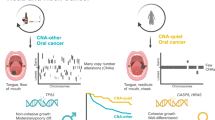
Abstract
In the past two decades, treatment for non-small-cell lung cancers (NSCLCs) and head and neck squamous cell carcinoma (HNSCC) has advanced considerably, owing largely to the characterization of distinct oncological subtypes, the development of targeted therapies for each subtype and the advent of immunotherapy. Data emerging over the past two decades suggest that NUT carcinoma, a highly aggressive malignancy driven by a NUT fusion oncoprotein and arising in the lungs, head and neck, and rarely in other sites, is a squamous cell carcinoma (SCC) based on transcriptional, histopathological, cell-of-origin and molecular characteristics. NUT carcinoma has an estimated incidence of 1,400 cases per year in the United States, surpassing that of some rare NSCLC and HNSCC subtypes. However, NUT carcinoma is currently not recognized as an SCC of the lungs or head and neck. The orphan classification of NUT carcinoma as a distinct entity leads to a lack of awareness of this malignancy among oncologists and surgeons, despite early diagnosis being crucial for this cancer type with a median survival of only ~6.5 months. Consequently, NUT carcinoma is underdiagnosed and often misdiagnosed, resulting in limited research and progress in developing effective treatments in one of the most aggressive forms of lung and head and neck cancer. With a growing number of targeted agents that can potentially be used to treat NUT carcinoma, improved recognition through reclassification and inclusion of NUT carcinoma as a squamous NSCLC or an HNSCC when arising in these locations will accelerate the development of effective therapies for this disease. Thus, in the Perspective, we propose such a reclassification of NUT carcinoma as an SCC and discuss the supporting evidence.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Yuan, M., Huang, L. L., Chen, J. H., Wu, J. & Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal. Transduct. Target. Ther. 4, 61 (2019).
Makarem, M. & Janne, P. A. Top advances of the year: targeted therapy for lung cancer. Cancer 130, 3239–3250 (2024).
Dotto, G. P. & Rustgi, A. K. Squamous cell cancers: a unified perspective on biology and genetics. Cancer Cell 29, 622–637 (2016).
Durall, R. T. et al. The BRD4–NUT fusion alone drives malignant transformation of NUT carcinoma. Cancer Res. 83, 3846–3860 (2023).
Grayson, A. R. et al. MYC, a downstream target of BRD–NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene 33, 1736–1742 (2014).
Huang, Y. et al. EZH2 cooperates with BRD4–NUT to drive NUT carcinoma growth by silencing key tumor suppressor genes. Cancer Res. 83, 3956–3973 (2023).
Wang, R. et al. Activation of SOX2 expression by BRD4–NUT oncogenic fusion drives neoplastic transformation in NUT midline carcinoma. Cancer Res. 74, 3332–3343 (2014).
Chau, N. G. et al. An anatomical site and genetic based prognostic model for patients with NUT midline carcinoma: analysis of 124 patients. JNCI Cancer Spectr. 4, pkz094 (2019).
Luo, J. et al. Initial chemotherapy for locally advanced and metastatic NUT carcinoma. J. Thorac. Oncol. 19, 829–838 (2024).
French, C. A., Badve, S., den Bakker, M. A. & Jain, D. in WHO Classification of Tumours: Thoracic Tumours 5th edn (eds Chan J. K. C. et al.) 364–367 (International Agency for Research on Cancer, 2021).
French, C. A. et al. BRD4–NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 63, 304–307 (2003).
French, C. A. et al. BRD–NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene 27, 2237–2242 (2008).
Wu, S. J. et al. Novel BRD2::NUTM1 fusion in NUT carcinoma with exceptional response to chemotherapy: a case report. JTO Clin. Res. Rep. 5, 100625 (2024).
French, C. A. et al. NSD3–NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. 4, 928–941 (2014).
Alekseyenko, A. A. et al. Ectopic protein interactions within BRD4–chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc. Natl Acad. Sci. USA 114, E4184–E92 (2017).
Shiota, H. et al. “Z4” complex member fusions in NUT carcinoma: implications for a novel oncogenic mechanism. Mol. Cancer Res. 16, 1826–1833 (2018).
Agaimy, A. et al. Misleading germ cell phenotype in pulmonary NUT carcinoma harboring the ZNF532–NUTM1 fusion. Am. J. Surg. Pathol. 46, 281–288 (2022).
