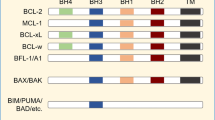
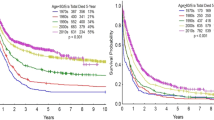
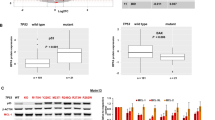
Abstract
Acute myeloid leukaemia (AML) remains a challenging haematological malignancy, with most patients developing resistance to standard-of-care (SOC) treatments. This resistance is often attributed to the overexpression of anti-apoptotic BCL-2 family proteins, which regulate the intrinsic apoptotic pathway by inhibiting pro-apoptotic effector proteins such as BAX and BAK. AML cells exploit this imbalance to evade apoptosis and sustain survival, necessitating the development of novel therapeutic strategies. BH3 mimetics are small-molecule inhibitors targeting the pro-survival BCL-2 family proteins and have emerged as promising agents in patients with AML who are unable to receive high-intensity induction chemotherapy. Co-treatment with the BCL-2-specific inhibitor venetoclax and various SOC therapies has been proven effective, with several combinations now approved by the US Food and Drug Administration for adults with AML who are ≥75 years of age and/or are ineligible for intensive induction chemotherapy, on the basis of improved response rates and survival outcomes compared with the previous SOC. In this Review, we highlight the transformative potential of BH3 mimetics in AML therapy, including ongoing studies investigating novel combination regimens and efforts to further refine treatment strategies, with the ultimate goal of improving outcomes for patients with AML.
Key points
-
Resistance to standard treatments for acute myeloid leukaemia (AML) is often attributed to the overexpression of anti-apoptotic BCL-2 family proteins, which inhibit apoptosis and sustain cancer cell survival, posing a major therapeutic challenge.
-
BH3 mimetics, which target pro-survival BCL-2 proteins, have demonstrated substantial anticancer activity, effectively overcoming resistance in patients with AML and improving therapeutic outcomes, especially when combined with hypomethylating agents or low-dose cytarabine.
-
Venetoclax, a potent BCL-2-specific BH3 mimetic, has revolutionized the management of patients with AML owing to improved efficacy when combined with standard therapies, leading to US Food and Drug Administration approval of this agent for specific groups of patients with AML.
-
Innovative sequential or combinatorial approaches targeting BCL-xL and MCL-1 dependencies will be crucial to addressing the current challenges, including thrombocytopenia and the limited ability to target resistant BCL-2 family proteins.
-
Techniques such as BH3 profiling, mitochondrial profiling and gene and/or protein expression profiling are transforming the management of patients with AML by enabling precise, personalized therapies and enhancing the optimization of therapeutic regimens, leading to improved patient outcomes.
-
The future treatment of patients with AML lies in developing novel drug combinations, optimizing BH3 mimetic dosages, mitigating adverse effects and exploring dual-action therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
11 September 2025
In the version of the article initially published, the initials “S.D.B., P.D., M.K.” were inadvertently omitted from the Author contributions section and have now been added. Additionally, the initials “C.D.N.” have now been amended to “C.D.D.”. These corrections have been made to the HTML and PDF versions of the article.
References
Melino, G. The Sirens’ song. Nature 412, 23 (2001).
Kroemer, G. et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 (2009).
Cotter, T. G. Apoptosis and cancer: the genesis of a research field. Nat. Rev. Cancer 9, 501–507 (2009).
Vitale, I. et al. Apoptotic cell death in disease-current understanding of the NCCD 2023. Cell Death Differ. 30, 1097–1154 (2023).
Kayagaki, N., Webster, J. D. & Newton, K. Control of cell death in health and disease. Annu. Rev. Pathol. 19, 157–180 (2024).
Newton, K., Strasser, A., Kayagaki, N. & Dixit, V. M. Cell death. Cell 187, 235–256 (2024).
Gregory, C. D. Hijacking homeostasis: regulation of the tumor microenvironment by apoptosis. Immunol. Rev. 319, 100–127 (2023).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Plati, J., Bucur, O. & Khosravi-Far, R. Apoptotic cell signaling in cancer progression and therapy. Integr. Biol. 3, 279–296 (2011).
Xu, G. & Shi, Y. Apoptosis signaling pathways and lymphocyte homeostasis. Cell Res. 17, 759–771 (2007).
Mustafa, M. et al. Apoptosis: a comprehensive overview of signaling pathways, morphological changes, and physiological significance and therapeutic implications. Cells 13, 1838 (2024).
Jin, Z. & El-Deiry, W. S. Overview of cell death signaling pathways. Cancer Biol. Ther. 4, 139–163 (2005).
Tian, X. et al. Targeting apoptotic pathways for cancer therapy. J. Clin. Invest. 134, e179570 (2024).
Wani, A. K. et al. Targeting apoptotic pathway of cancer cells with phytochemicals and plant-based nanomaterials. Biomolecules 13, 194 (2023).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Mohammad, R. M. et al. Broad targeting of resistance to apoptosis in cancer. Semin. Cancer Biol. 35, S78–S103 (2015).
Kaloni, D., Diepstraten, S. T., Strasser, A. & Kelly, G. L. BCL-2 protein family: attractive targets for cancer therapy. Apoptosis 28, 20–38 (2023).
US Food and Drug Administration. FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality. fda.gov https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-chronic-lymphocytic-leukemia-patients-specific-chromosomal-abnormality (2016).
US Food and Drug Administration. FDA approves venetoclax for CLL and SLL. fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-and-sll (2019).
US Food and Drug Administration. FDA grants regular approval to venetoclax in combination for untreated acute myeloid leukemia. fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-venetoclax-combination-untreated-acute-myeloid-leukemia (2020).
Warren, C. F. A., Wong-Brown, M. W. & Bowden, N. A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 10, 177 (2019).
