl4(DME)2 neutral transuranic An(IV) starting materials. Dalton Trans. 43, 1498–1501 (2014).
Whitefoot, M. A., Perales, D., Zeller, M. & Bart, S. C. Synthesis of non-aqueous neptunium(III) halide solvates from NpO2. Chem. Eur. J. 27, 18054–18057 (2021).
Goodwin, C. A. P., Janicke, M. T., Scott, B. L. & Gaunt, A. J. [AnI3(THF)4] (An = Np, Pu) preparation bypassing An0 metal precursors: access to Np3+/Pu3+ nonaqueous and organometallic complexes. J. Am. Chem. Soc. 143, 20680–20696 (2021).
Gaunt, A. J., Reilly, S. D., Hayton, T. W., Scott, B. L. & Neu, M. P. An entry route into non-aqueous plutonyl coordination chemistry. Chem. Commun. https://doi.org/10.1039/B618577K (2007).
Reilly, S. D., Scott, B. L. & Gaunt, A. J. [N(n-Bu)4]2[Pu(NO3)6] and [N(n-Bu)4]2[PuCl6]: starting materials to facilitate nonaqueous plutonium(IV) chemistry. Inorg. Chem. 51, 9165–9167 (2012).
Gaunt, A. J., Enriquez, A. E., Reilly, S. D., Scott, B. L. & Neu, M. P. Structural characterization of Pu[N(SiMe3)2]3, a synthetically useful nonaqueous plutonium(III) precursor. Inorg. Chem. 47, 26–28 (2008).
Gaunt, A. J. et al. Low-valent molecular plutonium halide complexes. Inorg. Chem. 47, 8412–8419 (2008).
Galley, S. S. et al. Conversion of americia to anhydrous trivalent americium halides. Organometallics 38, 606–609 (2019).
Long, B. N. et al. Altering the spectroscopy, electronic structure, and bonding of organometallic curium(III) upon coordination of 4,4′-bipyridine. Nat. Commun. 14, 3774 (2023).
Russo, D. R. et al. Berkelium–carbon bonding in a tetravalent berkelocene. Science 387, 974–978 (2025).
Goodwin, C. A. P. et al. N-Heterocyclic carbene to actinide d-based π-bonding correlates with observed metal–carbene bond length shortening versus lanthanide congeners. J. Am. Chem. Soc. 146, 10367–10380 (2024).
Clark, D. L., Sattelberger, A. P., Bott, S. G. & Vrtis, R. N. Lewis base adducts of uranium triiodide: a new class of synthetically useful precursors for trivalent uranium chemistry. Inorg. Chem. 28, 1771–1773 (1989).
Clark, D. L., Frankcom, T. M., Miller, M. M. & Watkin, J. G. Facile solution routes to hydrocarbon-soluble Lewis base adducts of thorium tetrahalides. synthesis, characterization, and X-ray structure of ThBr4(THF)4. Inorg. Chem. 31, 1628–1633 (2002).
Fetrow, T. V., Grabow, J. P., Leddy, J. & Daly, S. R. Convenient syntheses of trivalent uranium halide starting materials without uranium metal. Inorg. Chem. 60, 7593–7601 (2021).
Travia, N. E., Monreal, M. J., Scott, B. L. & Kiplinger, J. L. Thorium-mediated ring-opening of tetrahydrofuran and the development of a new thorium starting material: preparation and chemistry of ThI4(DME)2. Dalton Trans. 41, 14514–14523 (2012).
Cantat, T., Scott, B. L. & Kiplinger, J. L. Convenient access to the anhydrous thorium tetrachloride complexes ThCl4(DME)2, ThCl4(1,4-dioxane)2 and ThCl4(THF)3.5 using commercially available and inexpensive starting materials. Chem. Commun. 46, 919–921 (2010).
Monreal, M. J. et al. UI4(1,4-dioxane)2, [UCl4(1,4-dioxane)]2, and UI3(1,4-dioxane)1.5: stable and versatile starting materials for low- and high-valent uranium chemistry. Organometallics 30, 2031–2038 (2011).
Lopez, L. M., Uible, M. C., Zeller, M. & Bart, S. C. Lewis base adducts of NpCl4. Chem. Commun. 60, 5956–5959 (2024).
Windorff, C. J., Celis-Barros, C., Sperling, J. M., McKinnon, N. C. & Albrecht-Schmitt, T. E. Probing a variation of the inverse-trans-influence in americium and lanthanide tribromide tris(tricyclohexylphosphine oxide) complexes. Chem. Sci. 11, 2770–2782 (2020).
Wedal, J. C. et al. Synthesis of trimethyltriazacyclohexane (Me3tach) sandwich complexes of uranium, neptunium, and plutonium triiodides: (Me3tach)2AnI3. Inorg. Chem. 62, 5897–5905 (2023).
Baumgärtner, F., Fischer, E. O. & Laubereau, P. Über die existenz von tri-cyclopentadienyl-neptunium(IV)-halogenid. Naturwissenschaften 52, 560–560 (1965).
Crisler, L. R. & Eggerman, W. G. A novel synthesis of triscyclopentadienyl plutonium (III). J. Inorg. Nucl. Chem. 36, 1424–1426 (1974).
Laubereau, P. G. The formation of dicyclopentadienylberkeliumchloride. Inorg. Nucl. Chem. Lett. 6, 611–616 (1970).
Karraker, D. G. & Stone, J. A. Covalency of neptunium(IV) tris(cyclopentadienyl) compounds from Mössbauer spectra. Inorg. Chem. 18, 2205–2207 (1979).