French, C. A., Badve, S., den Bakker, M. A. & Jain, D. in WHO Classification of Tumours: Thoracic Tumours 5th edn (eds Borczuk, A. C. et al.) (International Agency for Research on Cancer, 2021).
Dickson, B. C. et al. NUTM1 gene fusions characterize a subset of undifferentiated soft tissue and visceral tumors. Am. J. Surg. Pathol. 42, 636–645 (2018).
Stevens, T. M. et al. NUTM1-rearranged neoplasia: a multi-institution experience yields novel fusion partners and expands the histologic spectrum. Mod. Pathol. 32, 764–773 (2019).
Van Treeck, B. J. et al. NUTM1-rearranged colorectal sarcoma: a clinicopathologically and genetically distinctive malignant neoplasm with a poor prognosis. Mod Pathol. 34, 1547–1557 (2021).
Sekine, S. et al. Recurrent YAP1–MAML2 and YAP1–NUTM1 fusions in poroma and porocarcinoma. J. Clin. Invest. 129, 3827–3832 (2019).
Xu, B. et al. NUTM1-fusion positive malignant neoplasms of the genitourinary tract: a report of six cases highlighting involvement of unusual anatomic locations and histologic heterogeneity. Genes. Chromosomes Cancer 61, 542–550 (2022).
Luo, W. et al. NUTM1-rearranged neoplasms-A heterogeneous group of primitive tumors with expanding spectrum of histology and molecular alterations—an updated review. Curr. Oncol. 28, 4485–4503 (2021).
Zheng, D. et al. Brd4::Nutm1 fusion gene initiates NUT carcinoma in vivo. Life Sci. Alliance 7, e202402602 (2024).
Haack, H. et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am. J. Surg. Pathol. 33, 984–991 (2009).
Kroening G. et al. Multiomic characterization and molecular profiling of nuclear protein in testis carcinoma. JCO Precis. Oncol. 8, e2400334 (2024).
Okamura, R. et al. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: implications for NTRK-targeted therapeutics. JCO Precis. Oncol. https://doi.org/10.1200/PO.18.00183 (2018).
Takeuchi, K. et al. RET, ROS1 and ALK fusions in lung cancer. Nat. Med. 18, 378–381 (2012).
Lassche, G. et al. Identification of fusion genes and targets for genetically matched therapies in a large cohort of salivary gland cancer patients. Cancers 14, 4156 (2022).
French, C. A. et al. Midline carcinoma of children and young adults with NUT rearrangement. J. Clin. Oncol. 22, 4135–4139 (2004).
Lee, A. C. et al. Disseminated mediastinal carcinoma with chromosomal translocation (15;19). A distinctive clinicopathologic syndrome. Cancer 72, 2273–2276 (1993).
Kubonishi, I. et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 51, 3327–3328 (1991).
Kees, U. R., Mulcahy, M. T. & Willoughby, M. L. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19). Am. J. Pediatr. Hematol. Oncol. 13, 459–464 (1991).
Travis, W. D. in Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart 185–186 (International Agency for Research on Cancer, 2004).
French, C. A. & den Bakker, M. A. in WHO Classification of Head and Neck Tumours 4th edn (eds Slootweg, P. J. et al.) 229–231 (International Agency for Research on Cancer, 2015).
Marx, A. et al. The 2021 WHO classification of tumors of the thymus and mediastinum: what is new in thymic epithelial, germ cell, and mesenchymal tumors? J. Thorac. Oncol. 17, 200–213 (2022).
French, C. A., Stelow, E. B. & Hiroshi, M. in WHO Classification of Tumours: Head and Neck Tumours Part A 5th edn (eds Bishop, J. A. et al.) 65–67 (International Agency for Research on Cancer, 2024).
Lee, J. K. et al. Complex chromosomal rearrangements by single catastrophic pathogenesis in NUT midline carcinoma. Ann. Oncol. 28, 890–897 (2017).
Bauer, D. E. et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin. Cancer Res. 18, 5773–5779 (2012).
Viswanathan, K. et al. The histological spectrum and immunoprofile of head and neck NUT carcinoma: a multicentre series of 30 cases. Histopathology 85, 317–326 (2024).
Farooq, A., Kerper, A. L., Boland, J. M. & Lo, Y. C. Nuclear protein in testis (NUT) carcinoma: a comprehensive immunohistochemical analysis of 57 cases with consideration of interpretation and pitfall recognition. Arch. Pathol. Lab. Med. 148, 898–904 (2023).
