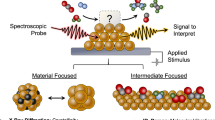
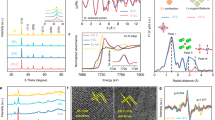
Abstract
Electrochemical organic oxidation reactions (OORs) play a pivotal role in various industrial processes and sustainable chemical production, transforming small molecules into value-added products. Understanding electrode surface dynamics, catalyst structures, reaction intermediates and product selectivity during OOR is crucial for both fundamental knowledge and the design of high-performance electrocatalysts and economically valuable reactions. The advancement of in situ and operando techniques, especially X-ray absorption and Raman and infrared spectroscopies, as well as differential electrochemical mass spectrometry, provide powerful tools for studying OORs. In situ methods reveal catalyst structural changes under applied bias and reaction-relevant conditions, whereas operando techniques simultaneously monitor both structure and activity in real operating conditions. This Review addresses the achievements towards closing the knowledge gap between fundamental, lab-scale studies and industrial, large-scale applications using in situ and operando techniques to uncover bulk catalyst structures, catalyst–surface–electrolyte dynamics and local transient product formation during anodic OORs. It also highlights the underlying principles of these techniques, offering perspectives on future prospects and challenges for advancing OOR applications, particularly in discovering next-generation efficient catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Change history
10 November 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41570-025-00785-5
References
Leech, M. C. & Lam, K. A practical guide to electrosynthesis. Nat. Rev. Chem. 6, 275–286 (2022).
Novaes, L. F. T. et al. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev. 50, 7941–8002 (2021).
Malapit, C. A. et al. Advances on the merger of electrochemistry and transition metal catalysis for organic synthesis. Chem. Rev. 122, 3180–3218 (2022).
Li, J., Zhang, Y., Kuruvinashetti, K. & Kornienko, N. Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis. Nat. Rev. Chem. 6, 303–319 (2022).
Ghosh, S. et al. Deciphering the role of nickel in electrochemical organic oxidation reactions. Adv. Energy Mater. 14, 2400696 (2024).
Chen, G., Li, X. & Feng, X. Upgrading organic compounds through the coupling of electrooxidation with hydrogen evolution. Angew. Chem. Int. Ed. 61, e202209014 (2022).
Liu, C., Chen, F., Zhao, B.-H., Wu, Y. & Zhang, B. Electrochemical hydrogenation and oxidation of organic species involving water. Nat. Rev. Chem. 8, 277–293 (2024).
Na, J. et al. General technoeconomic analysis for electrochemical coproduction coupling carbon dioxide reduction with organic oxidation. Nat. Commun. 10, 5193 (2019).
Zhong, W. et al. Electrocatalytic reduction of CO2 coupled with organic conversion to selectively synthesize high-value chemicals. Chem. Eur. J. 29, e202203228 (2023).
Bai, J. et al. Glycerol oxidation assisted electrocatalytic nitrogen reduction: ammonia and glyceraldehyde co-production on bimetallic RhCu ultrathin nanoflake nanoaggregates. J. Mater. Chem. A 7, 21149–21156 (2019).
Xu, G.-R. et al. Ruthenium(III) polyethyleneimine complexes for bifunctional ammonia production and biomass upgrading. J. Mater. Chem. A 7, 25433–25440 (2019).
Kahlstorf, T., Hausmann, J. N., Sontheimer, T. & Menezes, P. W. Challenges for hybrid water electrolysis to replace the oxygen evolution reaction on an industrial scale. Glob. Chall. 7, 2200242 (2023).
Wang, H.-Y., Sun, M.-L., Ren, J.-T. & Yuan, Z.-Y. Circumventing challenges: design of anodic electrocatalysts for hybrid water electrolysis systems. Adv. Energy Mater. 13, 2203568 (2023).
Wang, T. et al. Combined anodic and cathodic hydrogen production from aldehyde oxidation and hydrogen evolution reaction. Nat. Catal. 5, 66–73 (2022).
Deng, C. et al. Earth-abundant metal-based electrocatalysts promoted anodic reaction in hybrid water electrolysis for efficient hydrogen production: recent progress and perspectives. Adv. Energy Mater. 12, 2201047 (2022).
Wang, T. et al. Transforming electrocatalytic biomass upgrading and hydrogen production from electricity input to electricity output. Angew. Chem. Int. Ed. 61, e202115636 (2022).
Lee, K. J., Elgrishi, N., Kandemir, B. & Dempsey, J. L. Electrochemical and spectroscopic methods for evaluating molecular electrocatalysts. Nat. Rev. Chem. 1, 0039 (2017).
Kim, S.-J., Maligal-Ganesh, R. V., Mahmood, J., Babar, P. & Yavuz, C. T. Structural control over single-crystalline oxides for heterogeneous catalysis. Nat. Rev. Chem. 9, 397–414 (2025).
Zhu, Y., Wang, J., Chu, H., Chu, Y.-C. & Chen, H. M. In situ/operando studies for designing next-generation electrocatalysts. ACS Energy Lett. 5, 1281–1291 (2020).