Karraker, D. G. Cyclopentadienyl bonding in bis(cyclopentadienyl)neptunium(IV) compounds from 237Np Mössbauer spectra. Inorg. Chem. 22, 503–506 (1983).
Karraker, D. G., Stone, J. A., Jones, E. R. & Edelstein, N. Bis(cyclooctatetraenyl)neptunium(IV) and bis(cyclooctatetraenyl)plutonium(IV). J. Am. Chem. Soc. 92, 4841–4845 (1970).
Bagnall, K. W. et al. Anionic tris(cyclopentadienyl)actinide(IV) complexes. J. Chem. Soc. Dalton Trans. https://doi.org/10.1039/DT9820001999 (1982).
Bagnall, K. W., Plews, M. J. & Brown, D. Some oxygen donor complexes of cyclopentadienylneptunium(IV) trichloride. J. Less Common Met. 90, 29–35 (1983).
Bagnall, K. W., Payne, G. F. & Brown, D. Phosphine oxide complexes of cyclopentadienyl neptunium(IV) and plutonium(IV) N-thiocyanates. J. Less Common Met. 116, 333–339 (1986).
Bagnall, K. W., Payne, G. F., Alcock, N. W., Flanders, D. J. & Brown, D. Actinide structural studies. Part 8. Some new oxygen-donor complexes of trichloro(cyclopentadienyl)neptunium(IV); the crystal structure of trichloro(η5-cyclopentadienyl)bis(methyldiphenylphosphine oxide)neptunium(IV). J. Chem. Soc. Dalton Trans. https://doi.org/10.1039/DT9860000783 (1986).
De Ridder, D. J. A., Apostolidis, C., Rebizant, J., Kanellakopulos, B. & Maier, R. Tris(η5-cyclopentadienyl)phenolatoneptunium(IV). Acta Crystallogr. C 52, 1436–1438 (1996).
Fischer, E. O. & Hristidu, Y. Über aromatenkomplexe von metallen LVII. Uran-tetracyclopentadienyl. Z. Naturforsch. B 17, 275–276 (1962).
Fischer, E. O. & Treiber, A. Über aromatenkomplexe von metallen LIX. Thorium-tetra-cyclopentadienyl. Z. Naturforsch. B 17, 276–277 (1962).
Bagnall, K. W., Plews, M. J. & Brown, D. Tris(cyclopentadienyl)plutonium(IV) chloride and thiocyanate, (η5-C5H5)3PuCl and (η5-C5H5)3Pu(NCS). J. Organomet. Chem. 224, 263–266 (1982).
Goodwin, C. A. P. et al. [Am(C5Me4H)3]: an organometallic americium complex. Angew. Chem. Int. Ed. 58, 11695–11699 (2019).
Schumann, H., Glanz, M., Hemling, H. & Ekkehard Hahn, F. Organometallic compounds of the lanthanides. 93. Tetramethylcyclopentadienyl complexes of selected 4f-elements. Z. Anorg. Allg. Chem. 621, 341–345 (1995).
Evans, W. J., Rego, D. B. & Ziller, J. W. Synthesis, structure, and 15N NMR studies of paramagnetic lanthanide complexes obtained by reduction of dinitrogen. Inorg. Chem. 45, 10790–10798 (2006).
Siladke, N. A. et al. Actinide metallocene hydride chemistry: C–H activation in tetramethylcyclopentadienyl ligands to form [μ-η5-C5Me3H(CH2)-κC]2− tuck-over ligands in a tetrathorium octahydride complex. Organometallics 32, 6522–6531 (2013).
Windorff, C. J. et al. Small-scale metal-based syntheses of lanthanide iodide, amide, and cyclopentadienyl complexes as analogues for transuranic reactions. Inorg. Chem. 56, 11981–11989 (2017).
Long, B. N., Sperling, J. M., Windorff, C. J. & Albrecht-Schönzart, T. E. Characterization of the organoamericium complex Cp′3Am. Organometallics 42, 3048–3052 (2023).
Wedal, J. C. et al. Structural variations in cyclopentadienyl uranium(III) iodide complexes. J. Coord. Chem. 74, 74–91 (2020).
Carnall, W. T. & Wybourne, B. G. Electronic energy levels of the lighter actinides: U3+, Np3+, Pu3+, Am3+, and Cm3+. J. Chem. Phys. 40, 3428–3433 (1964).
Carnall, W. T. A systematic analysis of the spectra of trivalent actinide chlorides in D3h site symmetry. J. Chem. Phys. 96, 8713–8726 (1992).
Sonnenberger, D. C. & Gaudiello, J. Synthesis and cyclic voltammetric study of bis(pentamethylcyclopentadienyl)neptunium dichloride. J. Less Common Met. 126, 411–414 (1986).
Finke, R. G., Gaughan, G. & Voegeli, R. Organoactinide electrochemistry. A cyclic voltammetric and coulometric study of (C5Me5)2UCl2, [(C5Me5)2UCl2·THF]−Na+, (C5Me5)2UCl·THF and (C5Me5)2ThCl2. J. Organomet. Chem. 229, 179–184 (1982).
Dietrich, H. M., Ziller, J. W., Anwander, R. & Evans, W. J. Reactivity of (C5Me5)2UMe2 and (C5Me5)2UMeCl toward group 13 alkyls. Organometallics 28, 1173–1179 (2009).
Straub, T. et al. Intermolecular hydroamination of terminal alkynes catalyzed by organoactinide complexes. scope and mechanistic studies. Organometallics 20, 5017–5035 (2001).
