Abstract
Immune checkpoint inhibitors (ICIs) constitute a major breakthrough in the field of cancer therapy; their use has resulted in improved outcomes across various tumour types. However, ICIs can cause a diverse range of immune-related adverse events (irAEs) that present a considerable challenge to the efficacy and safety of these treatments. The gut microbiota has been demonstrated to have a crucial role in modulating the tumour immune microenvironment and thus influences the effectiveness of ICIs. Accumulating evidence indicates that alterations in the composition and function of the gut microbiota are also associated with an increased risk of irAEs, particularly ICI-induced colitis. Indeed, these changes in the gut microbiota can contribute to the pathogenesis of irAEs. In this Review, we first summarize the current clinical challenges posed by irAEs. We then focus on reported correlations between alterations in the gut microbiota and irAEs, especially ICI-induced colitis, and postulate mechanisms by which these microbial changes influence the occurrence of irAEs. Finally, we highlight the potential value of gut microbial changes as biomarkers for predicting irAEs and discuss gut microbial interventions that might serve as new strategies for the management of irAEs, including faecal microbiota transplantation, probiotic, prebiotic and/or postbiotic supplements, and dietary modulations.
Key points
-
Although immune checkpoint inhibitors (ICIs) have revolutionized cancer treatment, they often induce immune-related adverse events (irAEs) that are not only a common cause of treatment discontinuation, which might compromise oncological outcomes, but can also reduce patient quality of life and even have fatal outcomes.
-
Clinical cohort studies and preclinical data indicate that gut microbiota composition has a substantial influence on the risk and severity of irAEs.
-
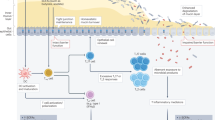
The mechanisms underlying the association of the gut microbiota with irAEs include antigen cross-reactivity, modulation of intestinal barrier integrity, interplay with the immune environment, regulation of microbial homeostasis and others.
-
Distinct gut microbial alterations could potentially be used to predict the occurrence of irAEs.
-
Gut microbial modulation strategies such as faecal microbiota transplantation, dietary modifications, and supplementation with probiotics, prebiotics and/or postbiotics, have promise for the treatment and perhaps even prevention of irAEs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Postow, M. A., Callahan, M. K. & Wolchok, J. D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 33, 1974–1982 (2015).
Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013).
Borghaei, H. et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N. Engl. J. Med. 373, 1627–1639 (2015).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Zhou, F., Qiao, M. & Zhou, C. The cutting-edge progress of immune-checkpoint blockade in lung cancer. Cell Mol. Immunol. 18, 279–293 (2021).
Ramos-Casals, M. et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 6, 38 (2020).
Khan, S. & Gerber, D. E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: a review. Semin. Cancer Biol. 64, 93–101 (2020).
Esfahani, K. et al. Moving towards personalized treatments of immune-related adverse events. Nat. Rev. Clin. Oncol. 17, 504–515 (2020).
Thapa, B. et al. Incidence and clinical pattern of immune related adverse effects (irAE) due to immune checkpoint inhibitors (ICI) [abstract]. J. Clin. Oncol. 37, e14151 (2019).
Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 16, 563–580 (2019).
Cook, S. et al. Immune-related adverse events and survival among patients with metastatic NSCLC treated with immune checkpoint inhibitors. JAMA Netw. Open. 7, e2352302 (2024).
Street, S. et al. The positive effect of immune checkpoint inhibitor-induced thyroiditis on overall survival accounting for immortal time bias: a retrospective cohort study of 6596 patients. Ann. Oncol. 32, 1050–1051 (2021).
Haratani, K. et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol. 4, 374–378 (2018).
Suijkerbuijk, K. P. M., van Eijs, M. J. M., van Wijk, F. & Eggermont, A. M. M. Clinical and translational attributes of immune-related adverse events. Nat. Cancer 5, 557–571 (2024).
Burke, K. P., Grebinoski, S., Sharpe, A. H. & Vignali, D. A. A. Understanding adverse events of immunotherapy: a mechanistic perspective. J. Exp. Med. 218, e20192179 (2021).
Singh, N., Hocking, A. M. & Buckner, J. H. Immune-related adverse events after immune check point inhibitors: understanding the intersection with autoimmunity. Immunol. Rev. 318, 81–88 (2023).
Schneider, B. J. et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J. Clin. Oncol. 39, 4073–4126 (2021).