Mertens, F., Wiebe, T., Adlercreutz, C., Mandahl, N. & French, C. A. Successful treatment of a child with t(15;19)-positive tumor. Pediatr. Blood Cancer 49, 1015–1017 (2007).
Li, W. & Chastain, K. NUT midline carcinoma with leukemic presentation mimicking CD34-positive acute leukemia. Blood 132, 456 (2018).
Numakura, S. et al. P63-negative pulmonary NUT carcinoma arising in the elderly: a case report. Diagn. Pathol. 15, 134 (2020).
Luo, J. et al. Presenting features and diagnostic delays of NUT carcinoma: a report from the NUT carcinoma registry. J. Thorac. Oncol. 19, S71–S72 (2024).
Hormann, F. M. et al. NUTM1 is a recurrent fusion gene partner in B-cell precursor acute lymphoblastic leukemia associated with increased expression of genes on chromosome band 10p12.31–12.2. Haematologica 104, e455–e459 (2019).
McEvoy, C. R., Fox, S. B. & Prall, O. W. J. Emerging entities in NUTM1-rearranged neoplasms. Genes Chromosomes Cancer 59, 375–385 (2020).
Li, J. et al. Emerging molecular subtypes and therapeutic targets in B-cell precursor acute lymphoblastic leukemia. Front. Med. 15, 347–371 (2021).
French, C. A. et al. BRD4 bromodomain gene rearrangement in aggressive carcinoma with translocation t(15;19). Am. J. Pathol. 159, 1987–1992 (2001).
Rahman, S. et al. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol. Cell Biol. 31, 2641–2652 (2011).
Gilan, O. et al. Functional interdependence of BRD4 and DOT1L in MLL leukemia. Nat. Struct. Mol. Biol. 23, 673–681 (2016).
Yokoyama, A. Molecular mechanisms of MLL-associated leukemia. Int. J. Hematol. 101, 352–361 (2015).
Alekseyenko, A. A. et al. The oncogenic BRD4–NUT chromatin regulator drives aberrant transcription within large topological domains. Genes. Dev. 29, 1507–1523 (2015).
Hammerman, P. S., Hayes, D. N. & Grandis, J. R. Therapeutic insights from genomic studies of head and neck squamous cell carcinomas. Cancer Discov. 5, 239–244 (2015).
Polo, V. et al. Squamous cell carcinomas of the lung and of the head and neck: new insights on molecular characterization. Oncotarget 7, 25050–25063 (2016).
Sands, J. M. et al. Next-generation sequencing informs diagnosis and identifies unexpected therapeutic targets in lung squamous cell carcinomas. Lung Cancer 140, 35–41 (2020).
Hai, J. Next generation mouse models of squamous cell lung cancer for translational immuno-oncology. Oncotarget 11, 4463–4464 (2020).
Kloker, L. D. et al. Clinical management of NUT carcinoma (NC) in Germany: analysis of survival, therapy response, tumor markers and tumor genome sequencing in 35 adult patients. Lung Cancer 189, 107496 (2024).
Koh, C. M. et al. Myc enforces overexpression of EZH2 in early prostatic neoplasia via transcriptional and post-transcriptional mechanisms. Oncotarget 2, 669–683 (2011).
Wu, X. et al. BRD4 regulates EZH2 transcription through upregulation of C-MYC and represents a novel therapeutic target in bladder cancer. Mol. Cancer Ther. 15, 1029–1042 (2016).
Balinth, S. et al. EZH2 regulates a SETDB1/DeltaNp63alpha axis via RUNX3 to drive a cancer stem cell phenotype in squamous cell carcinoma. Oncogene 41, 4130–4144 (2022).
Xu, M., Hou, Y., Li, N., Yu, W. & Chen, L. Targeting histone deacetylases in head and neck squamous cell carcinoma: molecular mechanisms and therapeutic targets. J. Transl. Med. 22, 418 (2024).
Yuan, G. et al. Elevated NSD3 histone methylation activity drives squamous cell lung cancer. Nature 590, 504–508 (2021).
Tamura, R. et al. Novel MXD4–NUTM1 fusion transcript identified in primary ovarian undifferentiated small round cell sarcoma. Genes Chromosomes Cancer 57, 557–563 (2018).
Diolaiti, D. et al. A recurrent novel MGA–NUTM1 fusion identifies a new subtype of high-grade spindle cell sarcoma. Cold Spring Harb. Mol. Case Stud. 4, a003194 (2018).
Le Loarer, F. et al. Clinicopathologic features of CIC–NUTM1 sarcomas, a new molecular variant of the family of CIC-fused sarcomas. Am. J. Surg. Pathol. 43, 268–276 (2019).