Spirlet, M. R., Rebizant, J., Apostolidis, C. & Kanellakopulos, B. Bis(cyclopentadienyl) actinide(IV) compounds. I. The structure of dichlorobis(pentamethyl-η5-cyclopentadienyl)uranium(IV) and dichlorobis(pentamethyl-η5-cyclopentadienyl)thorium(IV). Acta Crystallogr. C 48, 2135–2137 (1992).
Kovacs, A., Apostolidis, C. & Walter, O. Competing metal-ligand interactions in tris(cyclopentadienyl)-cyclohexylisonitrile complexes of trivalent actinides and lanthanides. Molecules 27, 3811 (2022).
Burns, J. H. & Baldwin, W. H. Molecular and crystal structure of the adduct of cyclohexylisonitrile and praseodymium tricyclopentadienide. J. Organomet. Chem. 120, 361–368 (1976).
Kanellakopulos, B., Aderhold, C., Dornberger, E., Müller, W. & Baybarz, R. D. The energy gap of the lowest manifolds in americium triscyclopentadienide. Radiochim. Acta 25, 89–92 (1978).
Arnold, P. L. et al. Subtle interactions and electron transfer between UIII, NpIII, or PuIII and uranyl mediated by the oxo group. Angew. Chem. Int. Ed. 55, 12797–12801 (2016).
Arnold, P. L., Patel, D., Pécharman, A.-F., Wilson, C. & Love, J. B. Equatorial ligand substitution by hydroxide in uranyl Pacman complexes of a Schiff-base pyrrole macrocycle. Dalton Trans. 39, 3501–3508 (2010).
Evans, W. J. Tutorial on the role of cyclopentadienyl ligands in the discovery of molecular complexes of the rare-earth and actinide metals in new oxidation states. Organometallics 35, 3088–3100 (2016).
Windorff, C. J. et al. Identification of the formal +2 oxidation state of plutonium: synthesis and characterization of {PuII[C5H3(SiMe3)2]3}−. J. Am. Chem. Soc. 139, 3970–3973 (2017).
Su, J. et al. Identification of the formal +2 oxidation state of neptunium: synthesis and characterization of {NpII[C5H3(SiMe3)2]3}1−. J. Am. Chem. Soc. 140, 7425–7428 (2018).
Langeslay, R. R., Fieser, M. E., Ziller, J. W., Furche, F. & Evans, W. J. Synthesis, structure, and reactivity of crystalline molecular complexes of the {[C5H3(SiMe3)2]3Th}1− anion containing thorium in the formal +2 oxidation state. Chem. Sci. 6, 517–521 (2015).
Windorff, C. J. et al. Expanding the chemistry of molecular U2+ complexes: synthesis, characterization, and reactivity of the {[C5H3(SiMe3)2]3U}− anion. Chem. Eur. J. 22, 772–782 (2016).
MacDonald, M. R. et al. Identification of the +2 oxidation state for uranium in a crystalline molecular complex, [K(2.2.2-cryptand)][(C5H4SiMe3)3U]. J. Am. Chem. Soc. 135, 13310–13313 (2013).
Zalkin, A., Brennan, J. G. & Andersen, R. A. Tris(trimethylsilylcyclopentadienyl)uranium(III). Acta Crystallogr. C 44, 2104–2106 (1988).
Krinsky, J. L., Minasian, S. G. & Arnold, J. Covalent lanthanide chemistry near the limit of weak bonding: observation of (CpSiMe3)3Ce–ECp* and a comprehensive density functional theory analysis of Cp3Ln−ECp (E = Al, Ga). Inorg. Chem. 50, 345–357 (2011).
Minasian, S. G. et al. A comparison of 4f vs 5f metal–metal bonds in (CpSiMe3)3M–ECp* (M = Nd, U; E = Al, Ga; Cp* = C5Me5): synthesis, thermodynamics, magnetism, and electronic structure. J. Am. Chem. Soc. 131, 13767–13783 (2009).
Peterson, J. K., MacDonald, M. R., Ziller, J. W. & Evans, W. J. Synthetic aspects of (C5H4SiMe3)3Ln rare-earth chemistry: formation of (C5H4SiMe3)3Lu via [(C5H4SiMe3)2Ln]+ metallocene precursors. Organometallics 32, 2625–2631 (2013).
Long, B. N., Sperling, J. M., Windorff, C. J., Huffman, Z. K. & Albrecht-Schonzart, T. E. Expanding transuranium organoactinide chemistry: synthesis and characterization of (Cp′3M)2(μ-4,4′-bpy) (M = Ce, Np, Pu). Inorg. Chem. 62, 6368–6374 (2023).
Long, B. N. et al. Cyclopentadienyl coordination induces unexpected ionic Am-N bonding in an americium bipyridyl complex. Nat. Commun. 13, 201 (2022).
Mehdoui, T., Berthet, J. C., Thuery, P. & Ephritikhine, M. CCDC 958634: experimental crystal structure determination. CCDC https://doi.org/10.5517/cc115jpf (2013).
Formanuik, A. et al. Double reduction of 4,4′-bipyridine and reductive coupling of pyridine by two thorium(III) single-electron transfers. Chem. Eur. J. 23, 2290–2293 (2017).
Nugent, L. J., Baybarz, R. D., Burnett, J. L. & Ryan, J. L. Electron-transfer and f-d absorption bands of some lanthanide and actinide complexes and the standard (II-III) oxidation potential for each member of the lanthanide and actinide series. J. Phys. Chem. 77, 1528–1539 (1973).
Zalkin, A. & Raymond, K. N. Structure of di-π-cyclooctatetraeneuranium (uranocene). J. Am. Chem. Soc. 91, 5667–5668 (1969).