Pastor, E. et al. Complementary probes for the electrochemical interface. Nat. Rev. Chem. 8, 159–178 (2024).
Magnussen, O. M. et al. In situ and operando X-ray scattering methods in electrochemistry and electrocatalysis. Chem. Rev. 124, 629–721 (2024).
Fu, X., Wan, C., Huang, Y. & Duan, X. Noble metal based electrocatalysts for alcohol oxidation reactions in alkaline media. Adv. Funct. Mater. 32, 2106401 (2022).
Zuo, S., Wu, Z., Zhang, H. & Lou, X. W. (D.) Operando monitoring and deciphering the structural evolution in oxygen evolution electrocatalysis. Adv. Energy Mater. 12, 2103383 (2022).
Mondal, I. et al. The (in)stability of heterostructures during the oxygen evolution reaction. Adv. Energy Mater. 14, 2400809 (2024).
Ghosh, S., Dasgupta, B., Walter, C., Menezes, P. W. & Driess, M. New avenues to chemical space for energy materials by the molecular precursor approach. Small Sci. 3, 2200115 (2023).
Yang, H., An, N., Kang, Z., Menezes, P. W. & Chen, Z. Understanding advanced transition metal-based two electron oxygen reduction electrocatalysts from the perspective of phase engineering. Adv. Mater. 36, 2400140 (2024).
Chen, Z. et al. Hydrogen-induced disproportionation of samarium-cobalt intermetallics enabling promoted hydrogen evolution reaction activity and durability in alkaline media. Adv. Funct. Mater. 34, 2402699 (2024).
Bagchi, D., Roy, S., Sarma, S., Ch, C. & Peter, S. Toward unifying the mechanistic concepts in electrochemical CO2 reduction from an integrated material design and catalytic perspective. Adv. Funct. Mater. 32, 2209023 (2022).
Bagchi, D. et al. Structure-tailored surface oxide on Cu–Ga intermetallics enhances CO2 reduction selectivity to methanol at ultralow potential. Adv. Mater. 34, 2109426 (2022).
Wu, J. et al. In situ characterization techniques for electrochemical nitrogen reduction reaction. ACS Nano 18, 20934–20956 (2024).
Heidary, N. & Kornienko, N. Operando vibrational spectroscopy for electrochemical biomass valorization. Chem. Commun. 56, 8726–8734 (2020).
Petrii, O. A. Pt–Ru electrocatalysts for fuel cells: a representative review. J. Solid State Electrochem. 12, 609–642 (2008).
Mostaghimi, A. H. B., Al-Attas, T. A., Kibria, M. G. & Siahrostami, S. A review on electrocatalytic oxidation of methane to oxygenates. J. Mater. Chem. A 8, 15575–15590 (2020).
Kahlstorf, T. et al. Water-soluble nickel and iron salts for hydroxymethylfurfural (HMF) and water oxidation: the simplest precatalysts? Green Chem. 25, 8679–8686 (2023).
Ghosh, S. et al. Evolution of carbonate-intercalated γ-NiOOH from a molecularly derived nickel sulfide (pre)catalyst for efficient water and selective organic oxidation. Small 19, 2206679 (2023).
Dasgupta, B. et al. A soft molecular single-source precursor approach to synthesize a nanostructured Co9S8 (pre)catalyst for efficient water oxidation and biomass valorization. J. Mater. Chem. A 12, 30522–30533 (2024).
Yang, H. et al. In situ reconstruction of helical iron borophosphate precatalyst toward durable industrial alkaline water electrolysis and selective oxidation of alcohols. Adv. Funct. Mater. 33, 2303702 (2023).
Yang, Y. et al. Hybrid oxidization of ethylene glycol on defective Ag-PtPd electrocatalyst beyond 3000 h stability at an industrial-scale current density. Adv. Funct. Mater. 35, 2418588 (2025).
Zhu, Y.-Q. et al. Identification of active sites formed on cobalt oxyhydroxide in glucose electrooxidation. Angew. Chem. Int. Ed. 62, e202219048 (2023).
Zhou, H. et al. Electrocatalytic upcycling of polyethylene terephthalate to commodity chemicals and H2 fuel. Nat. Commun. 12, 4679 (2021).
Chen, W. et al. Unveiling the electrooxidation of urea: intramolecular coupling of the N−N bond. Angew. Chem. Int. Ed. 60, 7297–7307 (2021).
Mondal, I. et al. Nanostructured intermetallic nickel silicide (pre)catalyst for anodic oxygen evolution reaction and selective dehydrogenation of primary amines. Adv. Energy Mater. 12, 2200269 (2022).
Han, S. et al. Membrane-free selective oxidation of thioethers with water over a nickel phosphide nanocube electrode. Cell Rep. Phys. Sci. 2, 100462 (2021).
Chung, M. et al. Direct propylene epoxidation via water activation over Pd-Pt electrocatalysts. Science 383, 49–55 (2024).