Barletta, J. A. et al. NUTM1-rearranged carcinoma of the thyroid: a distinct subset of NUT carcinoma characterized by frequent NSD3–NUTM1 fusions. Am. J. Surg. Pathol. 46, 1706–1715 (2022).
Allison, D. B. et al. Thyroid carcinoma with NSD3::NUTM1 fusion: a case with thyrocyte differentiation and colloid production. Endocr. Pathol. 33, 315–326 (2022).
den Bakker, M. A. et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am. J. Surg. Pathol. 33, 1253–1258 (2009).
Agaimy, A. et al. NUT carcinoma of the salivary glands: clinicopathologic and molecular analysis of 3 cases and a survey of NUT expression in salivary gland carcinomas. Am. J. Surg. Pathol. 42, 877–884 (2018).
Shehata, B. M. et al. NUT midline carcinoma in a newborn with multiorgan disseminated tumor and a 2-year-old with a pancreatic/hepatic primary. Pediatr. Dev. Pathol. 13, 481–485 (2010).
Bishop, J. A., Stelow, E. & French, C. A. in WHO Classification of Tumours: Paediatric Tumours 5th edn (ed. Thompson, L. D. R.) 908–909 (International Agency for Research on Cancer, 2022).
Iqbal, A. et al. Prognostic factors and survival outcomes in squamous cell carcinoma of the thyroid: a surveillance, epidemiology, and end results (SEER) database analysis. Cureus 16, e63326 (2024).
Gluck, G. et al. Comparative study of conventional urothelial carcinoma, squamous differentiation carcinoma and pure squamous carcinoma in patients with invasive bladder tumors. J. Med. Life 7, 211–214 (2014).
Ford, J. A., Bhatt, A., Kim, R. C., Larkins, M. & Burke, A. M. Primary squamous cell carcinoma of the pancreas: an update on a rare neoplasm from the SEER database. Front. Oncol. 13, 1272740 (2023).
Liang, K., Yuan, Y., Lv, B. & Ke, Z. Primary squamous cell carcinoma of renal parenchyma: a case report and literature review. Front. Oncol. 13, 1037156 (2023).
Sholl, L. M. et al. Primary pulmonary NUT midline carcinoma: clinical, radiographic, and pathologic characterizations. J. Thorac. Oncol. 10, 951–959 (2015).
Ferone, G. et al. SOX2 is the determining oncogenic switch in promoting lung squamous cell carcinoma from different cells of origin. Cancer Cell 30, 519–532 (2016).
French, C. A. & den Bakker, M. A. in WHO Classification of Head and Neck Tumours (eds El-Naggar et al.) 20–21 (International Agency for Research on Cancer, 2017).
Matsuda, K., Kashima, J. & Yatabe, Y. The isoform matters in NUT carcinoma: a diagnostic pitfall of p40 immunohistochemistry. J. Thorac. Oncol. 15, e176–e178 (2020).
Tilson, M. P. & Bishop, J. A. Utility of p40 in the differential diagnosis of small round blue cell tumors of the sinonasal tract. Head. Neck Pathol. 8, 141–145 (2014).
Zhuang, X. P. et al. Primary pulmonary NUT carcinoma: a clinicopathological analysis of seven cases. Zhonghua Bing. Li Xue Za Zhi 52, 1244–1248 (2023).
Comitani, F. et al. Diagnostic classification of childhood cancer using multiscale transcriptomics. Nat. Med. 29, 656–666 (2023).
Ruiz, E. J. et al. USP28 deletion and small-molecule inhibition destabilizes c-MYC and elicits regression of squamous cell lung carcinoma. eLife 10, e71596 (2021).
Boumahdi, S. et al. SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511, 246–250 (2014).
Rocco, J. W., Leong, C. O., Kuperwasser, N., DeYoung, M. P. & Ellisen, L. W. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9, 45–56 (2006).
Martin-Padron, J. et al. Plakophilin 1 enhances MYC translation, promoting squamous cell lung cancer. Oncogene 39, 5479–5493 (2020).
Justilien, V. et al. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 25, 139–151 (2014).
Akervall, J. et al. The gene ratios c-MYC:cyclin-dependent kinase (CDK)N2A and CCND1:CDKN2A correlate with poor prognosis in squamous cell carcinoma of the head and neck. Clin. Cancer Res. 9, 1750–1755 (2003).
Pickering, C. R. et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 3, 770–781 (2013).