Qian, S. et al. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 12, 985363 (2022).
Klener, P., Sovilj, D., Renesova, N. & Andera, L. BH3 mimetics in hematologic malignancies. Int. J. Mol. Sci. 22, 10157 (2021).
Gajkowska, B., Motyl, T., Olszewska-Badarczuk, H. & Godlewski, M. M. Expression of BAX in cell nucleus after experimentally induced apoptosis revealed by immunogold and embedment-free electron microscopy. Cell Biol. Int. 25, 725–733 (2001).
Nutt, L. K. et al. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J. Biol. Chem. 277, 20301–20308 (2002).
Roufayel, R., Younes, K., Al-Sabi, A. & Murshid, N. BH3-only proteins Noxa and Puma are key regulators of induced apoptosis. Life 12, 256 (2022).
Chen, M. et al. Eltrombopag directly activates BAK and induces apoptosis. Cell Death Dis. 14, 394 (2023).
Gonzalo, Ó et al. Study of the Bcl-2 interactome by BiFC reveals differences in the activation mechanism of Bax and Bak. Cells 12, 800 (2023).
O’Neill, K. L., Huang, K., Zhang, J., Chen, Y. & Luo, X. Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes. Dev. 30, 973–988 (2016).
Shimony, S., Stahl, M. & Stone, R. M. Acute myeloid leukemia: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 98, 502–526 (2023).
Wang, X. et al. The latest edition of WHO and ELN guidance and a new risk model for Chinese acute myeloid leukemia patients. Front. Med. 10, 1165445 (2023).
Huber, S. et al. AML classification in the year 2023: how to avoid a Babylonian confusion of languages. Leukemia 37, 1413–1420 (2023).
Jung, J. et al. Perspectives on acute myeloid leukemia diagnosis: a comparative analysis of the latest World Health Organization and the International Consensus Classifications. Leukemia 37, 2125–2128 (2023).
Salman, H. Comparative analysis of AML classification systems: evaluating the WHO, ICC, and ELN frameworks and their distinctions. Cancers 16, 2915 (2024).
Arber, D. A. et al. International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood 140, 1200–1228 (2022).
Dohner, H. et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 140, 1345–1377 (2022).
Li, Q. et al. FISH improves risk stratification in acute leukemia by identifying KMT2A abnormal copy number and rearrangements. Sci. Rep. 12, 9585 (2022).
Rausch, C. et al. Validation and refinement of the 2022 European LeukemiaNet genetic risk stratification of acute myeloid leukemia. Leukemia 37, 1234–1244 (2023).
Matos, S. et al. Screening a targeted panel of genes by next-generation sequencing improves risk stratification in real world patients with acute myeloid leukemia. Cancers 14, 3236 (2022).
Krizsán, S. et al. Next-generation sequencing-based genomic profiling of children with acute myeloid leukemia. J. Mol. Diagn. 25, 555–568 (2023).
Cassier, P. A., Castets, M., Belhabri, A. & Vey, N. Targeting apoptosis in acute myeloid leukaemia. Br. J. Cancer 117, 1089–1098 (2017).
Rücker, F. G. et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood 119, 2114–2121 (2012).
Qin, G. & Han, X. The prognostic value of TP53 mutations in adult acute myeloid leukemia: a meta-analysis. Transfus. Med. Hemother. 50, 234–244 (2023).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221 (2016).
Mehta, S. V., Shukla, S. N. & Vora, H. H. Overexpression of Bcl2 protein predicts chemoresistance in acute myeloid leukemia: its correlation with FLT3. Neoplasma 60, 666–675 (2013).
Andreeff, M. et al. Expression of Bcl-2-related genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia 13, 1881–1892 (1999).
Glaser, S. P. et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes. Dev. 26, 120–125 (2012).
Lachowiez, C. A. et al. Comparison and validation of the 2022 European LeukemiaNet guidelines in acute myeloid leukemia. Blood Adv. 7, 1899–1909 (2023).
Karakas, T. et al. High expression of bcl-2 mRNA as a determinant of poor prognosis in acute myeloid leukemia. Ann. Oncol. 9, 159–165 (1998).
Letai, A., Sorcinelli, M. D., Beard, C. & Korsmeyer, S. J. Antiapoptotic BCL-2 is required for maintenance of a model leukemia. Cancer Cell 6, 241–249 (2004).
Gorombei, P. et al. BCL-2 inhibitor ABT-737 effectively targets leukemia-initiating cells with differential regulation of relevant genes leading to extended survival in a NRAS/BCL-2 mouse model of high risk-myelodysplastic syndrome. Int. J. Mol. Sci. 22, 10658 (2021).
Moon, J. H. et al. BCL2 gene polymorphism could predict the treatment outcomes in acute myeloid leukemia patients. Leuk. Res. 34, 166–172 (2010).
Saygin, C. & Carraway, H. E. Emerging therapies for acute myeloid leukemia. J. Hematol. Oncol. 10, 93 (2017).
Tiribelli, M. et al. BCL-2 expression in AML patients over 65 years: impact on outcomes across different therapeutic strategies. J. Clin. Med. 10, 5096 (2021).
Lauria, F. et al. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia 11, 2075–2078 (1997).
Zhou, J. D. et al. BCL2 overexpression: clinical implication and biological insights in acute myeloid leukemia. Diagn. Pathol. 14, 68 (2019).
Lee, C. et al. Transcriptional signatures of the BCL2 family for individualized acute myeloid leukaemia treatment. Genome Med. 14, 111 (2022).
Konopleva, M. et al. The anti-apoptotic genes Bcl-X(L) and Bcl-2 are over-expressed and contribute to chemoresistance of non-proliferating leukaemic CD34+ cells. Br. J. Haematol. 118, 521–534 (2002).