Huang, C., Huang, Y., Liu, C., Yu, Y. & Zhang, B. Integrating hydrogen production with aqueous selective semi-dehydrogenation of tetrahydroisoquinolines over a Ni2P bifunctional electrode. Angew. Chem. Int. Ed. 58, 12014–12017 (2019).
Chong, X., Liu, C., Wang, C., Yang, R. & Zhang, B. Integrating hydrogen production and transfer hydrogenation with selenite promoted electrooxidation of α-nitrotoluenes to E-nitroethenes. Angew. Chem. Int. Ed. 60, 22010–22016 (2021).
Li, J. et al. Green electrosynthesis of 5,5′-azotetrazolate energetic materials plus energy-efficient hydrogen production using ruthenium single-atom catalysts. Adv. Mater. 34, 2203900 (2022).
Wu, X., Wang, Y. & Wu, Z.-S. Design principle of electrocatalysts for the electrooxidation of organics. Chem 8, 2594–2629 (2022).
Fan, Z. et al. Recent developments in electrode materials for the selective upgrade of biomass-derived platform molecules into high-value-added chemicals and fuels. Green Chem. 24, 7818–7868 (2022).
Román, A. M., Hasse, J. C., Medlin, J. W. & Holewinski, A. Elucidating acidic electro-oxidation pathways of furfural on platinum. ACS Catal. 9, 10305–10316 (2019).
Han, G., Li, G. & Sun, Y. Electrocatalytic dual hydrogenation of organic substrates with a Faradaic efficiency approaching 200%. Nat. Catal. 6, 224–233 (2023).
Fu, G. et al. Capturing critical gem-diol intermediates and hydride transfer for anodic hydrogen production from 5-hydroxymethylfurfural. Nat. Commun. 14, 8395 (2023).
Qian, Q. et al. Electrochemical biomass upgrading coupled with hydrogen production under industrial-level current density. Adv. Mater. 35, 2300935 (2023).
Dasgupta, B. et al. A knowledge-based molecular single-source precursor approach to nickel chalcogenide precatalysts for electrocatalytic water, alcohol, and aldehyde oxidations. ACS Nano 18, 33964–33976 (2024).
Yang, H. et al. Activation of nickel foam through in-liquid plasma-induced phosphorus incorporation for efficient quasi-industrial water oxidation and selective oxygenation of organics. Appl. Catal. B Environ. 324, 122249 (2023).
Yang, M. et al. Correlating the valence state with the adsorption behavior of a Cu-based electrocatalyst for furfural oxidation with anodic hydrogen production reaction. Adv. Mater. 35, 2304203 (2023).
Menezes, P. W. et al. Combination of highly efficient electrocatalytic water oxidation with selective oxygenation of organic substrates using manganese borophosphates. Adv. Mater. 33, 2004098 (2021).
Wang, W. et al. Vacancy-rich Ni(OH)2 drives the electrooxidation of amino C−N bonds to nitrile C≡N bonds. Angew. Chem. Int. Ed. 59, 16974–16981 (2020).
Gao, X. et al. Boosting urea electrooxidation on oxyanion-engineered nickel sites via inhibited water oxidation. Nat. Commun. 14, 5842 (2023).
Chung, M., Jin, K., Zeng, J. S., Ton, T. N. & Manthiram, K. Tuning single-atom dopants on manganese oxide for selective electrocatalytic cyclooctene epoxidation. J. Am. Chem. Soc. 144, 17416–17422 (2022).
Ghosh, S. et al. Nitridated nickel mesh as industrial water and alcohol oxidation catalyst: reconstruction and iron-incorporation matters. Adv. Energy Mater. 14, 2400356 (2024).
Zhou, H. et al. Scalable electrosynthesis of commodity chemicals from biomass by suppressing non-Faradaic transformations. Nat. Commun. 14, 5621 (2023).
Lee, J. H. et al. Tuning the activity and selectivity of electroreduction of CO2 to synthesis gas using bimetallic catalysts. Nat. Commun. 10, 3724 (2019).
Wang, D. & Botte, G. G. In situ X-ray diffraction study of urea electrolysis on nickel catalysts. ECS Electrochem. Lett. 3, H29 (2014).
Yang, Y. et al. Defect-promoted Ni-based layer double hydroxides with enhanced deprotonation capability for efficient biomass electrooxidation. Adv. Mater. 35, 2305573 (2023).
Zubair, M. et al. Vacancy mediated electrooxidation of 5-hydroxymethyl furfuryl using defect engineered layered double hydroxide electrocatalysts. Adv. Energy Mater. 14, 2400676 (2024).
Yang, Y. et al. Double hydroxide nanocatalysts for urea electrooxidation engineered toward environmentally benign products. Adv. Mater. 36, 2403187 (2024).
Roy, S. et al. Mechanistic insights into the promotional effect of Ni substitution in non-noble metal carbides for highly enhanced water splitting. Appl. Catal. B Environ. 298, 120560 (2021).
Timoshenko, J. & Roldan Cuenya, B. In situ/operando electrocatalyst characterization by X-ray absorption spectroscopy. Chem. Rev. 121, 882–961 (2021).