Avdeef, A., Raymond, K. N., Hodgson, K. O. & Zalkin, A. Two isostructural actinide π complexes. Crystal and molecular structure of bis(cyclooctatetraenyl)uranium(IV), U(C8H8)2, and bis(cyclooctatetraenyl)thorium(IV), Th(C8H8)2. Inorg. Chem. 11, 1083–1088 (1972).
Streitwieser, A. Jr. & Yoshida, N. Di-π-cyclooctatetraenethorium. J. Am. Chem. Soc. 91, 7528 (1969).
Kurras, E. & Kruger, C., CCDC 256935: experimental crystal structure determination. CCDC https://doi.org/10.5517/cc8mc7g (2005).
De Ridder, D. J. A., Rebizant, J., Apostolidis, C., Kanellakopulos, B. & Dornberger, E. Bis(cyclooctatetraenyl)neptunium(IV). Acta Crystallogr. C 52, 597–600 (1996).
Windorff, C. J. et al. A single small-scale plutonium redox reaction system yields three crystallographically-characterizable organoplutonium complexes. Inorg. Chem. 59, 13301–13314 (2020).
Magnani, N. et al. Magnetic memory effect in a transuranic mononuclear complex. Angew. Chem. Int. Ed. 50, 1696–1698 (2011).
Starks, D. F., Parsons, T. C., Streitwieser, A. & Edelstein, N. Bis(π-cyclooctatetraene)protactinium. Inorg. Chem. 13, 1307–1308 (1974).
Karraker, D. G. Bis(alkylcyclooctatetraenyl)actinide(IV) compounds. Inorg. Chem. 12, 1105–1108 (1973).
Streitwieser, A. Jr., Dempf, D., La Mar, G. N., Karraker, D. G. & Edelstein, N. Bis(1,3,5,7-tetramethylcyclooctatetraene)uranium(IV) and bis(1,3,5,7-tetramethylcyclooctatetraene)-neptunium(IV). Proton magnetic resonance spectrum and the question of f-orbital covalency. J. Am. Chem. Soc. 93, 7343–7344 (1971).
Solar, J. P., Burghard, H. P. G., Banks, R. H., Streitwieser, A. & Brown, D. Bis(η8-1,3,5,7-tetramethylcyclooctatetraene) compounds of protactinium, neptunium, and plutonium. Inorg. Chem. 19, 2186–2188 (1980).
Boussie, T. R., Eisenberg, D. C., Rigsbee, J., Streitwieser, A. & Zalkin, A. Structures of organo-f-element compounds differing in the oxidation state of the central metal: crystal structures of bis([8]annulene) complexes of cerium(IV), ytterbium(III), and uranium(III). Organometallics 10, 1922–1928 (1991).
Hodgson, K. O. & Raymond, K. N. Rotomeric configurations of a methyl-substituted cyclooctatetraene dianion complex of uranium(IV). Crystal and molecular structure of bis(1,3,5,7-tetramethylcyclooctatetraenyl)uranium(IV), U(C8H4(CH3)4)2. Inorg. Chem. 12, 458–466 (1973).
Thompson, S. G., Cunningham, B. B. & Seaborg, G. T. Chemical properties of berkelium. J. Am. Chem. Soc. 72, 2798–2801 (1950).
Bratsch, S. G. & Lagowski, J. J. Actinide thermodynamic predictions. 3. Thermodynamics of compounds and aquo-ions of the 2+, 3+, and 4+ oxidation states and standard electrode potentials at 298.15 K. J. Phys. Chem. 90, 307–312 (1986).
Russo, D. R. et al. Synthesis and characterization of isostructural annulated actinocenes. Chem. Commun. 61, 2504–2507 (2025).
Apostolidis, C. et al. A structurally characterized organometallic plutonium(IV) complex. Angew. Chem. Int. Ed. 56, 5066–5070 (2017).
Burton, N. C., Cloke, F. G. N., Hitchcock, P. B., de Lemos, H. C. & Sameh, A. A. Scandium, yttrium, uranium, and thorium derivatives of the 1,4-bis(trimethylsilyl)cyclo-octatetraene dianion; the X-ray crystal structure of [Sc2(η-C8H6{1,4-(SiMe3)2})2(µ-Cl)2(µ-THF)] (THF = tetrahydrofuran). J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39890001462 (1989).
Clegg, W. & A., M., CCDC 284020: experimental crystal structure determination. CCDC https://doi.org/10.5517/cc9jjy8 (2005).
Rausch, J. et al. One ligand fits all: lanthanide and actinide sandwich complexes comprising the 1,4-bis(trimethylsilyl)cyclooctatetraenyl (= COT′′) ligand. N. J. Chem. 39, 7656–7666 (2015).
Le Roy, J. J., Gorelsky, S. I., Korobkov, I. & Murugesu, M. Slow magnetic relaxation in uranium(III) and neodymium(III) cyclooctatetraenyl complexes. Organometallics 34, 1415–1418 (2015).
Lorenz, V. et al. Unprecedented bending and rearrangement of f-element sandwich complexes induced by superbulky cyclooctatetraenide ligands. J. Am. Chem. Soc. 133, 1257–1259 (2011).
Chen, Q. W., Ding, Y. S., Zhu, X. F., Wang, B. W. & Zheng, Z. Substituent positioning effects on the magnetic properties of sandwich-type erbium(III) complexes with bis(trimethylsilyl)-substituted cyclooctatetraenyl ligands. Inorg. Chem. 63, 9511–9519 (2023).
Karraker, D. G. Absorption spectrum of KAm(C8H8)2 in the solution. J. Inorg. Nucl. Chem. 39, 87–89 (1977).