Wei, Y. et al. Targeting Bcl-2 proteins in acute myeloid leukemia. Front. Oncol. 10, 584974 (2020).
Carter, B. Z. et al. Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica 107, 58–76 (2022).
Qiu, Y. et al. The GSK3β/Mcl-1 axis is regulated by both FLT3-ITD and Axl and determines the apoptosis induction abilities of FLT3-ITD inhibitors. Cell Death Discov. 9, 44 (2023).
Wei, A. H. et al. Targeting MCL-1 in hematologic malignancies: rationale and progress. Blood Rev. 44, 100672 (2020).
Chin, H. S. & Fu, N. Y. Physiological functions of Mcl-1: insights from genetic mouse models. Front. Cell Dev. Biol. 9, 704547 (2021).
Pei, S. et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 10, 536–551 (2020).
Diepstraten, S. T. et al. Putting the STING back into BH3-mimetic drugs for TP53-mutant blood cancers. Cancer Cell 42, 850–868 (2024).
Ferrarini, I., Rigo, A. & Visco, C. The mitochondrial anti-apoptotic dependencies of hematologic malignancies: from disease biology to advances in precision medicine. Haematologica 107, 790–802 (2022).
Oltersdorf, T. et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature 435, 677–681 (2005).
Tse, C. et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 68, 3421–3428 (2008).
Roberts, A. W. et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J. Clin. Oncol. 30, 488–496 (2012).
Schoenwaelder, S. M. et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood 118, 1663–1674 (2011).
Souers, A. J. et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 19, 202–208 (2013).
Coutre, S. et al. Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood 131, 1704–1711 (2018).
US Food and Drug Administration. FDA approves venetoclax for CLL or SLL, with or without 17 P deletion, after one prior therapy fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-venetoclax-cll-or-sll-or-without-17-p-deletion-after-one-prior-therapy (2018).
Garciaz, S., Hospital, M. A., Collette, Y. & Vey, N. Venetoclax resistance in acute myeloid leukemia. Cancers 16, 1091 (2024).
Wei, A. H. & Roberts, A. W. BCL2 inhibition: a new paradigm for the treatment of AML and beyond. Hemasphere 7, e912 (2023).
Lessene, G., Czabotar, P. E. & Colman, P. M. BCL-2 family antagonists for cancer therapy. Nat. Rev. Drug. Discov. 7, 989–1000 (2008).
Liu, J., Chen, Y., Yu, L. & Yang, L. Mechanisms of venetoclax resistance and solutions. Front. Oncol. 12, 1005659 (2022).
Vazquez, R. et al. Venetoclax combination therapy induces deep AML remission with eradication of leukemic stem cells and remodeling of clonal haematopoiesis. Blood Cancer J. 11, 62 (2021).
Esparza, S. et al. Venetoclax-induced tumour lysis syndrome in acute myeloid leukaemia. Br. J. Haematol. 188, 173–177 (2020).
Thijssen, R. et al. Intact TP-53 function is essential for sustaining durable responses to BH3-mimetic drugs in leukemias. Blood 137, 2721–2735 (2021).
Kim, K. et al. Outcomes of TP53-mutant acute myeloid leukemia with decitabine and venetoclax. Cancer 127, 3772–3781 (2021).
Lagadinou, E. D. et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell 12, 329–341 (2013).
Liu, F. et al. Cotargeting of mitochondrial complex I and Bcl-2 shows antileukemic activity against acute myeloid leukemia cells reliant on oxidative phosphorylation. Cancers 12, 2400 (2020).
Roca-Portoles, A. et al. Venetoclax causes metabolic reprogramming independent of BCL-2 inhibition. Cell Death Dis. 11, 616 (2020).
Lee, J. B. et al. Venetoclax enhances T cell-mediated antileukemic activity by increasing ROS production. Blood 138, 234–245 (2021).
Sena, L. A. et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38, 225–236 (2013).
Gong, X. et al. Venetoclax-based therapy for relapsed or refractory acute myeloid leukemia: latest updates from the 2023 ASH annual meeting. Exp. Hematol. Oncol. 13, 17 (2024).
Cerella, C., Dicato, M. & Diederich, M. BH3 mimetics in AML therapy: death and beyond? Trends Pharmacol. Sci. 41, 793–814 (2020).
Moujalled, D. M. et al. Acquired mutations in BAX confer resistance to BH3-mimetic therapy in acute myeloid leukemia. Blood 141, 634–644 (2023).
Glytsou, C. et al. Mitophagy promotes resistance to BH3 mimetics in acute myeloid leukemia. Cancer Discov. 13, 1656–1677 (2023).
Eldeeb, M. et al. A fetal tumor suppressor axis abrogates MLL-fusion-driven acute myeloid leukemia. Cell Rep. 42, 112099 (2023).
Parry, N., Wheadon, H. & Copland, M. The application of BH3 mimetics in myeloid leukemias. Cell Death Dis. 12, 222 (2021).
Chan, S. M. et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat. Med. 21, 178–184 (2015).
Konopleva, M. et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 6, 1106–1117 (2016).
Othman, J. et al. Molecular MRD is strongly prognostic in patients with NPM1-mutated AML receiving venetoclax-based nonintensive therapy. Blood 143, 336–341 (2024).
Griffioen, M. S., de Leeuw, D. C., Janssen, J. J. W. M. & Smit, L. Targeting acute myeloid leukemia with venetoclax; biomarkers for sensitivity and rationale for venetoclax-based combination therapies. Cancers 14, 3456 (2022).
Garciaz, S., Saillard, C., Hicheri, Y., Hospital, M. A. & Vey, N. Venetoclax in acute myeloid leukemia: molecular basis, evidences for preclinical and clinical efficacy and strategies to target resistance. Cancers 13, 5608 (2021).