Haanen, J. B. A. G. et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 33, 1217–1238 (2017).
Puzanov, I. et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. J. Immunother. Cancer 5, 95 (2017).
Thompson, J. A. et al. NCCN guidelines insights: management of immunotherapy-related toxicities, version 1. 2020. J. Natl Compr. Canc Netw. 18, 230–241 (2020).
Faje, A. T. et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 124, 3706–3714 (2018).
Goodman, R. S., Johnson, D. B. & Balko, J. M. Corticosteroids and cancer immunotherapy. Clin. Cancer Res. 29, 2580–2587 (2023).
Wahlström, A., Sayin, S. I., Marschall, H. U. & Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 24, 41–50 (2016).
Mishra, S. P. et al. A mechanism by which gut microbiota elevates permeability and inflammation in obese/diabetic mice and human gut. Gut 72, 1848–1865 (2023).
Arifuzzaman, M. et al. Inulin fibre promotes microbiota-derived bile acids and type 2 inflammation. Nature 611, 578–584 (2022).
Arima, K. et al. Western-style diet, pks Island-carrying Escherichia coli, and colorectal cancer: analyses from two large prospective cohort studies. Gastroenterology 163, 862–874 (2022).
Jiang, S. S. et al. Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer. Cell Host Microbe 31, 781–797.e9 (2023).
Gopalakrishnan, V., Helmink, B. A., Spencer, C. N., Reuben, A. & Wargo, J. A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 33, 570–580 (2018).
Wang, Y. et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 24, 1804–1808 (2018).
Andrews, M. C. et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 27, 1432–1441 (2021).
Hu, M. et al. Gut microbiome for predicting immune checkpoint blockade-associated adverse events. Genome Med. 16, 16 (2024).
Liu, X. et al. Gut microbiota composition in patients with advanced malignancies experiencing immune-related adverse events. Front. Immunol. 14, 1109281 (2023).
Liu, W. et al. Intestinal microbiome associated with immune-related adverse events for patients treated with anti-PD-1 inhibitors, a real-world study. Front. Immunol. 12, 756872 (2021).
Gao, Y. et al. Faecalibacterium prausnitzii abrogates intestinal toxicity and promotes tumor immunity to increase the efficacy of dual CTLA4 and PD-1 checkpoint blockade. Cancer Res. 83, 3710–3725 (2023).
Wang, T. et al. Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade-associated colitis by inhibiting group 3 innate lymphoid cells. Front. Immunol. 10, 01235 (2019).
Liu, X. et al. Gut microbiome metabolites, molecular mimicry, and species-level variation drive long-term efficacy and adverse event outcomes in lung cancer survivors. eBioMedicine 109, 105427 (2024).
Sun, S. et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl Acad. Sci. USA 117, 27509–27515 (2020).
Simpson, R. C. et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. 28, 2344–2352 (2022).
Zhang, M., Liu, J. & Xia, Q. Role of gut microbiome in cancer immunotherapy: from predictive biomarker to therapeutic target. Exp. Hematol. Oncol. 12, 84 (2023).
Sivan, A. et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 (2015).
Jia, D. et al. Microbial metabolite enhances immunotherapy efficacy by modulating T cell stemness in pan-cancer. Cell 187, 1651–1665.e21 (2024).
Cong, J. et al. Bile acids modified by the intestinal microbiota promote colorectal cancer growth by suppressing CD8+ T cell effector functions. Immunity 57, 876–889.e11 (2024).
Renga, G. et al. Optimizing therapeutic outcomes of immune checkpoint blockade by a microbial tryptophan metabolite. J. Immunother. Cancer 10, e003725 (2022).
Jing, Y., Yang, J., Johnson, D. B., Moslehi, J. J. & Han, L. Harnessing big data to characterize immune-related adverse events. Nat. Rev. Clin. Oncol. 19, 269–280 (2022).
Johnson, D. B., Nebhan, C. A., Moslehi, J. J. & Balko, J. M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022).
Lozano, A. X. et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. 28, 353–362 (2022).
Yasuda, Y. et al. CD4+ T cells are essential for the development of destructive thyroiditis induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci. Transl. Med. 13, eabb7495 (2021).
Hutchinson, J. A. et al. Virus-specific memory T cell responses unmasked by immune checkpoint blockade cause hepatitis. Nat. Commun. 12, 1439 (2021).