Saladi, S. V. et al. ACTL6A is co-amplified with p63 in squamous cell carcinoma to drive YAP activation, regenerative proliferation, and poor prognosis. Cancer Cell 31, 35–49 (2017).
Bass, A. J. et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 41, 1238–1242 (2009).
Brennan, J. A. et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 332, 712–717 (1995).
Werness, B. A., Levine, A. J. & Howley, P. M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 248, 76–79 (1990).
Reynoird, N. et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 29, 2943–2952 (2010).
Ezhkova, E. et al. Ezh2 orchestrates gene expression for the stepwise differentiation of tissue-specific stem cells. Cell 136, 1122–1135 (2009).
Behrens, C. et al. EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin. Cancer Res. 19, 6556–6565 (2013).
Xie, Q. et al. Increased expression of enhancer of Zeste Homolog 2 (EZH2) differentiates squamous cell carcinoma from normal skin and actinic keratosis. Eur. J. Dermatol. 24, 41–45 (2014).
Temam, S. et al. Epidermal growth factor receptor copy number alterations correlate with poor clinical outcome in patients with head and neck squamous cancer. J. Clin. Oncol. 25, 2164–2170 (2007).
Redon, R. et al. A simple specific pattern of chromosomal aberrations at early stages of head and neck squamous cell carcinomas: PIK3CA but not p63 gene as a likely target of 3q26-qter gains. Cancer Res. 61, 4122–4129 (2001).
Weiss, J. et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci. Transl. Med. 2, 62ra93 (2010).
Liao, S., Maertens, O., Cichowski, K. & Elledge, S. J. Genetic modifiers of the BRD4-NUT dependency of NUT midline carcinoma uncovers a synergism between BETis and CDK4/6is. Genes. Dev. 32, 1188–1200 (2018).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Morrison-Smith, C. D. et al. Combined targeting of the BRD4–NUT–p300 axis in NUT midline carcinoma by dual selective bromodomain inhibitor, NEO2734. Mol. Cancer Ther. 19, 1406–1414 (2020).
Zhang, X. et al. Therapeutic targeting of p300/CBP HAT domain for the treatment of NUT midline carcinoma. Oncogene 39, 4770–4779 (2020).
Kim, M. et al. Regulation of mouse embryonic stem cell neural differentiation by retinoic acid. Dev. Biol. 328, 456–471 (2009).
Guo, X., Stice, S. L., Boyd, N. L. & Chen, S. Y. A novel in vitro model system for smooth muscle differentiation from human embryonic stem cell-derived mesenchymal cells. Am. J. Physiol. Cell Physiol. 304, C289–C298 (2013).
Niederkohr, R. D., Cameron, M. J. & French, C. A. FDG PET/CT imaging of NUT midline carcinoma. Clin. Nucl. Med. 36, e124–e126 (2011).
Bishop, J. A. & Westra, W. H. NUT midline carcinomas of the sinonasal tract. Am. J. Surg. Pathol. 36, 1216–1221 (2012).
Thompson, L. D. Small round blue cell tumors of the sinonasal tract: a differential diagnosis approach. Mod. Pathol. 30, S1–S26 (2017).
Stelow, E. B. et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am. J. Surg. Pathol. 32, 828–834 (2008).
Pan, M. & Chang, J. S. Durable complete remission of PD-L1 positive NUT carcinoma treated with concurrent chemotherapy and radiation. Perm. J. 25, 1–3 (2020).
Ueki, H. et al. A case of NUT midline carcinoma with complete response to gemcitabine following cisplatin and docetaxel. J. Pediatr. Hematol. Oncol. 36, e476–e480 (2014).
Leeman, R. et al. NUT carcinoma without upfront surgical resection: a case report. J. Pediatr. Hematol. Oncol. 43, e707–e710 (2020).
Storck, S. et al. Pediatric NUT–midline carcinoma: therapeutic success employing a sarcoma based multimodal approach. Pediatr. Hematol. Oncol. 34, 231–237 (2017).
Murano, C. et al. Vimentin-positive and alpha-fetoprotein-elevated nuclear protein of the testis midline carcinoma: a case report and review of the literature. Intern. Med. 60, 3645–3649 (2021).
Parikh, S. A. et al. NUT midline carcinoma: an aggressive intrathoracic neoplasm. J. Thorac. Oncol. 8, 1335–1338 (2013).
Gupta, R. et al. NUT midline lung cancer: a rare case report with literature review. AME Case Rep. 6, 2 (2022).