Karraker, D. G. & Stone, J. A. Bis(cyclooctatetraenyl)neptunium(III) and -plutonium(III) compounds. J. Am. Chem. Soc. 96, 6885–6888 (1974).
Kot, W. K., Shalimoff, G. V., Edelstein, N. M., Edelman, M. A. & Lappert, M. F. [ThIII[η5-C5H3(SiMe3)2]3], an actinide compound with a 6d1 ground state. J. Am. Chem. Soc. 110, 986–987 (1988).
Parry, J. S., Cloke, F. G. N., Coles, S. J. & Hursthouse, M. B. Synthesis and characterization of the first sandwich complex of trivalent thorium: a structural comparison with the uranium analogue. J. Am. Chem. Soc. 121, 6867–6871 (1999).
Hodgson, K. O. & Raymond, K. N. An ion pair complex formed between bis(cyclooctatetraenyl)cerium(III) anion and an ether-coordinated potassium cation. The crystal and molecular structure of [K(CH3OCH2CH2)2O][Ce(C8H8)2]. Inorg. Chem. 11, 3030–3035 (1972).
Palumbo, C. T., Fieser, M. E., Ziller, J. W. & Evans, W. J. Reactivity of complexes of 4fn5d1 and 4fn+1 Ln2+ ions with cyclooctatetraene. Organometallics 36, 3721–3728 (2017).
Kotyk, C. M., MacDonald, M. R., Ziller, J. W. & Evans, W. J. Reactivity of the Ln2+ complexes [K(2.2.2-cryptand)][(C5H4SiMe3)3Ln]: reduction of naphthalene and biphenyl. Organometallics 34, 2287–2295 (2015).
Jena, R. et al. A rare isocyanide derived from an unprecedented neutral yttrium(II) bis(amide) complex. Chem. Sci. 14, 4257–4264 (2023).
Billow, B. S. et al. Synthesis and characterization of a neutral U(II) arene sandwich complex. J. Am. Chem. Soc. 140, 17369–17373 (2018).
Straub, M. D. et al. A uranium(II) arene complex that acts as a uranium(I) synthon. J. Am. Chem. Soc. 143, 19748–19760 (2021).
Keener, M. et al. Multielectron redox chemistry of uranium by accessing the +II oxidation state and enabling reduction to a U(I) synthon. J. Am. Chem. Soc. 145, 16271–16283 (2023).
Cloke, F. G. N. Zero oxidation state compounds of scandium, yttrium, and the lanthanides. Chem. Soc. Rev. 22, 17–24 (1993).
La Pierre, H. S., Scheurer, A., Heinemann, F. W., Hieringer, W. & Meyer, K. Synthesis and characterization of a uranium(II) monoarene complex supported by δ backbonding. Angew. Chem. Int. Ed. 53, 7158–7162 (2014).
Dutkiewicz, M. S. et al. Organometallic neptunium(III) complexes. Nat. Chem. 8, 797–802 (2016).
Ilango, S., Vidjayacoumar, B. & Gambarotta, S. Samarium complexes of a σ-/π-pyrrolide/arene based macrocyclic ligand. Dalton Trans. 39, 6853–6857 (2010).
Murillo, J. et al. Synthesis and comparison of iso-structural f-block metal complexes (Ce, U, Np, Pu) featuring η6-arene interactions. Chem. Sci. 14, 7438–7446 (2023).
Pattenaude, S. A., Anderson, N. H., Bart, S. C., Gaunt, A. J. & Scott, B. L. Non-aqueous neptunium and plutonium redox behaviour in THF — access to a rare Np(III) synthetic precursor. Chem. Commun. 54, 6113–6116 (2018).
Pividori, D. et al. Uranium going the soft way: low-valent uranium(III) coordinated to an arene-anchored tris-thiophenolate ligand. Inorg. Chem. 60, 16455–16465 (2021).
La Pierre, H. S., Kameo, H., Halter, D. P., Heinemann, F. W. & Meyer, K. Coordination and redox isomerization in the reduction of a uranium(III) monoarene complex. Angew. Chem. Int. Ed. 53, 7154–7157 (2014).
Mills, D. P. & Evans, P. f-Block phospholyl and arsolyl chemistry. Chem. Eur. J. 27, 6645–6665 (2021).
Le Floch, P. Phosphaalkene, phospholyl and phosphinine ligands: new tools in coordination chemistry and catalysis. Coord. Chem. Rev. 250, 627–681 (2006).
Černá, M. et al. Isostructural σ-hydrocarbyl phospholide complexes of uranium, neptunium, and plutonium. Chem. Commun. 58, 13278–13281 (2022).
Gradoz, P. et al. Synthesis, crystal structure and some derivatives of the chlorotris(tetramethylphospholyl)uranium. J. Chem. Soc. Chem. Commun. https://doi.org/10.1039/C39920001720 (1992).
Izod, K., Liddle, S. T. & Clegg, W. A convenient route to lanthanide triiodide THF solvates. Crystal structures of LnI3(THF)4 [Ln = Pr] and LnI3(THF)3.5 [Ln = Nd, Gd, Y]. Inorg. Chem. 43, 214–218 (2004).
Carpenter, S. H. et al. Employing a template synthesis to access diastereopure Np(IV) and U(IV) complexes and analysis of their 5f orbitals in bonding. Inorg. Chem. Front. 11, 3731–3743 (2024).
Fichter, S. et al. Enantiomerically pure tetravalent neptunium amidinates: synthesis and characterization. Chem. Eur. J. 26, 8867–8870 (2020).