Collier, J. L. et al. Single-cell profiling reveals unique features of diabetogenic T cells in anti-PD-1-induced type 1 diabetes mice. J. Exp. Med. 220, e20221920 (2023).
Friedman, C. F., Proverbs-Singh, T. A. & Postow, M. A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors. JAMA Oncol. 2, 1346–1353 (2016).
Kennedy, L. B. & Salama, A. K. S. A review of cancer immunotherapy toxicity. CA Cancer J. Clin. 70, 86–104 (2020).
Bergqvist, V. et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol. Immunother. 66, 581–592 (2017).
Abu-Sbeih, H. et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: a multi-center study. J. Immunother. Cancer 6, 142 (2018).
Abu-Sbeih, H. et al. Early introduction of selective immunosuppressive therapy associated with favorable clinical outcomes in patients with immune checkpoint inhibitor-induced colitis. J. Immunother. Cancer 7, 93 (2019).
Feliu, V. et al. Distant antimetastatic effect of enterotropic colon cancer-derived α4β7+CD8+ T cells. Sci. Immunol. 8, eadg8841 (2023).
Fidelle, M. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296 (2023).
van Not, O. J. et al. Association of immune-related adverse event management with survival in patients with advanced melanoma. JAMA Oncol. 8, 1794–1801 (2022).
Bauché, D. et al. Antitumor efficacy of combined CTLA4/PD-1 blockade without intestinal inflammation is achieved by elimination of FcγR interactions. J. Immunother. Cancer 8, e001584 (2020).
Perez-Ruiz, E. et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 569, 428–432 (2019).
Badran, Y. R. et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J. Immunother. Cancer 7, 226 (2019).
Hailemichael, Y. et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 40, 509–523 (2022).
Fa’ak, F. et al. Selective immune suppression using interleukin-6 receptor inhibitors for management of immune-related adverse events. J. Immunother. Cancer 11, e006814 (2023).
Bishu, S. et al. Efficacy and outcome of tofacitinib in immune checkpoint inhibitor colitis. Gastroenterology 160, 932–934.e3 (2021).
Routy, B. et al. Fecal microbiota transplantation plus anti-PD-1 immunotherapy in advanced melanoma: a phase I trial. Nat. Med. 29, 2121–2132 (2023).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT04883762 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06206707 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT03819296 (2024).
Martinez Chanza, N. et al. Safety and efficacy of immune checkpoint inhibitors in advanced urological cancers with pre-existing autoimmune disorders: a retrospective international multicenter study. J. Immunother. Cancer 8, e000538 (2020).
Johnson, D. B. et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2, 234–240 (2016).
Tison, A. et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 71, 2100–2111 (2019).
Abdel-Wahab, N., Shah, M., Lopez-Olivo, M. A. & Suarez-Almazor, M. E. Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease. Ann. Intern. Med. 168, 121–130 (2018).
Liu, X. et al. Risk factors for immune-related adverse events: what have we learned and what lies ahead? Biomark. Res. 9, 79 (2021).
Abu-Sbeih, H. et al. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis. J. Immunother. Cancer 7, 242 (2019).
Jing, Y. et al. Association of antibiotic treatment with immune-related adverse events in patients with cancer receiving immunotherapy. J. Immunother. Cancer 10, e003779 (2022).
Mohiuddin, J. J. et al. Association of antibiotic exposure with survival and toxicity in patients with melanoma receiving immunotherapy. J. Natl Cancer Inst. 113, 162–170 (2021).
McCulloch, J. A. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 28, 545–556 (2022).
Hamada, K. et al. Turicibacter and Acidaminococcus predict immune-related adverse events and efficacy of immune checkpoint inhibitor. Front. Immunol. 14, 1164724 (2023).
Hirata, Y. et al. Gut microbiota shifts from onset to remission in immune checkpoint inhibitor-induced enterocolitis: a case report. Gut Pathog. 16, 33 (2024).
Zhou, G., Zhang, N., Meng, K. & Pan, F. Interaction between gut microbiota and immune checkpoint inhibitor-related colitis. Front. Immunol. 13, 1001623 (2022).
Lo, B. C. et al. Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors. Science 383, 62–70 (2024).
Sun, Y. et al. Gut firmicutes: relationship with dietary fiber and role in host homeostasis. Crit. Rev. Food Sci. Nutr. 63, 12073–12088 (2022).