Bagchi, D. et al. Operando investigation of the origin of C─C coupling in electrochemical CO2 reduction upon releasing bonding strength, structural ordering in Pd─Cu catalyst. Adv. Energy Mater. 14, 2402237 (2024).
Hausmann, J. N., Mebs, S., Dau, H., Driess, M. & Menezes, P. W. Oxygen evolution activity of amorphous cobalt oxyhydroxides: interconnecting precatalyst reconstruction, long-range order, buffer-binding, morphology, mass transport, and operation temperature. Adv. Mater. 34, 2207494 (2022).
Wai, M. H. et al. Influence of surface formate species on methane selectivity for carbon dioxide methanation over nickel hydroxyapatite catalyst. ChemCatChem 12, 6410–6419 (2020).
Reith, L. et al. In situ detection of iron in oxidation states ≥ IV in cobalt-iron oxyhydroxide reconstructed during oxygen evolution reaction. Adv. Energy Mater. 13, 2203886 (2023).
Holstein, W. L. & Rosenfeld, H. D. In-situ X-ray absorption spectroscopy study of Pt and Ru chemistry during methanol electrooxidation. J. Phys. Chem. B 109, 2176–2186 (2005).
Sakamoto, T. et al. Operando XAFS study of carbon supported Ni, NiZn, and Co catalysts for hydrazine electrooxidation for use in anion exchange membrane fuel cells. Electrochim. Acta 163, 116–122 (2015).
Zhou, Z. et al. Strain-induced in situ formation of NiOOH species on Co–Co bond for selective electrooxidation of 5-hydroxymethylfurfural and efficient hydrogen production. Appl. Catal. B Environ. 305, 121072 (2022).
Zhou, B. et al. Activity origin and alkalinity effect of electrocatalytic biomass oxidation on nickel nitride. J. Energy Chem. 61, 179–185 (2021).
Manzoli, M. et al. Structure–reactivity relationship in Co3O4 promoted Au/CeO2 catalysts for the CH3OH oxidation reaction revealed by in situ FTIR and operando EXAFS studies. J. Mater. Chem. A 5, 2083–2094 (2017).
Lin, R. et al. Identification and manipulation of dynamic active site deficiency-induced competing reactions in electrocatalytic oxidation processes. Energy Environ. Sci. 15, 2386–2396 (2022).
Melke, J. et al. Electrooxidation of ethanol on Pt. An in situ and time-resolved XANES study. J. Phys. Chem. C 116, 2838–2849 (2012).
Li, Z. et al. Alcohols electrooxidation coupled with H2 production at high current densities promoted by a cooperative catalyst. Nat. Commun. 13, 147 (2022).
Pan, Y. et al. Unveiling the synergistic effect of multi-valence Cu species to promote formaldehyde oxidation for anodic hydrogen production. Chem 9, 963–977 (2023).
Lum, Y. et al. Tuning OH binding energy enables selective electrochemical oxidation of ethylene to ethylene glycol. Nat. Catal. 3, 14–22 (2020).
Yang, Y. et al. Operando methods in electrocatalysis. ACS Catal. 11, 1136–1178 (2021).
Chen, Z. et al. Thermal migration towards constructing W-W dual-sites for boosted alkaline hydrogen evolution reaction. Nat. Commun. 13, 763 (2022).
Li, X., Wang, S., Li, L., Sun, Y. & Xie, Y. Progress and perspective for in situ studies of CO2 reduction. J. Am. Chem. Soc. 142, 9567–9581 (2020).
Zhang, D., Wang, R. (J.), Wang, X. & Gogotsi, Y. In situ monitoring redox processes in energy storage using UV–Vis spectroscopy. Nat. Energy 8, 567–576 (2023).
Yan, Y. et al. Nonredox trivalent nickel catalyzing nucleophilic electrooxidation of organics. Nat. Commun. 14, 7987 (2023).
Lazanas, A. C. & Prodromidis, M. I. Electrochemical impedance spectroscopy─a tutorial. ACS Meas. Sci. Au 3, 162–193 (2023).
Song, Y. et al. Bifunctional integrated electrode for high-efficient hydrogen production coupled with 5-hydroxymethylfurfural oxidation. Appl. Catal. B Environ. 312, 121400 (2022).
Qi, Y. et al. Insights into the activity of nickel boride/nickel heterostructures for efficient methanol electrooxidation. Nat. Commun. 13, 4602 (2022).
Chen, W. et al. Activity origins and design principles of nickel-based catalysts for nucleophile electrooxidation. Chem 6, 2974–2993 (2020).
Li, S. et al. Coordination environment tuning of nickel sites by oxyanions to optimize methanol electro-oxidation activity. Nat. Commun. 13, 2916 (2022).
Zhu, B. et al. Unraveling a bifunctional mechanism for methanol-to-formate electro-oxidation on nickel-based hydroxides. Nat. Commun. 14, 1686 (2023).
Dasgupta, B. et al. A facile molecular approach to amorphous nickel pnictides and their reconstruction to crystalline potassium-intercalated γ-NiOOHx enabling high-performance electrocatalytic water oxidation and selective oxidation of 5-hydroxymethylfurfural. Small 19, 2301258 (2023).