Kloditz, R. et al. Series of tetravalent actinide amidinates: structure determination and bonding analysis. Inorg. Chem. 59, 15670–15680 (2020).
Zwick, B. D., Sattelberger, A. P. & Avens, L. R. in Transuranium Elements: A Half Century (eds Morss, L. R. & Fuger, J.) 239–246 (American Chemical Society, 1992)
Myers, A. J., Tarlton, M. L., Kelley, S. P., Lukens, W. W. & Walensky, J. R. Synthesis and utility of neptunium(III) hydrocarbyl complex. Angew. Chem. Int. Ed. 58, 14891–14895 (2019).
Kihara, S. et al. A critical evaluation of the redox properties of uranium, neptunium and plutonium ions in acidic aqueous solutions. Pure Appl. Chem. 71, 1771–1807 (1999).
Behrle, A. C. et al. Uranium(III) and thorium(IV) alkyl complexes as potential starting materials. Chem. Commun. 52, 14373–14375 (2016).
Baker, R. J. The coordination and organometallic chemistry of UI3 and U{N(SiMe3)2}3: synthetic reagents par excellence. Coord. Chem. Rev. 256, 2843–2871 (2012).
Goodwin, C. A. P. & Mills, D. P. in Specialist Periodical Reports: Organometallic Chemistry Organometallic Chemistry Vol. 41, 123–156 (Royal Society of Chemistry, 2017).
Hervé, A. et al. UIII–CN versus UIV–NC coordination in tris(silylamide) complexes. Inorg. Chem. 54, 2474–2490 (2015).
Bradley, D. C., Ghotra, J. S. & Hart, F. A. Tris-[bis-trimethylsilylamido]-monochloro-thorium(IV); a 4-coordinated thorium compound. Inorg. Nucl. Chem. Lett. 10, 209–211 (1974).
Turner, H. W., Andersen, R. A., Zalkin, A. & Templeton, D. H. Chloro-, methyl-, and (tetrahydroborato)tris((hexamethyldisilyl)amido)thorium(IV) and uranium(IV). Crystal structure of (tetrahydroborato)tris((hexamethyldisilyl)amido)thorium(IV). Inorg. Chem. 18, 1221–1224 (1979).
Smiles, D. E., Wu, G., Kaltsoyannis, N. & Hayton, T. W. Thorium–ligand multiple bonds via reductive deprotection of a trityl group. Chem. Sci. 6, 3891–3899 (2015).
Staun, S. L. et al. Expanding the nonaqueous chemistry of neptunium: synthesis and structural characterization of [Np(NR2)3Cl], [Np(NR2)3Cl]−, and [Np{N(R)(SiMe2CH2)}2(NR2)]− (R = SiMe3). Inorg. Chem. 60, 2740–2748 (2021).
Goodwin, C. A. P., Tuna, F., McInnes, E. J. L. & Mills, D. P. Exploring synthetic routes to heteroleptic UIII, UIV, and ThIV bulky bis(silyl) amide complexes. Eur. J. Inorg. Chem. https://doi.org/10.1002/ejic.201800036 (2018).
Roger, M. et al. U(SMes*)n, (n = 3, 4) and Ln(SMes*)3 (Ln = La, Ce, Pr, Nd): lanthanide(III)/actinide(III) differentiation in agostic interactions and an unprecedented η3 ligation mode of the arylthiolate ligand, from X-ray diffraction and DFT analysis. J. Am. Chem. Soc. 128, 8790–8802 (2006).
Berg, J. M. et al. Early actinide alkoxide chemistry. Synthesis, characterization, and molecular structures of Th(IV) and U(IV) aryloxide complexes. J. Am. Chem. Soc. 114, 10811–10821 (1992).
Simpson, S. J., Turner, H. W. & Andersen, R. A. Hydrogen-deuterium exchange: perdeuteriohydridotris(hexamethyldisilylamido)thorium(IV) and -uranium(IV). J. Am. Chem. Soc. 101, 7728–7729 (1979).
Bell, N. L., Maron, L. & Arnold, P. L. Thorium mono- and bis(imido) complexes made by reprotonation of cyclo-metalated amides. J. Am. Chem. Soc. 137, 10492–10495 (2015).
Gregson, M. et al. The inverse-trans-influence in tetravalent lanthanide and actinide bis(carbene) complexes. Nat. Commun. 8, 14137 (2017).
Goodwin, C. A. P. et al. Carbene complexes of neptunium. J. Am. Chem. Soc. 144, 9764–9774 (2022).
Murillo, J. et al. Carbene complexes of plutonium: structure, bonding, and divergent reactivity to lanthanide analogs. J. Am. Chem. Soc. 146, 4098–4111 (2024).
de Frémont, P., Marion, N. & Nolan, S. P. Carbenes: synthesis, properties, and organometallic chemistry. Coord. Chem. Rev. 253, 862–892 (2009).
Nelson, D. J. & Nolan, S. P. Quantifying and understanding the electronic properties of N-heterocyclic carbenes. Chem. Soc. Rev. 42, 6723–6753 (2013).
Arnold, P. L. & Casely, I. J. f-Block N-heterocyclic carbene complexes. Chem. Rev. 109, 3599–3611 (2009).
Gregson, M., Wooles, A. J., Cooper, O. J. & Liddle, S. T. Covalent uranium carbene chemistry. Comments Inorg. Chem. 35, 262–294 (2015).
Ephritikhine, M. Uranium carbene compounds. C. R. Chim. 16, 391–405 (2013).
Nakai, H., Hu, X., Zakharov, L. N., Rheingold, A. L. & Meyer, K. Synthesis and characterization of N-heterocyclic carbene complexes of uranium(III). Inorg. Chem. 43, 855–857 (2004).