Pan, X., Raaijmakers, J. M. & Carrión, V. J. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 31, 959–971 (2023).
Brinkmann, S., Spohn, M. S. & Schäberle, T. F. Bioactive natural products from Bacteroidetes. Nat. Product. Rep. 39, 1045–1065 (2022).
Zeng, Y. et al. Dynamic gut microbiota changes in patients with advanced malignancies experiencing secondary resistance to immune checkpoint inhibitors and immune-related adverse events. Front. Oncol. 13, 1144534 (2023).
Sakai, K. et al. Intestinal microbiota and gene expression reveal similarity and dissimilarity between immune-mediated colitis and ulcerative colitis. Front. Oncol. 11, 763468 (2021).
Dubin, K. et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 (2016).
Zhang, Y. et al. Correlation of the gut microbiome and immune-related adverse events in gastrointestinal cancer patients treated with immune checkpoint inhibitors. Front. Cell Infect. Microbiol. 13, 1099063 (2023).
Usyk, M. et al. Bacteroides vulgatus and Bacteroides dorei predict immune-related adverse events in immune checkpoint blockade treatment of metastatic melanoma. Genome Med. 13, 160 (2021).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Gavzy, S. J. et al. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 15, 2291164 (2023).
Wang, F., Yin, Q., Chen, L. & Davis, M. M. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc. Natl Acad. Sci. USA 115, 157–161 (2017).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283 (2014).
Li, G. et al. Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 13, 1968257 (2021).
Chen, G. et al. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. eBioMedicine 30, 317–325 (2018).
Kelly et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 (2015).
Hao, F. et al. Butyrate enhances CPT1A activity to promote fatty acid oxidation and iTreg differentiation. Proc. Natl Acad. Sci. USA 118, e2014681118 (2021).
Furusawa, Y. et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450 (2013).
Kang, X. et al. Roseburia intestinalis generated butyrate boosts anti-PD-1 efficacy in colorectal cancer by activating cytotoxic CD8+ T cells. Gut 72, 2112–2122 (2023).
Coutzac, C. et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 11, 2168 (2020).
Zhu, X. et al. Microbial metabolite butyrate promotes anti-PD-1 antitumor efficacy by modulating T cell receptor signaling of cytotoxic CD8 T cell. Gut Microbes 15, 2249143 (2023).
Luu, M. et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12, 4077 (2021).
Liu, L. et al. Bacteroides vulgatus attenuates experimental mice colitis through modulating gut microbiota and immune responses. Front. Immunol. 13, 1036196 (2022).
Yang, J. et al. High soluble fiber promotes colorectal tumorigenesis through modulating gut microbiota and metabolites in mice. Gastroenterology 166, 323–337.e7 (2024).
Chen, Y. et al. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARα-CYP4X1 axis in colonic macrophages. J. Exp. Clin. Cancer Res. 41, 1 (2022).
Jia, W., Xie, G. & Jia, W. Bile acid–microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 15, 111–128 (2017).
Sinha, S. R. et al. Dysbiosis-induced secondary bile acid deficiency promotes intestinal inflammation. Cell Host Microbe 27, 659–670.e5 (2020).
Yan, Y. et al. Bacteroides uniformis-induced perturbations in colonic microbiota and bile acid levels inhibit TH17 differentiation and ameliorate colitis developments. NPJ Biofilms Microbiomes 9, 56 (2023).
Agus, A., Planchais, J. & Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 (2018).
Agus, A., Clément, K. & Sokol, H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70, 1174–1182 (2021).
Li, M. et al. Indole-3-acetic acid alleviates DSS-induced colitis by promoting the production of R-equol from Bifidobacterium pseudolongum. Gut Microbes 16, 2329147 (2024).
Fong, W. et al. Lactobacillus gallinarum-derived metabolites boost anti-PD1 efficacy in colorectal cancer by inhibiting regulatory T cells through modulating IDO1/Kyn/AHR axis. Gut 72, 2272–2285 (2023).
Rothhammer, V. et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 (2016).
Montgomery, T. L. et al. Lactobacillus reuteri tryptophan metabolism promotes host susceptibility to CNS autoimmunity. Microbiome 10, 198 (2022).
Mager, L. F. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020).
Johnson, E. L. et al. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 11, 2471 (2020).