Zhao, B. et al. Hollow NiSe nanocrystals heterogenized with carbon nanotubes for efficient electrocatalytic methanol upgrading to boost hydrogen Co-production. Adv. Funct. Mater. 31, 2008812 (2021).
Mondal, A., Shit, S. C. & Mondal, I. A MnNi-heterometallic perovskite hydroxide (pre)catalyst for electrochemical alcohol oxidation: insight into the active phase. J. Mater. Chem. A 13, 2327–2334 (2025).
Huang, H. et al. Ni, Co hydroxide triggers electrocatalytic production of high-purity benzoic acid over 400 mA cm−2. Energy Environ. Sci. 13, 4990–4999 (2020).
Xie, Y., Zhou, Z., Yang, N. & Zhao, G. An overall reaction integrated with highly selective oxidation of 5-hydroxymethylfurfural and efficient hydrogen evolution. Adv. Funct. Mater. 31, 2102886 (2021).
Huang, Y., Chong, X., Liu, C., Liang, Y. & Zhang, B. Boosting hydrogen production by anodic oxidation of primary amines over a NiSe nanorod electrode. Angew. Chem. Int. Ed. 57, 13163–13166 (2018).
Deng, X. et al. Understanding the roles of electrogenerated Co3+ and Co4+ in selectivity-tuned 5-hydroxymethylfurfural oxidation. Angew. Chem. Int. Ed. 60, 20535–20542 (2021).
Zhang, Y. et al. Coupling glucose-assisted Cu(I)/Cu(II) redox with electrochemical hydrogen production. Adv. Mater. 33, 2104791 (2021).
Zhao, H.-F. et al. In-situ electrochemical transformed Cu oxide from Cu sulfide for efficient upgrading of biomass derived 5-hydroxymethylfurfural in anion exchange membrane electrolyzer. ChemSusChem 15, e202201625 (2022).
Zhou, B. et al. Room-temperature chemical looping hydrogen production mediated by electrochemically induced heterogeneous Cu(I)/Cu(II) redox. Chem Catal. 1, 1493–1504 (2021).
Si, D. et al. Hydrogen anode/cathode co-productions-coupled anode alcohol selective oxidation and distinctive H/e transfer pathways. Appl. Catal. B Environ. 331, 122664 (2023).
Hausmann, J. N. & Menezes, P. W. Effect of surface-adsorbed and intercalated (oxy)anions on the oxygen evolution reaction. Angew. Chem. 134, e202207279 (2022).
Chen, Z., Yang, H., Kang, Z., Driess, M. & Menezes, P. W. The pivotal role of s-, p-, and f-block metals in water electrolysis: status quo and perspectives. Adv. Mater. 34, 2108432 (2022).
Wen, Q. et al. In situ chalcogen leaching manipulates reactant interface toward efficient amine electrooxidation. ACS Nano 16, 9572–9582 (2022).
Heidary, N. & Kornienko, N. Operando Raman probing of electrocatalytic biomass oxidation on gold nanoparticle surfaces. Chem. Commun. 55, 11996–11999 (2019).
Heidary, N. & Kornienko, N. Electrochemical biomass valorization on gold-metal oxide nanoscale heterojunctions enables investigation of both catalyst and reaction dynamics with operando surface-enhanced Raman spectroscopy. Chem. Sci. 11, 1798–1806 (2020).
Yang, Y.-Y. et al. Electrocatalysis of ethanol on a Pd electrode in alkaline media: an in situ attenuated total reflection surface-enhanced infrared absorption spectroscopy study. ACS Catal. 4, 798–803 (2014).
Monyoncho, E. A. et al. Ethanol electro-oxidation on palladium revisited using polarization modulation infrared reflection absorption spectroscopy (PM-IRRAS) and density functional theory (DFT): why is it difficult to break the C–C bond? ACS Catal. 6, 4894–4906 (2016).
Chen, D.-J. & Tong, Y. J. Irrelevance of carbon monoxide poisoning in the methanol oxidation reaction on a PtRu electrocatalyst. Angew. Chem. Int. Ed. 54, 9394–9398 (2015).
Pech-Rodríguez, W. J. et al. Electrochemical and in situ FTIR study of the ethanol oxidation reaction on PtMo/C nanomaterials in alkaline media. Appl. Catal. B Environ. 203, 654–662 (2017).
de Souza, J. P. I., Queiroz, S. L., Bergamaski, K., Gonzalez, E. R. & Nart, F. C. Electro-oxidation of ethanol on Pt, Rh, and PtRh electrodes. a study using DEMS and in-Situ FTIR techniques. J. Phys. Chem. B 106, 9825–9830 (2002).
Torrero, J., Peña, M. A., Retuerto, M., Pascual, L. & Rojas, S. Infrared study of the electrooxidation of ethanol in alkaline electrolyte with Pt/C, PtRu/C and Pt3Sn. Electrochim. Acta 319, 312–322 (2019).