Mehdoui, T., Berthet, J. C., Thuéry, P. & Ephritikhine, M. The remarkable efficiency of N-heterocyclic carbenes in lanthanide(III)/actinide(III) differentiation. Chem. Commun. https://doi.org/10.1039/B503526K (2005).
Goodwin, C. A. P. et al. Structural and spectroscopic comparison of soft-Se vs. hard-O donor bonding in trivalent americium/neodymium molecules. Angew. Chem. Int. Ed. 60, 9459–9466 (2021).
Cross, J. N. et al. Comparing the 2,2′-biphenylenedithiophosphinate binding of americium with neodymium and europium. Angew. Chem. Int. Ed. 55, 12755–12759 (2016).
Cary, S. K. et al. A series of dithiocarbamates for americium, curium, and californium. Dalton Trans. 47, 14452–14461 (2018).
Denning, R. G. et al. Covalency in the uranyl ion: a polarized x-ray spectroscopic study. J. Chem. Phys. 117, 8008–8020 (2002).
Kovács, A., Konings, R. J., Gibson, J. K., Infante, I. & Gagliardi, L. Quantum chemical calculations and experimental investigations of molecular actinide oxides. Chem. Rev. 115, 1725–1759 (2015).
Ephritikhine, M. The vitality of uranium molecular chemistry at the dawn of the XXIst century. Dalton Trans. https://doi.org/10.1039/B603463B (2006).
Hayton, T. W. Metal-ligand multiple bonding in uranium: structure and reactivity. Dalton Trans. 39, 1145–1158 (2010).
Wildman, E. P., Balázs, G., Wooles, A. J., Scheer, M. & Liddle, S. T. Thorium-phosphorus triamidoamine complexes containing Th-P single- and multiple-bond interactions. Nat. Commun. 7, 12884 (2016).
Du, J. et al. Thorium-nitrogen multiple bonds provide evidence for pushing-from-below for early actinides. Nat. Commun. 10, 4203 (2019).
Du, J. et al. Thorium- and uranium-azide reductions: a transient dithorium-nitride versus isolable diuranium-nitrides. Chem. Sci. 10, 3738–3745 (2019).
Ren, W., Zi, G., Fang, D.-C. & Walter, M. D. Thorium oxo and sulfido metallocenes: synthesis, structure, reactivity, and computational studies. J. Am. Chem. Soc. 133, 13183–13196 (2011).
Smiles, D. E., Wu, G., Hrobárik, P. & Hayton, T. W. Use of 77Se and 125Te NMR spectroscopy to probe covalency of the actinide-chalcogen bonding in [Th(En){N(SiMe3)2}3]− (E = Se, Te; n = 1, 2) and their oxo-uranium(VI) congeners. J. Am. Chem. Soc. 138, 814–825 (2016).
Smiles, D. E., Wu, G., Hrobárik, P. & Hayton, T. W. Synthesis, thermochemistry, bonding, and 13C NMR chemical shift analysis of a phosphorano-stabilized carbene of thorium. Organometallics 36, 4519–4524 (2017).
Staun, S. L., Sergentu, D.-C., Wu, G., Autschbach, J. & Hayton, T. W. Use of 15N NMR spectroscopy to probe covalency in a thorium nitride. Chem. Sci. 10, 6431–6436 (2019).
Sergentu, D.-C. et al. Probing the electronic structure of a thorium nitride complex by solid-state 15N NMR spectroscopy. Inorg. Chem. 59, 10138–10145 (2020).
Ma, G., Ferguson, M. J., McDonald, R. & Cavell, R. G. Actinide metals with multiple bonds to carbon: synthesis, characterization, and reactivity of U(IV) and Th(IV) bis(iminophosphorano)methandiide pincer carbene complexes. Inorg. Chem. 50, 6500–6508 (2011).
Rupasinghe, D. M. R. Y. P. et al. Actinide–oxygen multiple bonds from air: synthesis and characterization of a thorium oxo supported by redox-active ligands. J. Am. Chem. Soc. 144, 17423–17431 (2022).
King, D. M. et al. Synthesis and structure of a terminal uranium nitride complex. Science 337, 717–720 (2012).
King, D. M. et al. Isolation and characterization of a uranium(VI)-nitride triple bond. Nat. Chem. 5, 482–488 (2013).
Hayton, T. W. Recent developments in actinide-ligand multiple bonding. Chem. Commun. 49, 2956–2973 (2013).
Gibson, J. K. Actinide gas-phase chemistry: reactions of An+ and AnO+ [An = Th, U, Np, Pu, Am] with nitriles and butylamine. Inorg. Chem. 38, 165–173 (1999).
Marçalo, J. & Gibson, J. K. Gas-phase energetics of actinide oxides: an assessment of neutral and cationic monoxides and dioxides from thorium to curium. J. Phys. Chem. A 113, 12599–12606 (2009).
Infante, I. et al. Ionization energies for the actinide mono- and dioxides series, from Th to Cm: theory versus experiment. J. Phys. Chem. A 114, 6007–6015 (2010).
Pereira, C. C., Marsden, C. J., Marçalo, J. & Gibson, J. K. Actinide sulfides in the gas phase: experimental and theoretical studies of the thermochemistry of AnS (An = Ac, Th, Pa, U, Np, Pu, Am and Cm). Phys. Chem. Chem. Phys. 13, 12940–12958 (2011).
Dau, P. D., Vasiliu, M., Peterson, K. A., Dixon, D. A. & Gibson, J. K. Remarkably high stability of late actinide dioxide cations: extending chemistry to pentavalent berkelium and californium. Chem. Eur. J. 23, 17369–17378 (2017).