Heaver, S. L. et al. Characterization of inositol lipid metabolism in gut-associated Bacteroidetes. Nat. Microbiol. 7, 986–1000 (2022).
Brown, E. M. et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe 25, 668–680 (2019).
Huang, X. et al. Multi-kingdom gut microbiota analyses define bacterial-fungal interplay and microbial markers of pan-cancer immunotherapy across cohorts. Cell Host Microbe 31, 1930–1943.e4 (2023).
Nakatsu, G. et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541.e5 (2018).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020).
Johnson, D. B. et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 25, 1243–1250 (2019).
Kang, J. H., Bluestone, J. A. & Young, A. Predicting and preventing immune checkpoint inhibitor toxicity: targeting cytokines. Trends Immunol. 42, 293–311 (2021).
Johnson, D. B. et al. Fulminant myocarditis with combination immune checkpoint blockade. N. Engl. J. Med. 375, 1749–1755 (2016).
Berner, F. et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol. 5, 1043–1047 (2019).
Berner, F. et al. Autoreactive napsin A-specific T cells are enriched in lung tumors and inflammatory lung lesions during immune checkpoint blockade. Sci. Immunol. 7, eabn9644 (2022).
Samstein, R. M. et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 51, 202–206 (2019).
Zhang, L., Shi, Y. & Han, X. Immunogenomic correlates of immune-related adverse events for anti-programmed cell death 1 therapy. Front. Immunol. 13, 1032221 (2022).
Bomze, D., Ali, O. H., Bate, A. & Flatz, L. Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden. JAMA Oncol. 5, 1633–1635 (2019).
Balachandran, V. P. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017).
Bessell, C. A. et al. Commensal bacteria stimulate antitumor responses via T cell cross-reactivity. JCI Insight 5, e135597 (2020).
Mehandru, S. & Colombel, J.-F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 18, 83–84 (2020).
Bamias, G. et al. Immunological characteristics of colitis associated with anti-CTLA-4 antibody therapy. Cancer Invest. 35, 443–455 (2017).
Fan, S. et al. Akkermansia muciniphila: a potential booster to improve the effectiveness of cancer immunotherapy. J. Cancer Res. Clin. Oncol. 149, 13477–13494 (2023).
Vergalito, F. et al. Akkermansia muciniphila: new insights into resistance to gastrointestinal stress, adhesion, and protein interaction with human mucins through optimised in vitro trials and bioinformatics tools. Front. Microbiol. 15, 1462220 (2024).
Mo, C. et al. The influence of Akkermansia muciniphila on intestinal barrier function. Gut Pathog. 16, 41 (2024).
Zhuge, A. et al. Akkermansia muciniphila-derived acetate activates the hepatic AMPK/SIRT1/PGC-1α axis to alleviate ferroptosis in metabolic-associated fatty liver disease. Acta Pharm. Sin. B 15, 151–167 (2025).
Geremia, A., Biancheri, P., Allan, P., Corazza, G. R. & Di Sabatino, A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun. Rev. 13, 3–10 (2014).
Mohebali, N. et al. Faecalibacterium prausnitzii, Bacteroides faecis and Roseburia intestinalis attenuate clinical symptoms of experimental colitis by regulating Treg/Th17 cell balance and intestinal barrier integrity. Biomed. Pharmacother. 167, 115568 (2023).
Guo, M. et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes 15, 2190304 (2023).
Zuo, L. et al. Bifidobacterium infantis attenuates colitis by regulating T cell subset responses. World J. Gastroenterol. 20, 18316–18329 (2014).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Li, Y. et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review. J. Transl. Med. 22, 293 (2024).
Liu, X. et al. Regulatory T cells and M2 macrophages present diverse prognostic value in gastric cancer patients with different clinicopathologic characteristics and chemotherapy strategies. J. Transl. Med. 17, 192 (2019).
Xu, W. et al. The appearance of Tregs in cancer nest is a promising independent risk factor in colon cancer. J. Cancer Res. Clin. Oncol. 139, 1845–1852 (2013).
Dykema, A. G. et al. Lung tumor-infiltrating Treg have divergent transcriptional profiles and function linked to checkpoint blockade response. Sci. Immunol. 8, eadg1487 (2023).
Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 182, 655–671.e22 (2020).
Zhou, Y. et al. Intestinal toxicity to CTLA-4 blockade driven by IL-6 and myeloid infiltration. J. Exp. Med. 220, e20221333 (2023).