Ong, F., Kim, K. & Konopleva, M. Y. Venetoclax resistance: mechanistic insights and future strategies. Cancer Drug. Resist. 5, 380–400 (2022).
Roberts, A. W., Wei, A. H. & Huang, D. C. S. BCL2 and MCL1 inhibitors for hematologic malignancies. Blood 138, 1120–1136 (2021).
Wang, Z. et al. Efficacy of a novel BCL-xL degrader, DT2216, in preclinical models of JAK2-mutated post-MPN AML. Blood https://doi.org/10.1182/blood.2024027117 (2025).
Pratz, K. W. et al. Long-term follow-up of VIALE-A: venetoclax and azacitidine in chemotherapy-ineligible untreated acute myeloid leukemia. Am. J. Hematol. 99, 615–624 (2024).
Chen, X. et al. Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov. 9, 890–909 (2019).
Lin, K. H. et al. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci. Rep. 6, 27696 (2016).
Zhang, Q. et al. Activation of RAS/MAPK pathway confers MCL-1 mediated acquired resistance to BCL-2 inhibitor venetoclax in acute myeloid leukemia. Signal. Transduct. Target. Ther. 7, 51 (2022).
Ramsey, H. E. et al. A novel MCL1 inhibitor combined with venetoclax rescues venetoclax-resistant acute myelogenous leukemia. Cancer Discov. 8, 1566–1581 (2018).
Wang, H., Guo, M., Wei, H. & Chen, Y. Targeting MCL-1 in cancer: current status and perspectives. J. Hematol. Oncol. 14, 67 (2021).
Desai, P. et al. A phase 1 first-in-human study of the MCL-1 inhibitor AZD5991 in patients with relapsed/refractory hematologic malignancies. Clin. Cancer Res. 30, 4844–4855 (2024).
Zhang, H. et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat. Cancer 1, 826–839 (2020).
Bisaillon, R. et al. Genetic characterization of ABT-199 sensitivity in human AML. Leukemia 34, 63–74 (2020).
Hanley, J. A. & McNeil, B. J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 143, 29–36 (1982).
Samra, B., Konopleva, M., Isidori, A., Daver, N. & DiNardo, C. Venetoclax-based combinations in acute myeloid leukemia: current evidence and future directions. Front. Oncol. 10, 562558 (2020).
Nakao, M. et al. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 10, 1911–1918 (1996).
Garciaz, S. & Hospital, M. A. FMS-like tyrosine kinase 3 inhibitors in the treatment of acute myeloid leukemia: an update on the emerging evidence and safety profile. Onco Targets Ther. 16, 31–45 (2023).
Stuani, L. et al. Mitochondrial metabolism supports resistance to IDH mutant inhibitors in acute myeloid leukemia. J. Exp. Med. 218, e20200924 (2021).
Zhu, R. et al. FLT3 tyrosine kinase inhibitors synergize with BCL-2 inhibition to eliminate FLT3/ITD acute leukemia cells through BIM activation. Signal. Transduct. Target. Ther. 6, 186 (2021).
Singh Mali, R. et al. Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models. Haematologica 106, 1034–1046 (2021).
Ma, J. et al. Inhibition of Bcl-2 synergistically enhances the antileukemic activity of midostaurin and gilteritinib in preclinical models of FLT3-mutated acute myeloid leukemia. Clin. Cancer Res. 25, 6815–6826 (2019).
Short, N. J. et al. Advances in the treatment of acute myeloid leukemia: new drugs and new challenges. Cancer Discov. 10, 506–525 (2020).
Chyla, B. et al. Genetic biomarkers of sensitivity and resistance to venetoclax monotherapy in patients with relapsed acute myeloid leukemia. Am. J. Hematol. 93, E202–E205 (2018).
Daver, N. A.-O. X. et al. Venetoclax plus gilteritinib for FLT3-mutated relapsed/refractory acute myeloid leukemia. J Clin Oncol (2022).
Short, N. J. et al. Azacitidine, venetoclax, and gilteritinib in newly diagnosed and relapsed or refractory FLT3-mutated AML. J. Clin. Oncol. 42, 1499–1508 (2024).
Nechiporuk, T. et al. The TP53 apoptotic network is a primary mediator of resistance to BCL2 inhibition in AML cells. Cancer Discov. 9, 910–925 (2019).
DiNardo, C. D. et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 135, 791–803 (2020).
Fischer, M. Census and evaluation of p53 target genes. Oncogene 36, 3943–3956 (2017).
Aubrey, B. J., Kelly, G. L., Janic, A., Herold, M. J. & Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 25, 104–113 (2018).
DiNardo, C. D. et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 19, 216–228 (2018).
Shi, X. et al. Nuclear NAD+ homeostasis governed by NMNAT1 prevents apoptosis of acute myeloid leukemia stem cells. Sci. Adv. 7, eabf3895 (2021).
Stevens, B. M. et al. Fatty acid metabolism underlies venetoclax resistance in acute myeloid leukemia stem cells. Nat. Cancer 1, 1176–1187 (2020).
Sharon, D. et al. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci. Transl. Med. 11, eaax2863 (2019).
Thevarajan, I., Zolkiewski, M. & Zolkiewska, A. Human CLPB forms ATP-dependent complexes in the mitochondrial intermembrane space. Int. J. Biochem. Cell Biol. 127, 105841 (2020).
Cavdar Koc, E. et al. A new face on apoptosis: death-associated protein 3 and PDCD9 are mitochondrial ribosomal proteins. FEBS Lett. 492, 166–170 (2001).
Wang, F., Zhang, D., Li, P. & Gao, Y. Mitochondrial protein translation: emerging roles and clinical significance in disease. Front. Cell Dev. Biol. 9, 675465 (2021).