Fossella, F. V. et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J. Clin. Oncol. 18, 2354–2362 (2000).
Klingberg, D. et al. Association of chemotherapy dose intensity and age with outcomes in patients with Ewing’s family sarcoma. Asia Pac. J. Clin. Oncol. 21, 87–94 (2023).
Davis, A., Mahar, A., Wong, K., Barnet, M. & Kao, S. Prolonged disease control on nivolumab for primary pulmonary NUT carcinoma. Clin. Lung Cancer 22, e665–e667 (2021).
Riess, J. W. et al. Genomic profiling of solid tumors harboring BRD4–NUT and response to immune checkpoint inhibitors. Transl. Oncol. 14, 101184 (2021).
Jung, M. et al. Clinicopathological and preclinical findings of NUT carcinoma: a multicenter study. Oncologist 24, e740–e748 (2019).
Xie, X. H. et al. Clinical features, treatment, and survival outcome of primary pulmonary NUT midline carcinoma. Orphanet J. Rare Dis. 15, 183 (2020).
Tontsch-Grunt, U. et al. Therapeutic impact of BET inhibitor BI 894999 treatment: backtranslation from the clinic. Br. J. Cancer 127, 577–586 (2022).
Piha-Paul, S. A. et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr. 4, pkz093 (2020).
Lewin, J. et al. Phase Ib trial with birabresib, a small-molecule inhibitor of bromodomain and extraterminal proteins, in patients with selected advanced solid tumors. J. Clin. Oncol. 36, 3007–3014 (2018).
Shapiro, G. I. et al. A phase 1 study of RO6870810, a novel bromodomain and extra-terminal protein inhibitor, in patients with NUT carcinoma, other solid tumours, or diffuse large B-cell lymphoma. Br. J. Cancer 124, 744–753 (2020).
Hilton, J. et al. Initial results from a phase I/IIa trial evaluating BMS-986158, an inhibitor of the bromodomain and extra-terminal (BET) proteins, in patients (pts) with advanced cancer. Ann. Oncol. 29 (2018).
Stathis, A. et al. Clinical response of carcinomas harboring the BRD4–NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 6, 492–500 (2016).
French, C. A. et al. Report of the first international symposium on NUT carcinoma. Clin. Cancer Res. 28, 2493–2505 (2022).
Yamamoto, T. et al. BRD4 promotes metastatic potential in oral squamous cell carcinoma through the epigenetic regulation of the MMP2 gene. Br. J. Cancer 123, 580–590 (2020).
Wu, Y. et al. Therapeutic targeting of BRD4 in head neck squamous cell carcinoma. Theranostics 9, 1777–1793 (2019).
Zhang, W. et al. Combinational therapeutic targeting of BRD4 and CDK7 synergistically induces anticancer effects in head and neck squamous cell carcinoma. Cancer Lett. 469, 510–523 (2020).
Wu, Q. et al. BRD4 drives esophageal squamous cell carcinoma growth by promoting RCC2 expression. Oncogene 41, 347–360 (2022).
Fisher, M. L. et al. BRD4 regulates transcription factor deltaNp63alpha to drive a cancer stem cell phenotype in squamous cell carcinomas. Cancer Res. 81, 6246–6258 (2021).
Ohnesorge, P. V. et al. Efficacy of oncolytic herpes simplex virus T-VEC combined with BET inhibitors as an innovative therapy approach for NUT carcinoma. Cancers 14, 2761 (2022).
Kloker, L. D. et al. Case report: immunovirotherapy as a novel add-on treatment in a patient with thoracic NUT carcinoma. Front. Oncol. 12, 995744 (2022).
Sotiriadis, S. et al. Multimodal therapy approaches for NUT carcinoma by dual combination of oncolytic virus talimogene laherparepvec with small molecule inhibitors. Viruses 16, 775 (2024).
Pham, C., Lang, D. & Iams, W. T. Successful treatment and retreatment with erdafitinib for a patient with FGFR3-TACC3 fusion squamous NSCLC: a case report. JTO Clin. Res. Rep. 4, 100511 (2023).
Wang, C. G., Peiris, M. N., Meyer, A. N., Nelson, K. N. & Donoghue, D. J. Oncogenic driver FGFR3–TACC3 requires five coiled-coil heptads for activation and disulfide bond formation for stability. Oncotarget 14, 133–145 (2023).
Amin, S. E. et al. DEK::AFF2 fusion-associated squamous cell carcinoma: a case series with literature review on an emerging and challenging entity. Head Neck Pathol. 18, 86 (2024).