Siwicki, M. et al. Resident Kupffer cells and neutrophils drive liver toxicity in cancer immunotherapy. Sci. Immunol. 6, abi7083 (2021).
Kim, S. T. et al. Distinct molecular and immune hallmarks of inflammatory arthritis induced by immune checkpoint inhibitors for cancer therapy. Nat. Commun. 15, 5621 (2022).
Llewellyn, H. P. et al. T cells and monocyte-derived myeloid cells mediate immunotherapy-related hepatitis in a mouse model. J. Hepatol. 75, 1083–1095 (2021).
Goldszmid, R. S. et al. Microbiota modulation of myeloid cells in cancer therapy. Cancer Immunol. Res. 3, 103–109 (2015).
Shmuel-Galia, L. et al. Dysbiosis exacerbates colitis by promoting ubiquitination and accumulation of the innate immune adaptor STING in myeloid cells. Immunity 54, 1137–1153.e8 (2021).
Porbahaie, M. et al. Short-chain fatty acids inhibit the activation of T lymphocytes and myeloid cells and induce innate immune tolerance. Benef. Microbes 14, 401–419 (2023).
Saez, A. et al. Innate lymphoid cells in intestinal homeostasis and inflammatory bowel disease. Int. J. Mol. Sci. 22, 7618 (2021).
Gury-BenAri, M. et al. The spectrum and regulatory landscape of intestinal innate lymphoid cells are shaped by the microbiome. Cell 166, 1231–1246 (2016).
Si, W. et al. Lactobacillus rhamnosus GG induces STING-dependent IL-10 in intestinal monocytes and alleviates inflammatory colitis in mice. J. Clin. Invest. 135, e174910 (2025).
Culp, E. J. & Goodman, A. L. Cross-feeding in the gut microbiome: ecology and mechanisms. Cell Host Microbe 31, 485–499 (2023).
Wang, S. et al. Microbial collaborations and conflicts: unraveling interactions in the gut ecosystem. Gut Microbes 16, 2296603 (2023).
Ruff, W. E., Greiling, T. M. & Kriegel, M. A. Host–microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 18, 521–538 (2020).
Callahan, M. K. et al. Evaluation of serum IL-17 levels during ipilimumab therapy: correlation with colitis [abstract]. J. Clin. Oncol. 29, 2505 (2011).
Regen, T. et al. IL-17 controls central nervous system autoimmunity through the intestinal microbiome. Sci. Immunol. 6, eaaz6563 (2021).
Castillo-dela Cruz, P. et al. Intestinal IL-17R signaling constrains IL-18-driven liver inflammation by the regulation of microbiome-derived products. Cell Rep. 29, 2270–2283.e7 (2019).
Chandra, V. et al. Gut epithelial Interleukin-17 receptor A signaling can modulate distant tumors growth through microbial regulation. Cancer Cell 42, 85–100.e6 (2024).
Maharshak, N. et al. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes 4, 316–324 (2014).
Huang, J. et al. IL-10 deficiency accelerates type 1 diabetes development via modulation of innate and adaptive immune cells and gut microbiota in BDC2.5 NOD mice. Front. Immunol. 12, 702955 (2021).
Nuñez, N. G. et al. Immune signatures predict development of autoimmune toxicity in patients with cancer treated with immune checkpoint inhibitors. Med 4, 113–129.e7 (2023).
Smits, L. P., Bouter, K. E., de Vos, W. M., Borody, T. J. & Nieuwdorp, M. Therapeutic potential of fecal microbiota transplantation. Gastroenterology 145, 946–953 (2013).
Vaughn, B. P. et al. Effectiveness and safety of colonic and capsule fecal microbiota transplantation for recurrent clostridioides difficile infection. Clin. Gastroenterol. Hepatol. 21, 1330–1337 (2023).
Erez, N., Baruch, I. Y., Ben-Betzalel, G. & Ortenberg, R. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
Elkrief, A. et al. Immune-related colitis is associated with fecal microbial dysbiosis and can be mitigated by fecal microbiota transplantation. Cancer Immunol. Res. 12, 308–321 (2024).
Fasanello, M. K., Robillard, K. T., Boland, P. M., Bain, A. J. & Kanehira, K. Use of fecal microbial transplantation for immune checkpoint inhibitor colitis. ACG Case Rep. J. 7, e00360 (2020).