Fang, X., Wang, L., Shen, P. K., Cui, G. & Bianchini, C. An in situ Fourier transform infrared spectroelectrochemical study on ethanol electrooxidation on Pd in alkaline solution. J. Power Sources 195, 1375–1378 (2010).
García, G. et al. Ethanol oxidation on PtRuMo/C catalysts: in situ FTIR spectroscopy and DEMS studies. Int. J. Hydrog. Energy 37, 7131–7140 (2012).
Wang, L. et al. In situ FTIR spectroelectrochemical study on the mechanism of ethylene glycol electrocatalytic oxidation at a Pd electrode. Phys. Chem. Chem. Phys. 13, 2667–2673 (2011).
Falase, A., Garcia, K., Lau, C. & Atanassov, P. Electrochemical and in situ IR characterization of PtRu catalysts for complete oxidation of ethylene glycol and glycerol. Electrochem. Commun. 13, 1488–1491 (2011).
Holade, Y., Morais, C., Servat, K., Napporn, T. W. & Kokoh, K. B. Toward the electrochemical valorization of glycerol: Fourier transform infrared spectroscopic and chromatographic studies. ACS Catal. 3, 2403–2411 (2013).
Schnaidt, J., Heinen, M., Denot, D., Jusys, Z. & Jürgen Behm, R. Electrooxidation of glycerol studied by combined in situ IR spectroscopy and online mass spectrometry under continuous flow conditions. J. Electroanal. Chem. 661, 250–264 (2011).
Fernández, P. S., Martins, M. E. & Camara, G. A. New insights about the electro-oxidation of glycerol on platinum nanoparticles supported on multi-walled carbon nanotubes. Electrochim. Acta 66, 180–187 (2012).
Houache, M. S. E. et al. Electrochemical valorization of glycerol on Ni-rich bimetallic NiPd nanoparticles: insight into product selectivity using in situ polarization modulation infrared-reflection absorption spectroscopy. ACS Sustain. Chem. Eng. 7, 14425–14434 (2019).
Gomes, J. F. & Tremiliosi-Filho, G. Spectroscopic studies of the glycerol electro-oxidation on polycrystalline Au and Pt surfaces in acidic and alkaline media. Electrocatalysis 2, 96–105 (2011).
Zalineeva, A. et al. Self-supported PdxBi catalysts for the electrooxidation of glycerol in alkaline media. J. Am. Chem. Soc. 136, 3937–3945 (2014).
Liu, W.-J. et al. Efficient electrochemical production of glucaric acid and H2 via glucose electrolysis. Nat. Commun. 11, 265 (2020).
Koley, P. et al. Elucidation of active sites and mechanistic pathways of a heteropolyacid/Cu-metal–organic framework catalyst for selective oxidation of 5-hydroxymethylfurfural via ex situ X-ray absorption spectroscopy and in situ attenuated total reflection-infrared studies. ACS Catal. 13, 6076–6092 (2023).
Zhang, L. et al. A lattice-oxygen-involved reaction pathway to boost urea oxidation. Angew. Chem. Int. Ed. 58, 16820–16825 (2019).
Kwon, Y., Birdja, Y., Spanos, I., Rodriguez, P. & Koper, M. T. M. Highly selective electro-oxidation of glycerol to dihydroxyacetone on platinum in the presence of bismuth. ACS Catal. 2, 759–764 (2012).
Huang, L. et al. Combined EC-NMR and in situ FTIR spectroscopic studies of glycerol electrooxidation on Pt/C, PtRu/C, and PtRh/C. ACS Catal. 6, 7686–7695 (2016).
Ishiyama, T., Imamura, T. & Morita, A. Theoretical studies of structures and vibrational sum frequency generation spectra at aqueous interfaces. Chem. Rev. 114, 8447–8470 (2014).
Yang, Y. & Mu, T. Electrochemical oxidation of biomass derived 5-hydroxymethylfurfural (HMF): pathway, mechanism, catalysts and coupling reactions. Green Chem. 23, 4228–4254 (2021).
Zhang, N. et al. Electrochemical oxidation of 5-hydroxymethylfurfural on nickel nitride/carbon nanosheets: reaction pathway determined by in situ sum frequency generation vibrational spectroscopy. Angew. Chem. Int. Ed. 58, 15895–15903 (2019).
Kutz, R. B., Braunschweig, B., Mukherjee, P., Dlott, D. D. & Wieckowski, A. Study of ethanol electrooxidation in alkaline electrolytes with isotope labels and sum-frequency generation. J. Phys. Chem. Lett. 2, 2236–2240 (2011).
Jin, L. et al. Boosting the electrocatalytic urea oxidation performance by amorphous–crystalline Ni-TPA@NiSe heterostructures and mechanism discovery. ACS Catal. 13, 837–847 (2023).
Clark, E. L., Singh, M. R., Kwon, Y. & Bell, A. T. Differential electrochemical mass spectrometer cell design for online quantification of products produced during electrochemical reduction of CO2. Anal. Chem. 87, 8013–8020 (2015).
Kim, C., Weng, L.-C. & Bell, A. T. Impact of pulsed electrochemical reduction of CO2 on the formation of C2+ products over Cu. ACS Catal. 10, 12403–12413 (2020).