Xu, Y. & Ye, H. Progress in understanding the mechanisms of resistance to BCL-2 inhibitors. Exp. Hematol. Oncol. 11, 31 (2022).
Kuusanmäki, H. et al. Phenotype-based drug screening reveals association between venetoclax response and differentiation stage in acute myeloid leukemia. Haematologica 105, 708–720 (2020).
Lachowiez, C. A. & DiNardo, C. D. Mutation- and MRD-informed treatments for transplant-ineligible patients. Hematol. Am. Soc. Hematol. Educ. Program 2024, 168–177 (2024).
Kadia, T. M. et al. Phase II study of venetoclax added to cladribine plus low-dose cytarabine alternating with 5-azacitidine in older patients with newly diagnosed acute myeloid leukemia. J. Clin. Oncol. 40, 3848–3857 (2022).
Rivera, D. et al. Implications of RAS mutational status in subsets of patients with newly diagnosed acute myeloid leukemia across therapy subtypes. Am. J. Hematol. 97, 1599–1606 (2022).
Bhatt, S. et al. Reduced mitochondrial apoptotic priming drives resistance to BH3 mimetics in acute myeloid leukemia. Cancer Cell 38, 872–890.e876 (2020).
Zhang, X. et al. Not BCL2 mutation but dominant mutation conversation contributed to acquired venetoclax resistance in acute myeloid leukemia. Biomark. Res. 9, 30 (2021).
Thomalla, D. et al. Deregulation and epigenetic modification of BCL2-family genes cause resistance to venetoclax in hematologic malignancies. Blood 140, 2113–2126 (2022).
Guirguis, A. A. et al. RNA methylation: where to from here for hematologic malignancies? Exp. Hematol. 143, 104694 (2024).
Calderon, A., Han, C., Karma, S. & Wang, E. Non-genetic mechanisms of drug resistance in acute leukemias. Trends Cancer 10, 38–51 (2024).
Kufe, D. W., Munroe, D., Herrick, D., Egan, E. & Spriggs, D. Effects of 1-beta-D-arabinofuranosylcytosine incorporation on eukaryotic DNA template function. Mol. Pharmacol. 26, 128–134 (1984).
Hiddemann, W. Cytosine arabinoside in the treatment of acute myeloid leukemia: the role and place of high-dose regimens. Ann. Hematol. 62, 119–128 (1991).
Wang, X. et al. Chemotherapy-induced differential cell cycle arrest in B-cell lymphomas affects their sensitivity to Wee1 inhibition. Haematologica 103, 466–476 (2018).
Niu, X. et al. Binding of released bim to Mcl-1 is a mechanism of intrinsic resistance to ABT-199 which can be overcome by combination with daunorubicin or cytarabine in AML cells. Clin. Cancer Res. 22, 4440–4451 (2016).
Xie, C. et al. Obatoclax potentiates the cytotoxic effect of cytarabine on acute myeloid leukemia cells by enhancing DNA damage. Mol. Oncol. 9, 409–421 (2015).
Wei, A. H. et al. Venetoclax combined with low-dose cytarabine for previously untreated patients with acute myeloid leukemia: results from a phase Ib/II study. J. Clin. Oncol. 37, 1277–1284 (2019).
Wei, A. H. et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood 135, 2137–2145 (2020).
Gozzo, L. et al. Off-label use of venetoclax in patients with acute myeloid leukemia: single center experience and data from pharmacovigilance database. Front. Pharmacol. 12, 748766 (2021).
Wei, A. H. et al. Long-term follow-up of VIALE-C in patients with untreated AML ineligible for intensive chemotherapy. Blood 140, 2754–2756 (2022).
Alsouqi, A., Geramita, E. & Im, A. Treatment of acute myeloid leukemia in older adults. Cancers https://doi.org/10.3390/cancers15225409 (2023).
Baylin, S. B. & Jones, P. A. A decade of exploring the cancer epigenome - biological and translational implications. Nat. Rev. Cancer 11, 726–734 (2011).
Robertson, K. D. DNA methylation and human disease. Nat. Rev. Genet. 6, 597–610 (2005).
Stomper, J., Rotondo, J. C., Greve, G. & Lübbert, M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: mechanisms of resistance and novel HMA-based therapies. Leukemia 35, 1873–1889 (2021).
Tsao, T. et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann. Hematol. 91, 1861–1870 (2012).
Bogenberger, J. M. et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia 28, 1657–1665 (2014).
Bogenberger, J. M. et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk. Lymphoma 56, 226–229 (2015).
DiNardo, C. D. et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 133, 7–17 (2019).
Male, H. J. & Lin, T. L. The approach of HMA plus VEN with or without BMT for all patients with AML. Hematol. Am. Soc. Hematol. Educ. Program 2023, 186–191 (2023).
DiNardo, C. D. et al. Azacitidine and Venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 383, 617–629 (2020).
Ucciero, A. et al. Venetoclax with hypomethylating agents in newly diagnosed acute myeloid leukemia: a systematic review and meta-analysis of survival data from real-world studies. Cancers 15, 4618 (2023).
Bataller, A. et al. Prognostic risk signature in patients with acute myeloid leukemia treated with hypomethylating agents and venetoclax. Blood Adv. 8, 927–935 (2024).
Dohner, H. et al. Genetic risk classification for adults with AML receiving less-intensive therapies: the 2024 ELN recommendations. Blood 144, 2169–2173 (2024).
Wan, Y., Dai, N., Tang, Z. & Fang, H. Small-molecule Mcl-1 inhibitors: emerging anti-tumor agents. Eur. J. Med. Chem. 146, 471–482 (2018).
Hormi, M. et al. Pairing MCL-1 inhibition with venetoclax improves therapeutic efficiency of BH3-mimetics in AML. Eur. J. Haematol. 105, 588–596 (2020).