Halsey, T. M. et al. Microbiome alteration via fecal microbiota transplantation is effective for refractory immune checkpoint inhibitor-induced colitis. Sci. Transl. Med. 15, eabq4006 (2023).
DeFilipp, Z. et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 381, 2043–2050 (2019).
Gulati, A. S., Nicholson, M. R., Khoruts, A. & Kahn, S. A. Fecal microbiota transplantation across the lifespan: balancing efficacy, safety, and innovation. Am. J. Gastroenterol. 118, 435–439 (2023).
Li, Z. et al. Critical role of the gut microbiota in immune responses and cancer immunotherapy. J. Hematol. Oncol. 17, 33 (2024).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT05832606 (2025).
US National Library of Medicine. ClinicalTrials.gov clinicaltrials.gov/study/NCT06438588 (2024).
Cortellino, S. et al. Fasting renders immunotherapy effective against low-immunogenic breast cancer while reducing side effects. Cell Rep. 40, 111256 (2022).
Rangan, P. et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 26, 2704–2719.e6 (2019).
Wu, J. et al. Intermittent fasting alleviates risk markers in a murine model of ulcerative colitis by modulating the gut microbiome and metabolome. Nutrients 14, 5311 (2022).
Falade, A. S. et al. Case report: fulminant celiac disease with combination immune checkpoint therapy. Front. Immunol. 13, 871452 (2022).
Alsaadi, D., Shah, N. J., Charabaty, A. & Atkins, M. B. A case of checkpoint inhibitor-induced celiac disease. J. Immunother. Cancer 7, 203 (2019).
Martinez, K. B., Leone, V. & Chang, E. B. Western diets, gut dysbiosis, and metabolic diseases: are they linked? Gut Microbes 8, 130–142 (2017).
Kaźmierczak-Siedlecka, K. et al. Next-generation probiotics – do they open new therapeutic strategies for cancer patients? Gut Microbes 14, 2035659 (2022).
Ting, N. L.-N., Lau, H. C.-H. & Yu, J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut 71, 1412–1425 (2022).
Yu, Y., Dunaway, S., Champer, J., Kim, J. & Alikhan, A. Changing our microbiome: probiotics in dermatology. Br. J. Dermatol. 182, 39–46 (2020).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
Tan, H. et al. Preliminary safety assessment of a new Bacteroides fragilis isolate. Food Chem. Toxicol. 135, 110934 (2020).
Khan, M. T. et al. Synergy and oxygen adaptation for development of next-generation probiotics. Nature 620, 381–385 (2023).
Tanoue, T. et al. A defined commensal consortium elicits CD8 T cells and anti-cancer immunity. Nature 565, 600–605 (2019).
Merenstein, D. et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 15, 2185034 (2023).
Holscher, H. D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8, 172–184 (2017).
Yang, Y. et al. Starch from Pueraria lobata and the amylose fraction alleviates dextran sodium sulfate induced colitis in mice. Carbohydr. Polym. 302, 120329 (2023).
Chang, A. E. et al. Targeting the gut microbiome to mitigate immunotherapy-induced colitis in cancer. Trends Cancer 7, 583–593 (2021).
Messaoudene, M. et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087 (2022).
Salminen, S. et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667 (2021).
Cuevas-González, P. F., Liceaga, A. M. & Aguilar-Toalá, J. E. Postbiotics and paraprobiotics: from concepts to applications. Food Res. Int. 136, 109502 (2020).
Mehta, J. P., Ayakar, S. & Singhal, R. S. The potential of paraprobiotics and postbiotics to modulate the immune system: a review. Microbiol. Res. 275, 127449 (2023).
Kim, Y. et al. Fecal microbiota transplantation improves anti-PD-1 inhibitor efficacy in unresectable or metastatic solid cancers refractory to anti-PD-1 inhibitor. Cell Host Microbe 32, 1380–1393 (2024).
Montalban-Arques, A. et al. Commensal Clostridiales strains mediate effective anti-cancer immune response against solid tumors. Cell Host Microbe 29, 1573–1588 (2021).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862.e26 (2023).
Si, W. et al. Lactobacillus rhamnosus GG induces cGAS/STING-dependent type I interferon and improves response to immune checkpoint blockade. Gut 71, 521–533 (2022).