Mondal, S. et al. Distortion-induced interfacial charge transfer at single cobalt atom secured on ordered intermetallic surface enhances pure oxygen production. ACS Nano 17, 23169–23180 (2023).
Queiroz, A. C. et al. Electrochemical mass spectrometry: evolution of the cell setup for on-line investigation of products and screening of electrocatalysts for carbon dioxide reduction. ChemElectroChem 9, e202101408 (2022).
Wang, H., Rus, E. & Abruña, H. D. New double-band-electrode channel flow differential electrochemical mass spectrometry cell: application for detecting product formation during methanol electrooxidation. Anal. Chem. 82, 4319–4324 (2010).
Hauke, P., Klingenhof, M., Wang, X., de Araújo, J. F. & Strasser, P. Efficient electrolysis of 5-hydroxymethylfurfural to the biopolymer-precursor furandicarboxylic acid in a zero-gap MEA-type electrolyzer. Cell Rep. Phys. Sci. 2, 100650 (2021).
Zeng, R. et al. Methanol oxidation using ternary ordered intermetallic electrocatalysts: a DEMS study. ACS Catal. 10, 770–776 (2020).
Dutta, N., Bagchi, D., Chawla, G. & Peter, S. C. A guideline to determine faradaic efficiency in electrochemical CO2 reduction. ACS Energy Lett. 9, 323–328 (2024).
Zhu, Z., Luo, R. & Zhao, E. W. Operando NMR methods for studying electrocatalysis. Magn. Reson. Lett. 4, 100096 (2024).
Boisseau, R., Bussy, U., Giraudeau, P. & Boujtita, M. In situ ultrafast 2D NMR spectroelectrochemistry for real-time monitoring of redox reactions. Anal. Chem. 87, 372–375 (2015).
Wang, J. et al. Small-sized Pt nanoparticles supported on hybrid structures of MoS2 nanoflowers/graphene nanosheets: highly active composite catalyst toward efficient ethanol oxidation reaction studied by in situ electrochemical NMR spectroscopy. Appl. Catal. B Environ. 259, 118060 (2019).
Beatrice Richter, J., Eßbach, C., Senkovska, I., Kaskel, S. & Brunner, E. Quantitative in situ 13C NMR studies of the electro-catalytic oxidation of ethanol. Chem. Commun. 55, 6042–6045 (2019).
Wang, X. L. et al. Operando NMR spectroscopic analysis of proton transfer in heterogeneous photocatalytic reactions. Nat. Commun. 7, 11918 (2016).
Huffman, B. L., Bredar, A. R. C. & Dempsey, J. L. Origins of non-ideal behaviour in voltammetric analysis of redox-active monolayers. Nat. Rev. Chem. 8, 628–643 (2024).
Rusling, J. F. & Suib, S. L. Characterizing materials with cyclic voltammetry. Adv. Mater. 6, 922–930 (1994).
Van Acker, T., Theiner, S., Bolea-Fernandez, E., Vanhaecke, F. & Koellensperger, G. Inductively coupled plasma mass spectrometry. Nat. Rev. Methods Primers 3, 52 (2023).
Kasian, O., Geiger, S., Mayrhofer, K. J. J. & Cherevko, S. Electrochemical on-line ICP-MS in electrocatalysis research. Chem. Rec. 19, 2130–2142 (2019).
Yan, K. et al. On-line inductively coupled plasma mass spectrometry reveals material degradation dynamics of Au and Cu catalysts during electrochemical CO2 reduction. J. Am. Chem. Soc. 147, 4079–4088 (2025).
Gentil, T. C. et al. Stability of supported Pd-based ethanol oxidation reaction electrocatalysts in alkaline media. J. Catal. 440, 115816 (2024).
Kormányos, A., Speck, F. D., Mayrhofer, K. J. J. & Cherevko, S. Influence of fuels and pH on the dissolution stability of bifunctional PtRu/C alloy electrocatalysts. ACS Catal. 10, 10858–10870 (2020).
Minichová, M. et al. Electrochemical dissolution of PtRu/C: effect of potential, fuels, and temperature. Electrochim. Acta 502, 144764 (2024).
Kormányos, A. et al. Electrocatalytic oxidation of 2-propanol on PtxIr100−x bifunctional electrocatalysts – a thin-film materials library study. J. Catal. 396, 387–394 (2021).
Ranninger, J. et al. On-line monitoring of dissolution processes in nonaqueous electrolytes – A case study with platinum. Electrochem. Commun. 114, 106702 (2020).
Liu, S. et al. Organic matrix effects in inductively coupled plasma mass spectrometry: a tutorial review. Appl. Spectrosc. Rev. 57, 461–489 (2022).
Zheng, Y. et al. Hierarchically structured CuNiP/CuOx-VP nanoarrays construction by heteroatom doping boosting glycerol valorization at industrial-level current density. Adv. Funct. Mater. 35, 2412810 (2025).