Carter, B. Z. et al. Combined inhibition of BCL-2 and MCL-1 overcomes BAX deficiency-mediated resistance of TP53-mutant acute myeloid leukemia to individual BH3 mimetics. Blood Cancer J. 13, 57 (2023).
Aid, Z. et al. High caspase 3 and vulnerability to dual BCL2 family inhibition define ETO2::GLIS2 pediatric leukemia. Leukemia 37, 571–579 (2023).
Rasmussen, M. L. et al. MCL-1 inhibition by selective BH3 mimetics disrupts mitochondrial dynamics causing loss of viability and functionality of human cardiomyocytes. iScience 23, 101015 (2020).
Bolomsky, A. et al. MCL-1 inhibitors, fast-lane development of a new class of anti-cancer agents. J. Hematol. Oncol. 13, 173 (2020).
Szlávik, Z. et al. Structure-guided discovery of a selective Mcl-1 inhibitor with cellular activity. J. Med. Chem. 62, 6913–6924 (2019).
Yi, X. et al. AMG-176, an Mcl-1 antagonist, shows preclinical efficacy in chronic lymphocytic leukemia. Clin. Cancer Res. 26, 3856–3867 (2020).
Hird, A. W. & Tron, A. E. Recent advances in the development of Mcl-1 inhibitors for cancer therapy. Pharmacol. Ther. 198, 59–67 (2019).
Tron, A. E. et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 9, 5341 (2018).
Yue, X., Chen, Q. & He, J. Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int. 20, 524 (2020).
Pan, R. et al. Synthetic lethality of combined Bcl-2 inhibition and p53 activation in AML: mechanisms and superior antileukemic efficacy. Cancer Cell 32, 748–760 (2017).
Bogenberger, J. et al. Combined venetoclax and alvocidib in acute myeloid leukemia. Oncotarget 8, 107206–107222 (2017).
Knorr, K. L. et al. MLN4924 induces Noxa upregulation in acute myelogenous leukemia and synergizes with Bcl-2 inhibitors. Cell Death Differ. 22, 2133–2142 (2015).
Han, L. et al. Concomitant targeting of BCL2 with venetoclax and MAPK signaling with cobimetinib in acute myeloid leukemia models. Haematologica 105, 697–707 (2020).
Zeidner, J. F. et al. Phase 2a study of SLS009, a highly selective CDK9 inhibitor, in combination with azacitidine and venetoclax for relapsed/refractory acute myeloid leukemia after prior venetoclax treatment. Blood 144, 2877–2877 (2024).
Lee, D. J. & Zeidner, J. F. Cyclin-dependent kinase (CDK) 9 and 4/6 inhibitors in acute myeloid leukemia (AML): a promising therapeutic approach. Expert. Opin. Investig. Drugs 28, 989–1001 (2019).
Zeidner, J. F. & Karp, J. E. Clinical activity of alvocidib (flavopiridol) in acute myeloid leukemia. Leuk. Res. 39, 1312–1318 (2015).
Cidado, J. et al. AZD4573 is a highly selective CDK9 inhibitor that suppresses MCL-1 and induces apoptosis in hematologic cancer cells. Clin. Cancer Res. 26, 922–934 (2020).
Chantkran, W. et al. Interrogation of novel CDK2/9 inhibitor fadraciclib (CYC065) as a potential therapeutic approach for AML. Cell Death Discov. 7, 137 (2021).
Baker, A. et al. The CDK9 inhibitor dinaciclib exerts potent apoptotic and antitumor effects in preclinical models of MLL-rearranged acute myeloid leukemia. Cancer Res. 76, 1158–1169 (2016).
Wang, X. et al. Deletion of MCL-1 causes lethal cardiac failure and mitochondrial dysfunction. Genes. Dev. 27, 1351–1364 (2013).
Thomas, R. L. et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes. Dev. 27, 1365–1377 (2013).
Jonas, B. A. et al. A phase 1b study of venetoclax and alvocidib in patients with relapsed/refractory acute myeloid leukemia. Hematol. Oncol. 41, 743–752 (2023).
Short, N. J. et al. A phase 1/2 study of azacitidine, venetoclax and pevonedistat in newly diagnosed secondary AML and in MDS or CMML after failure of hypomethylating agents. J. Hematol. Oncol. 16, 73 (2023).
Daver, N. et al. Preliminary results from a phase Ib study evaluating BCL-2 inhibitor venetoclax in combination with MEK inhibitor cobimetinib or MDM2 inhibitor idasanutlin in patients with relapsed or refractory (R/R) AML. Blood 130, 813 (2017).
Puglisi, M. et al. A phase I study of the safety, pharmacokinetics and efficacy of navitoclax plus docetaxel in patients with advanced solid tumors. Future Oncol. 17, 2747–27580 (2021).
Nor Hisam, N. S. et al. Combination therapy of navitoclax with chemotherapeutic agents in solid tumors and blood cancer: a review of current evidence. Pharmaceutics 13, 1353 (2021).
Pullarkat, V. A. et al. Venetoclax and navitoclax in combination with chemotherapy in patients with relapsed or refractory acute lymphoblastic leukemia and lymphoblastic lymphoma. Cancer Discov. 11, 1440–1453 (2021).
Marinoff, A. E. et al. Venetoclax in combination with chemotherapy as treatment for pediatric advanced hematologic malignancies. Pediatr. Blood Cancer 70, e30335 (2023).
Stahl, M. et al. Clinical and molecular predictors of response and survival following venetoclax therapy in relapsed/refractory AML. Blood Adv. 5, 1552–1564 (2021).
Haupt, Y., Maya, R., Kazaz, A. & Oren, M. Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299 (1997).