McLean-Holden, A. C. et al. NUT carcinoma in a patient with unusually long survival and false negative FISH results. Head Neck Pathol. 15, 698–703 (2021).
Begum, S. & Westra, W. H. Basaloid squamous cell carcinoma of the head and neck is a mixed variant that can be further resolved by HPV status. Am. J. Surg. Pathol. 32, 1044–1050 (2008).
Fujimaki, M. et al. Histological subtypes and characteristic structures of HPV-associated oropharyngeal carcinoma: study with Japanese cases. Diagn. Pathol. 8, 211 (2013).
Petrini, P. et al. NUT rearrangement is uncommon in human thymic epithelial tumors. J. Thorac. Oncol. 7, 744–750 (2012).
Gokmen-Polar, Y., Cano, O. D., Kesler, K. A., Loehrer, P. J. & Badve, S. NUT midline carcinomas in the thymic region. Mod. Pathol. 27, 1649–1656 (2014).
Chen, S. et al. A prognostic model for elderly patients with squamous non-small cell lung cancer: a population-based study. J. Transl. Med. 18, 436 (2020).
Schaefer, I. M. et al. CIC–NUTM1 fusion: a case which expands the spectrum of NUT-rearranged epithelioid malignancies. Genes Chromosomes Cancer 57, 446–451 (2018).
Sturm, D. et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell 164, 1060–1072 (2016).
Andersson, A. K. et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat. Genet. 47, 330–337 (2015).
Gu, Z. et al. Genomic analyses identify recurrent MEF2D fusions in acute lymphoblastic leukaemia. Nat. Commun. 7, 13331 (2016).
Liu, Y. F. et al. Genomic profiling of adult and pediatric B-cell acute lymphoblastic leukemia. eBioMedicine 8, 173–183 (2016).
Lilljebjorn, H. et al. Identification of ETV6–RUNX1-like and DUX4-rearranged subtypes in paediatric B-cell precursor acute lymphoblastic leukaemia. Nat. Commun. 7, 11790 (2016).
Liu, Y. et al. The genomic landscape of pediatric and young adult T-lineage acute lymphoblastic leukemia. Nat. Genet. 49, 1211–1218 (2017).
Chaturvedi, A. K. et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 29, 4294–4301 (2011).
Chau, N. G. et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer 122, 3632–3640 (2016).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–49 (2021).
Windon, M. J. et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults. Cancer 124, 2993–9 (2018).
SEER*Explorer: an interactive website for SEER cancer statistics. National Cancer Institute https://seer.cancer.gov/statistics-network/explorer/ (2024).
Crook, T. et al. Status of c-myc, p53 and retinoblastoma genes in human papillomavirus positive and negative squamous cell carcinomas of the anus. Oncogene 6, 1251–1257 (1991).
Sarbia, M. et al. Expression of Bcl-2 and amplification of c-myc are frequent in basaloid squamous cell carcinomas of the esophagus. Am. J. Pathol. 155, 1027–1032 (1999).
Tonon, G. et al. High-resolution genomic profiles of human lung cancer. Proc. Natl Acad. Sci. USA 102, 9625–9630 (2005).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011).
Hussenet, T. et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS ONE 5, e8960 (2010).
Hussenet, T. & du Manoir, S. SOX2 in squamous cell carcinoma: amplifying a pleiotropic oncogene along carcinogenesis. Cell Cycle 9, 1480–1486 (2010).
Sheu, J. J. et al. Functional genomic analysis identified epidermal growth factor receptor activation as the most common genetic event in oral squamous cell carcinoma. Cancer Res. 69, 2568–2576 (2009).
Koole, K. et al. FGFR1 is a potential prognostic biomarker and therapeutic target in head and neck squamous cell carcinoma. Clin. Cancer Res. 22, 3884–3893 (2016).
Murugan, A. K., Hong, N. T., Fukui, Y., Munirajan, A. K. & Tsuchida, N. Oncogenic mutations of the PIK3CA gene in head and neck squamous cell carcinomas. Int. J. Oncol. 32, 101–111 (2008).
Schwartz, B. E. et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 71, 2686–2696 (2011).
Shiota, H. et al. Chemical screen identifies diverse and novel histone deacetylase inhibitors as repressors of NUT function: implications for NUT carcinoma pathogenesis and treatment. Mol. Cancer Res. 19, 1818–1830 (2021).