Shaikh, F. Y. et al. Fecal microbiome composition correlates with pathologic complete response in patients with operable esophageal cancer treated with combined chemoradiotherapy and immunotherapy. Cancers 16, 3644 (2024).
Jin, Y. et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J. Thorac. Oncol. 14, 1378–1389 (2019).
Huang, J. et al. Ginseng polysaccharides alter the gut microbiota and kynurenine/tryptophan ratio, potentiating the antitumour effect of antiprogrammed cell death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1) immunotherapy. Gut 71, 734–745 (2022).
Ghosh, S., Whitley, C. S., Haribabu, B. & Jala, V. R. Regulation of intestinal barrier function by microbial metabolites. Cell Mol. Gastroenterol. Hepatol. 11, 1463–1482 (2021).
Johannet, P. et al. Baseline serum autoantibody signatures predict recurrence and toxicity in melanoma patients receiving adjuvant immune checkpoint blockade. Clin. Cancer Res. 28, 4121–4130 (2022).
Kobayashi, T. et al. Anti-pituitary antibodies and susceptible human leukocyte antigen alleles as predictive biomarkers for pituitary dysfunction induced by immune checkpoint inhibitors. J. Immunother. Cancer 9, e002493 (2021).
Tahir, S. A. et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc. Natl Acad. Sci. USA 116, 22246–22251 (2019).
Oh, D. Y. et al. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res. 77, 1322–1330 (2017).
Goswami, S. et al. A composite T cell biomarker in pre-treatment blood samples correlates with detection of immune-related adverse events. Cancer Cell 40, 249–251 (2022).
Akturk, H. K. et al. Analysis of human leukocyte antigen DR alleles, immune-related adverse events, and survival associated with immune checkpoint inhibitor use among patients with advanced malignant melanoma. JAMA Netw. Open. 5, e2246400 (2022).
Chang, H. et al. HLA-B27 association of autoimmune encephalitis induced by PD-L1 inhibitor. Ann. Clin. Transl. Neurol. 7, 2243–2250 (2020).
Groha, S. et al. Germline variants associated with toxicity to immune checkpoint blockade. Nat. Med. 28, 2584–2591 (2022).
Sasson, S. C. et al. Interferon-gamma-producing CD8+ tissue resident memory T cells are a targetable hallmark of immune checkpoint inhibitor-colitis. Gastroenterology 161, 1229–1244.e9 (2021).
Reschke, R. et al. Checkpoint blockade-induced dermatitis and colitis are dominated by tissue-resident memory T cells and Th1/Tc1 cytokines. Cancer Immunol. Res. 10, 1167–1174 (2022).
Ju, M. et al. Prophylactic IL-23 blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. J. Immunother. Cancer 12, e009345 (2024).
Tarhini, A. A. et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J. Immunother. Cancer 3, 39 (2015).
Acknowledgements
The work of the authors is supported by grants from the National Key R&D Program of China (2020YFA0509200 to J.-Y.F.), the National Natural Science Foundation of China (82250005 and 82330086 to J.-Y.F.) and the Shanghai Municipal Hospital Gastroenterology clinical competence improvement and advancement specialist alliance (SHDC22024302 to J.-Y.F.).
Author information
Authors and Affiliations
Contributions
Y.-.Q.G. researched data for the article, Y.-.Q.G. and Y.-J.T. wrote the article, and J.-Y.F. contributed substantially to discussion of the content and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks M. Fidelle, J. Yu and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, YQ., Tan, YJ. & Fang, JY. Roles of the gut microbiota in immune-related adverse events: mechanisms and therapeutic intervention. Nat Rev Clin Oncol 22, 499–516 (2025). https://doi.org/10.1038/s41571-025-01026-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01026-w
This article is cited by
-
Establishment of a microbial abundance prognostic model for colorectal cancer
BMC Cancer (2026)
-
Harnessing the microbiome for cancer therapy
Nature Reviews Microbiology (2026)
-
Biomaterials mediated 3R (remove-remodel-repair) strategy: holistic management of Helicobacter pylori infection
Journal of Nanobiotechnology (2025)
-
The impact of concomitant medications on treatment outcomes in patients with cancer receiving immune checkpoint inhibitors
Nature Reviews Cancer (2025)
-
The role of metabolites in the causal effect of immune cell phenotypes on immunotherapy toxicity risk
Discover Oncology (2025)