Sun, Y., Wang, J., Qi, Y., Li, W. & Wang, C. Efficient electrooxidation of 5-hydroxymethylfurfural using co-doped Ni3S2 catalyst: promising for H2 production under industrial-level current density. Adv. Sci. 9, 2200957 (2022).
Li, W. et al. Electrocatalytic upgrading of polyethylene terephthalate plastic to formic acid at an industrial-scale current density via Ni-MOF@MnCo-OH catalyst. Chem. Eng. J. 480, 148087 (2024).
Mondal, I. et al. In-liquid plasma-mediated manganese oxide electrocatalysts for quasi-industrial water oxidation and selective dehydrogenation. ACS Nano 17, 14043–14052 (2023).
Yu, H. et al. Ambient γ-rays-mediated noble-metal deposition on defect-rich manganese oxide for glycerol-assisted H2 evolution at industrial-level current density. Angew. Chem. Int. Ed. 62, e202314569 (2023).
Li, S. et al. Doped Mn enhanced NiS electrooxidation performance of HMF into FDCA at industrial-level current density. Adv. Funct. Mater. 33, 2214488 (2023).
Chen, D., Li, W., Liu, J. & Sun, L. Bio-inspired proton relay for promoting continuous 5-hydroxymethylfurfural electrooxidation in a flowing system. Energy Environ. Sci. 18, 3120–3128 (2025).
Moysiadou, A., Lee, S., Hsu, C.-S., Chen, H. M. & Hu, X. Mechanism of oxygen evolution catalyzed by cobalt oxyhydroxide: cobalt superoxide species as a key intermediate and dioxygen release as a rate-determining step. J. Am. Chem. Soc. 142, 11901–11914 (2020).
Xie, Y. et al. In situ investigation on life-time dynamic structure–performance correlation toward electrocatalyst service behavior in water splitting. Adv. Funct. Mater. 32, 2111777 (2022).
Dionigi, F. et al. In-situ structure and catalytic mechanism of NiFe and CoFe layered double hydroxides during oxygen evolution. Nat. Commun. 11, 2522 (2020).
Hausmann, J. N. & Menezes, P. W. A rising mismatch between system complexity, characterization, and theory in electrocatalysis: challenges and solutions. Appl. Catal. B Environ. 342, 123447 (2024).
Spurgeon, S. R. et al. Towards data-driven next-generation transmission electron microscopy. Nat. Mater. 20, 274–279 (2021).
Yao, Z. et al. Machine learning for a sustainable energy future. Nat. Rev. Mater. 8, 202–215 (2023).
Zheng, H., Lu, X. & He, K. In situ transmission electron microscopy and artificial intelligence enabled data analytics for energy materials. J. Energy Chem. 68, 454–493 (2022).
Li, H., Jiao, Y., Davey, K. & Qiao, S.-Z. Data-driven machine learning for understanding surface structures of heterogeneous catalysts. Angew. Chem. Int. Ed. 62, e202216383 (2023).
Badreldin, A., Bouhali, O. & Abdel-Wahab, A. Complimentary computational cues for water electrocatalysis: a DFT and ML perspective. Adv. Funct. Mater. 34, 2312425 (2024).
Dinic, F. et al. Applied machine learning for developing next-generation functional materials. Adv. Funct. Mater. 31, 2104195 (2021).
Neukermans, S. et al. A versatile in-situ electron paramagnetic resonance spectro-electrochemical approach for electrocatalyst research. ChemElectroChem 7, 4578–4586 (2020).
Hunger, M. & Weitkamp, J. In situ IR, NMR, EPR, and UV/Vis spectroscopy: tools for new insight into the mechanisms of heterogeneous catalysis. Angew. Chem. Int. Ed. 40, 2954–2971 (2001).
Hollmann, D. et al. From the precursor to the active state: monitoring metamorphosis of electrocatalysts during water oxidation by in situ spectroscopy. ChemElectroChem 4, 2117–2122 (2017).
Long, C. et al. Operando toolbox for heterogeneous interface in electrocatalysis. Chem Catal. 1, 509–522 (2021).
Wang, Y., Skaanvik, S. A., Xiong, X., Wang, S. & Dong, M. Scanning probe microscopy for electrocatalysis. Matter 4, 3483–3514 (2021).
Acknowledgements
This work is funded by the German Federal Ministry of Education and Research (BMBF) in the framework of the project Catlab (03EW0015A/B) and the project PrometH2eus (03HY105C). The authors also acknowledge support from the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation) under Germany’s Excellence Strategy – EXC 2008/1 – 390540038 – UniSysCat.
Author information
Authors and Affiliations
Contributions
P.W.M. conceived the idea, proposed the topic and designed the Review structure. B.D. and D.B. collaboratively planned, organized and co-authored the initial draft. T.S., M.D. and P.W.M. were involved in reviewing, discussing and supervising the Review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dasgupta, B., Bagchi, D., Sontheimer, T. et al. Dynamics in electrochemical organic oxidation reactions from in situ and operando techniques. Nat Rev Chem 9, 766–789 (2025). https://doi.org/10.1038/s41570-025-00767-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41570-025-00767-7