Dutkiewicz, M. S. et al. A terminal neptunium(V)-mono(oxo) complex. Nat. Chem. 14, 342–349 (2022).
King, D. M. et al. Single-molecule magnetism in a single-ion triamidoamine uranium(V) terminal mono-oxo complex. Angew. Chem. Int. Ed. 52, 4921–4924 (2013).
Pyykkö, P. Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: a summary. J. Phys. Chem. A 119, 2326–2337 (2015).
La Pierre, H. S. & Meyer, K. Uranium–ligand multiple bonding in uranyl analogues, [L═;U═L]n+, and the inverse trans influence. Inorg. Chem. 52, 529–539 (2013).
Lewis, A. J., Carroll, P. J. & Schelter, E. J. Stable uranium(VI) methyl and acetylide complexes and the elucidation of an inverse trans influence ligand series. J. Am. Chem. Soc. 135, 13185–13192 (2013).
Denning, R. G. in Complexes, Clusters and Crystal Chemistry Structure and Bonding Vol. 79, 215–276 (Springer, 1992).
Denning, R. G. Electronic structure and bonding in actinyl ions and their analogs. J. Phys. Chem. A 111, 4125–4143 (2007).
Tatsumi, K. & Hoffmann, R. Bent cis d0 MoO22+ vs. linear trans d0f0 UO22+: a significant role for nonvalence 6p orbitals in uranyl. Inorg. Chem. 19, 2656–2658 (1980).
Seed, J. A. et al. Anomalous magnetism of uranium(IV)-oxo and -imido complexes reveals unusual doubly degenerate electronic ground states. Chem 7, 1666–1680 (2021).
Hayton, T. W. et al. Synthesis of imido analogs of the uranyl ion. Science 310, 1941–1943 (2005).
Brown, J. L. et al. A linear trans-bis(imido) neptunium(V) actinyl analog: NpV(NDipp)2(tBu2bipy)2Cl (Dipp = 2,6-iPr2C6H3). J. Am. Chem. Soc. 137, 9583–9586 (2015).
Jilek, R. E. et al. A general and modular synthesis of monoimidouranium(IV) dihalides. Inorg. Chem. 50, 4235–4237 (2011).
Jilek, R. E. et al. A direct route to bis(imido)uranium(V) halides via metathesis of uranium tetrachloride. J. Am. Chem. Soc. 134, 9876–9878 (2012).
Wooles, A. J. et al. Uranium(III)-carbon multiple bonding supported by arene δ-bonding in mixed-valence hexauranium nanometre-scale rings. Nat. Commun. 9, 2097 (2018).
Baker, C. F., Seed, J. A., Adams, R. W., Lee, D. & Liddle, S. T. 13C carbene nuclear magnetic resonance chemical shift analysis confirms CeIV=C double bonding in cerium(IV)–diphosphonioalkylidene complexes. Chem. Sci. 15, 238–249 (2024).
Cooper, O. J. et al. Uranium-carbon multiple bonding: facile access to the pentavalent uranium carbene [U{C(PPh2NSiMe3)2}(Cl)2(I)] and comparison of UV=C and UIV=C bonds. Angew. Chem. Int. Ed. 50, 2383–2386 (2011).
Mills, D. P. et al. Synthesis of a uranium(VI)-carbene: reductive formation of uranyl(V)-methanides, oxidative preparation of a [R2C═U═O]2+ analogue of the [O═U═O]2+ uranyl ion (R = Ph2PNSiMe3), and comparison of the nature of UIV═C, U(V)═C, and UVI═C double bonds. J. Am. Chem. Soc. 134, 10047–10054 (2012).
Otte, K. S. et al. Divergent stabilities of tetravalent cerium, uranium, and neptunium imidophosphorane complexes. Angew. Chem. Int. Ed. 62, e202306580 (2023).
Otte, K. S. et al. Proton-coupled electron transfer at the Pu5+/4+ couple. J. Am. Chem. Soc. 146, 21859–21867 (2024).
Morss, L. R. Thermochemical properties of yttrium, lanthanum, and the lanthanide elements and ions. Chem. Rev. 76, 827–841 (1976).
Niklas, J. E. et al. A tetrahedral neptunium(V) complex. Nat. Chem. 16, 1490–1495 (2024).
Gardner, B. M. et al. Evidence for single metal two electron oxidative addition and reductive elimination at uranium. Nat. Commun. 8, 1898 (2017).
Klinkenberg, P. F. A. Spectral structure of trebly ionized thorium, Th IV. Physica B+C 151, 552–567 (1988).
Guerra, M., Amaro, P., Santos, J. P. & Indelicato, P. Relativistic calculations of screening parameters and atomic radii of neutral atoms. At. Data Nucl. Data Tables 117–118, 439–457 (2017).
Acknowledgements
We thank the Royal Society for generously supporting C.A.P.G. with a University Research Fellowship (URF\211271) and the Engineering and Physical Sciences Research Council (EP/Y006534/1) for the funding to B.L.L.R. We also thank the University of Manchester School of Natural Sciences and Department of Chemistry for providing PhD studentships (under EPSRC DTP EP/W524347/1) to R.E.M. and C.N.D.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. C.A.P.G. and B.L.L.R. discussed the content. All authors wrote the article then reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks Scott Daly and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Réant, B.L.L., Deakin, C.N., MacKenzie, R.E. et al. Transuranium organometallic chemistry. Nat Rev Chem 9, 578–600 (2025). https://doi.org/10.1038/s41570-025-00732-4
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41570-025-00732-4