Acknowledgements
The work of J.L., G.I.S. and C.A.F. is funded by K12TR004381 (Harvard Catalyst, and the Harvard Clinical and Translational Science Center; J.L.), Lowe Center of Thoracic Oncology (J.L.), Dana–Farber Department of Medical Oncology (J.L.), R01 CA124633 (C.A.F.), U01 CA294062 (C.A.F.), R01 CA285308 (G.I.S. and C.A.F.) and R21 CA277316 (J.L., G.I.S. and C.A.F.). The Dana–Farber/Brigham and Women’s Hospital NUT Carcinoma Program receives philanthropic support from the Friends of Jay Dion Memorial Classic, the Ryan Richards Foundation, the McDevitt Strong Foundation, the Max Vincze Foundation, the Victor Family Foundation, the Alexandra Hallock Memorial Fund, and the Fortisure Foundation Fund for NUT Carcinoma.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the manuscript concepts and content. J.L. and C.A.F. researched data for the article, wrote the manuscript and drafted the initial figures. J.B., S.G.D., G.J.H., L.M.S., E.B.S., L.D.R.T. and G.I.S. reviewed and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.L. reports honoraria from Cancer GRACE, Community Cancer Education Inc., Physicians’ Education Resource, Targeted Oncology and VJ Oncology; advisory board participation for Amgen, Astellas and AstraZeneca; institutional research support from Erasca, Genentech, Kronos Bio, Novartis and Revolution Medicines; and personal fees from Blueprint Medicines, Daiichi Sankyo and Erasca. A patent filed by Memorial Sloan Kettering Cancer Center related to multimodal features to predict response to immunotherapy (PCT/US2023/115872) is pending. G.J.H. reports grants or contracts from ACCRF, Actuate Therapeutics, ASCO CCF, Bicara, Bristol Myers Squibb, Elevar Therapeutics, Exicure, Gateway for Cancer Research, Genentech, GSK, ImmunityBio, Kartos, Kite (a Gilead company), KSQ Therapeutics, Kura Oncology, Regeneron, Repertoire, Sanofi Genzyme, Secura Bio and V Foundation; and advisory roles for and/or honoraria from Bicara, Bio-Rad, Boxer Capital, Bristol Myers Squibb, Coherus, Elevar, Exicure, General Catalyst, Guardian Bio, KSQ Therapeutics, Kura Oncology, Massachusetts Medical Society, Merck, Naveris, Nextech, Prelude, Rain, Regeneron, Remix, Replimune, Sanofi Genzyme, SIRPant and Surface Oncology. S.G.D. reports honoraria from and/or advisory board participation for Amgen, Bayer, InhibRx and Jazz Pharmaceuticals; and travel expenses from LOXO Oncology, Roche and Salarius. G.I.S. reports personal fees from Artios, Bayer, Bicycle Therapeutics, Blueprint Medicines, Boehringer Ingelheim, Concarlo Holdings, Cybrexa Therapeutics, CytomX Therapeutics, ImmunoMet, Janssen, Kymera Therapeutics, Merck KGaA/EMD-Serono, Syros, Xinthera and Zentalis; grants from Bristol Myers Squibb, Eli Lilly, Merck KGaA/EMD-Serono, Pfizer and Tango; has a patent for “Dosage regimen for sapacitabine and seliciclib”, issued to Cyclacel Pharmaceuticals and G.I.S., and a patent for “Compositions and methods for predicting response and resistance to CDK4/6 inhibition”, issued to Liam Cornell and G.I.S. C.A.F. reports research funding and consultancy fees from Boehringer Ingelheim.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, J., Bishop, J.A., DuBois, S.G. et al. Hiding in plain sight: NUT carcinoma is an unrecognized subtype of squamous cell carcinoma of the lungs and head and neck. Nat Rev Clin Oncol 22, 292–306 (2025). https://doi.org/10.1038/s41571-025-00986-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-00986-3
This article is cited by
-
Fusion oncoproteins orchestrate tumorigenesis and sustain malignant progression via a positive feedback mechanism
Cell & Bioscience (2026)
-
Photodynamic therapeutic activity of novel porphyrins against lung squamous cell carcinoma
BMC Cancer (2025)
-
So It’s Squamous: What’s Next? Ancillary Testing to Better Classify Head and Neck Tumors with Squamous Differentiation
Head and Neck Pathology (2025)
-
Expanding the spectrum of AFF2 carcinoma: clinical, morphological, immunohistochemical, and molecular characteristics of five cases harboring alternate fusions
Virchows Archiv (2025)