Khurana, A. & Shafer, D. A. MDM2 antagonists as a novel treatment option for acute myeloid leukemia: perspectives on the therapeutic potential of idasanutlin (RG7388). Onco Targets Ther. 12, 2903–2910 (2019).
Wang, H., Guo, M., Wei, H. & Chen, Y. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal. Transduct. Target. Ther. 8, 92 (2023).
Zhang, Q., Bykov, V. J. N., Wiman, K. G. & Zawacka-Pankau, J. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 9, 439 (2018).
Garcia-Manero, G. et al. Eprenetapopt combined with venetoclax and azacitidine in TP53-mutated acute myeloid leukaemia: a phase 1, dose-finding and expansion study. Lancet Haematol. 10, e272–e283 (2023).
Perl, A. E. et al. Venetoclax in combination with gilteritinib in patients with relapsed/refractory acute myeloid leukemia: a phase 1b study. Blood 134, 3910 (2019).
Yilmaz, M. et al. Quizartinib with decitabine and venetoclax (triplet) is highly active in patients with FLT3-ITD mutated acute myeloid leukemia (AML). J. Clin. Oncol. 39, e19019 (2021).
Milnerowicz, S., Maszewska, J., Skowera, P., Stelmach, M. & Lejman, M. AML under the scope: current strategies and treatment involving FLT3 inhibitors and venetoclax-based regimens. Int. J. Mol. Sci. 24, 15849 (2023).
Daver, N., Alotaibi, A. S., Bücklein, V. & Subklewe, M. T-cell-based immunotherapy of acute myeloid leukemia: current concepts and future developments. Leukemia 35, 1843–1863 (2021).
Miari, K. E., Guzman, M. L., Wheadon, H. & Williams, M. T. S. Macrophages in acute myeloid leukaemia: significant players in therapy resistance and patient outcomes. Front. Cell Dev. Biol. 9, 692800 (2021).
Li, W., Wang, F., Guo, R., Bian, Z. & Song, Y. Targeting macrophages in hematological malignancies: recent advances and future directions. J. Hematol. Oncol. 15, 110 (2022).
Perzolli, A., Koedijk, J. B., Zwaan, C. M. & Heidenreich, O. Targeting the innate immune system in pediatric and adult AML. Leukemia 38, 1191–1201 (2024).
Chao, M. P. et al. Therapeutic targeting of the macrophage immune checkpoint CD47 in myeloid malignancies. Front. Oncol. 9, 1380 (2019).
Majeti, R. et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299 (2009).
Sallman, D. A. et al. The first-in-class anti-CD47 antibody Hu5F9-G4 is active and well tolerated alone or with azacitidine in AML and MDS patients: initial phase 1b results. J. Clin. Oncol. https://doi.org/10.1200/JCO.2019.37.15_suppl.7009 (2019).
Donio, M. J. et al. Pre-clinical combination of AO-176, a highly differentiated clinical stage CD47 antibody, with either azacitidine or venetoclax significantly enhances DAMP induction and phagocytosis of acute myeloid leukemia. Blood 136, 9–10 (2020).
Daver, N. G. et al. The ENHANCE-3 study: venetoclax and azacitidine plus magrolimab or placebo for untreated AML unfit for intensive therapy. Blood https://doi.org/10.1182/blood.2024027506 (2025).
Teicher, B. A. & Fricker, S. P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 16, 2927–2931 (2010).
López-Gil, J. C., Martin-Hijano, L., Hermann, P. C. & Sainz, B. The CXCL12 crossroads in cancer stem cells and their niche. Cancers 13, 469 (2021).
Zhou, W., Guo, S., Liu, M., Burow, M. E. & Wang, G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem. 26, 3026–3041 (2019).
Krause, D. S. Evading eviction: leukemic stem cell squatters. Blood 138, 1007–1008 (2021).
Konopleva, M., Tabe, Y., Zeng, Z. & Andreeff, M. Therapeutic targeting of microenvironmental interactions in leukemia: mechanisms and approaches. Drug. Resist. Updat. 12, 103–113 (2009).
Yu, X. et al. CD44 loss of function sensitizes AML cells to the BCL-2 inhibitor venetoclax by decreasing CXCL12-driven survival cues. Blood 138, 1067–1080 (2021).
Jaffrézou, J. P. et al. Daunorubicin-induced apoptosis: triggering of ceramide generation through sphingomyelin hydrolysis. EMBO J. 15, 2417–2424 (1996).
Al-Aamri, H. M. et al. Time dependent response of daunorubicin on cytotoxicity, cell cycle and DNA repair in acute lymphoblastic leukaemia. BMC Cancer 19, 179 (2019).
Kim, Y. H., Park, J. W., Lee, J. Y., Surh, Y. J. & Kwon, T. K. Bcl-2 overexpression prevents daunorubicin-induced apoptosis through inhibition of XIAP and Akt degradation. Biochem. Pharmacol. 66, 1779–1786 (2003).
Dariushnejad, H. et al. ABT-737, synergistically enhances daunorubicin-mediated apoptosis in acute myeloid leukemia cell lines. Adv. Pharm. Bull. 4, 185–189 (2014).
Daver, N., Schlenk, R. F., Russell, N. H. & Levis, M. J. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33, 299–312 (2019).
Tecik, M. & Adan, A. Therapeutic targeting of FLT3 in acute myeloid leukemia: current status and novel approaches. Onco Targets Ther. 15, 1449–1478 (2022).
Yoshimoto, G. et al. FLT3-ITD up-regulates MCL-1 to promote survival of stem cells in acute myeloid leukemia via FLT3-ITD-specific STAT5 activation. Blood 114, 5034–5043 (2009).
Seipel, K., Marques, M. A. T., Sidler, C., Mueller, B. U. & Pabst, T. MDM2- and FLT3-inhibitors in the treatment of. Haematologica 103, 1862–1872 (2018).
Yilmaz, M. et al. Hypomethylating agent and venetoclax with FLT3 inhibitor “triplet” therapy in older/unfit patients with FLT3 mutated AML. Blood Cancer J. 12, 77 (2022).
Zhang, W. et al. Sorafenib induces apoptosis of AML cells via Bim-mediated activation of the intrinsic apoptotic pathway. Leukemia 22, 808–818 (2008).
Rahmani, M., Davis, E. M., Bauer, C., Dent, P. & Grant, S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J. Biol. Chem. 280, 35217–35227 (2005).
Rahmani, M. et al. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood 119, 6089–6098 (2012).
Moujalled, D. M. et al. Combining BH3-mimetics to target both BCL-2 and MCL1 has potent activity in pre-clinical models of acute myeloid leukemia. Leukemia 33, 905–917 (2019).
Lin, V. S., Xu, Z. F., Huang, D. C. S. & Thijssen, R. BH3 mimetics for the treatment of B-cell malignancies-insights and lessons from the clinic. Cancers 12, 3353 (2020).
Olesinski, E. A. et al. BH3 profiling identifies BCL-2 dependence in adult patients with early T-cell progenitor acute lymphoblastic leukemia. Blood Adv. 7, 2917–2923 (2023).
Ishizawa, J. et al. Mitochondrial profiling of acute myeloid leukemia in the assessment of response to apoptosis modulating drugs. PLoS ONE 10, e0138377 (2015).
Soderquist, R. S. et al. Systematic mapping of BCL-2 gene dependencies in cancer reveals molecular determinants of BH3 mimetic sensitivity. Nat. Commun. 9, 3513 (2018).
Pacchiardi, K. et al. Prospective feasibility of a minimal BH3 profiling assay in acute myeloid leukemia. Cytom. B108, 86–94 (2025).
Iyer, P., Jasdanwala, S. S., Wang, Y., Bhatia, K. & Bhatt, S. Decoding acute myeloid leukemia: a clinician’s guide to functional profiling. Diagnostics 14, 2560 (2024).
Daver, N. A.-O. X. et al. Venetoclax and idasanutlin in relapsed/refractory AML: a nonrandomized, open-label phase 1b trial. Blood 141, 1265–1276 (2023).
Campos, L. et al. High expression of bcl-2 protein in acute myeloid leukemia cells is associated with poor response to chemotherapy. Blood 81, 3091–3096 (1993).
Keith, F. J., Bradbury, D. A., Zhu, Y. M. & Russell, N. H. Inhibition of bcl-2 with antisense oligonucleotides induces apoptosis and increases the sensitivity of AML blasts to Ara-C. Leukemia 9, 131–138 (1995).
Bensi, L. et al. Bcl-2 oncoprotein expression in acute myeloid leukemia. Haematologica 80, 98–102 (1995).
Wang, J. L. et al. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res. 60, 1498–1502 (2000).
Wang, J. L. et al. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc. Natl Acad. Sci. USA 97, 7124–7129 (2000).
Pan, R. et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 4, 362–375 (2014).
Venetoclax receives 3rd breakthrough therapy designation from the FDA for the combination treatment of patients with untreated acute myeloid leukemia not eligible for standard induction chemotherapy. AbbVie https://news.abbvie.com/2016-01-28-Venetoclax-Receives-3rd-Breakthrough-Therapy-Designation-from-the-FDA-for-the-Combination-Treatment-of-Patients-with-Untreated-Acute-Myeloid-Leukemia-not-Eligible-for-Standard-Induction-Chemotherapy (2016).
Acknowledgements
The work of A.P.K. was supported by the NUHS Seed Grant, National University of Singapore (NUHSRO/2023/039/RO5+6/Seed-Mar/04). The work of C.G. is supported by NIH grant 4R00CA252602-04, a V Foundation award V2023-008 and a New Jersey Health Foundation grant PC 99-24.
Author information
Authors and Affiliations
Contributions
A.G., E.W., H.Y.L., D.J.J.T., M.J.M., J.D.G., C.D.D. and A.P.K. researched data for the manuscript, A.G., E.W., H.Y.L., D.J.J.T., A.J.I., Y.G., C.E.L., W.S., C.G., L.S., T.Y., T.Y.Z., V.E.K., B.D.S., T.M., S.D.B., P.D., M.K., M.J.M., J.D.G., C.D.D. and A.P.K. wrote the manuscript, and all authors made a significant contribution to discussions of content and edited and/or reviewed the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
B.D.S. is a consultant and adviser of Servier. M.K. is a consultant of AbbVie, Adaptive, AmMax, Curis, Janssen, Kyowa Kirin, Menarini/Stemline Therapeutics, Novartis, Sanofi Aventis, Servier and Vincerx and is an adviser of AbbVie, Auxenion GmbH, Dark Blue Therapeutics, Legend, MEI Pharma, Menarini/Stemline Therapeutics, Novartis and Syndax and receives research funding from AbbVie, Janssen and Klondike Biopharma. C.D.D. is a consultant and/or adviser of Abbvie, Astellas, AstraZeneca, BMS, Daiichi Sankyo, GenMab, GSK, Rigel, Ryvu, Schrodinger, Servier and Solu Therapeutics and has received research funding from Abbvie, Astex, Beigene, BMS, Jazz, ImmuneOnc, Remix, Servier, Schrodinger and Systimmune. A.P.K. serves on the advisory board for AUM Biosciences. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks J. Zeidner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Glaviano, A., Weisberg, E., Lam, H.Y. et al. Apoptosis-targeting BH3 mimetics: transforming treatment for patients with acute myeloid leukaemia. Nat Rev Clin Oncol 22, 847–868 (2025). https://doi.org/10.1038/s41571-025-01068-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41571-025-01068-0