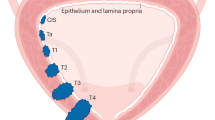
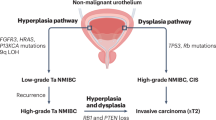
Abstract
Bladder cancer remains a substantial global health burden. Localized, non-metastatic bladder cancer encompasses a spectrum from non-muscle-invasive bladder cancer (NMIBC) to muscle-invasive bladder cancer (MIBC). Advances in risk stratification, biomarker discovery and therapeutic innovation are reshaping the management of localized bladder cancers. Regarding NMIBC, enhanced cystoscopy techniques, en bloc transurethral resection and a multitude of novel systemic or intravesical treatment strategies, including immunomodulatory, viral, molecularly targeted and/or cytotoxic therapies as well as novel drug delivery mechanisms and chemoablation, are expanding treatment options, particularly for patients with Bacillus Calmette–Guérin-unresponsive disease. For patients with MIBC, refinements in surgical techniques and new neoadjuvant and/or adjuvant systemic therapies, particularly perioperative immunotherapy and potentially antibody–drug conjugates, continue to improve oncological outcomes. Bladder-preserving approaches such as trimodal therapy or even active surveillance following neoadjuvant therapy are also gaining clinical traction, offering selected patients with MIBC an alternative to radical cystectomy. Advances in the identification and application of circulating tumour DNA-based and tumour tissue-based biomarkers will help to further support personalized treatment and follow-up strategies. In this Review, we discuss progress and changes in the management of localized bladder cancer over the past decade and highlight ongoing innovations and future research directions that will shape clinical practice in the coming decade.
Key points
-
Urine biomarkers have not yet changed diagnosis and surveillance approaches for bladder cancer; cystoscopy remains the gold standard approach.
-
Management of non-muscle-invasive bladder cancer (NMIBC) relies on risk-adapted strategies, although recent guideline modifications reallocating small high-grade Ta tumours from the high-risk to intermediate-risk category remain controversial.
-
Therapeutic innovations are expanding treatment options for Bacillus Calmette–Guérin (BCG)-unresponsive NMIBC, with regulatory approval of pembrolizumab, nadofaragene firadenovec and nogapendekin alfa inbakicept. Several clinical trials are investigating other novel therapies for both BCG-unresponsive and BCG-naive NMIBC.
-
Radical cystectomy remains the standard-of-care treatment for muscle-invasive bladder cancer, with no clear differences in oncological outcomes between open and robotic-assisted approaches. Standard pelvic lymph node dissection is recommended, given that no survival benefit has been demonstrated with the use of an extended template.
-
Perioperative chemoimmunotherapy has demonstrated superiority over neoadjuvant cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer and has therefore become a standard of care. Bladder-sparing approaches will become increasingly important as more-effective neoadjuvant therapies are introduced.
-
Detectable circulating tumour DNA after neoadjuvant therapy or radical cystectomy correlates with inferior clinical outcomes, supporting further investigations in ongoing trials to test the potential role of circulating tumour DNA status in guiding intensification or de-intensification of adjuvant therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
19 January 2026
A Correction to this paper has been published: https://doi.org/10.1038/s41571-026-01121-6
References
Wéber, A. et al. Global burden of bladder cancer mortality in 2020 and 2040 according to GLOBOCAN estimates. World J. Urol. 42, 237 (2024).
Sloan, F. A., Yashkin, A. P., Akushevich, I. & Inman, B. A. The cost to medicare of bladder cancer care. Eur. Urol. Oncol. 3, 515–522 (2020).
Svatek, R. S. et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur. Urol. 66, 253–262 (2014).
Alfred Witjes, J. et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur. Urol. 85, 17–31 (2024).
Galsky, M. D. et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat. Med. https://doi.org/10.1038/s41591-023-02568-1 (2023).
Ditonno, F. et al. Trimodal therapy vs radical cystectomy in patients with muscle-invasive bladder cancer: a systematic review and meta-analysis of comparative studies. BJU Int. https://doi.org/10.1111/bju.16366 (2024).
Gontero, P. et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (TaT1 and carcinoma in situ) — a summary of the 2024 guidelines update. Eur. Urol. 86, 531–549 (2024).
Subiela, J. D. et al. Unlocking the potential of adequate Bacillus Calmette–Guérin immunotherapy in very-high-risk non-muscle-invasive bladder carcinoma: a multicenter analysis of oncological outcomes and risk dynamics. Eur. Urol. Oncol. 7, 1367–1375 (2024).
Holzbeierlein, J. M. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline: 2024 amendment. J. Urol. 211, 533–538 (2024).
Chang, S. S. et al. Diagnosis and treatment of non-muscle invasive bladder cancer: AUA/SUO guideline. J. Urol. 196, 1021–1029 (2016).
Bree, K. K. et al. All high-grade Ta tumors should be classified as high risk: Bacillus Calmette–Guérin response in high-grade Ta tumors. J. Urol. 208, 284–291 (2022).
Babjuk, M. et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur. Urol. 81, 75–94 (2022).
Annapureddy, D. et al. Impact of tumor stage on oncologic outcomes of high-grade Bacillus Calmette–Guérin unresponsive non-muscle-invasive bladder cancer undergoing bladder-sparing therapies. Eur. Urol. Focus https://doi.org/10.1016/j.euf.2025.01.001 (2025).
Beijert, I. J., Hentschel, A. E., Bründl, J., Compérat, E. M. & Plass, K. Prognosis of primary papillary Ta grade 3 bladder cancer in the non-muscle-invasive spectrum. Eur. Urol. Oncol. 6, 214–221 (2023).
St-Laurent, M.-P., Suderman, J. & Black, P. C. Where to draw the line: stratifying risk for high-grade Ta bladder cancer. Eur. Urol. Oncol. https://doi.org/10.1016/j.euo.2023.02.003 (2023).
Soria, F. & Gontero, P. Intermediate-risk non-muscle-invasive bladder cancer: time to tidy up the mess? Eur. Urol. Oncol. 5, 517–518 (2022).
Tan, W. S. et al. Intermediate-risk non-muscle-invasive bladder cancer: updated consensus definition and management recommendations from the International Bladder Cancer Group. Eur. Urol. Oncol. 5, 505–516 (2022).
Maas, M., Todenhöfer, T. & Black, P. C. Urine biomarkers in bladder cancer — current status and future perspectives. Nat. Rev. Urol. 20, 597–614 (2023).
Tomiyama, E., Fujita, K., Hashimoto, M., Uemura, H. & Nonomura, N. Urinary markers for bladder cancer diagnosis: a review of current status and future challenges. Int. J. Urol. 31, 208–219 (2024).
Lotan, Y. et al. Optimal trial design for studying urinary markers in bladder cancer: a collaborative review. Eur. Urol. Oncol. 1, 223–230 (2018).
Schroeck, F. R. et al. Clinical trial protocol for ‘replace cysto’: replacing invasive cystoscopy with urine testing for non-muscle-invasive bladder cancer surveillance — a multicenter, randomized, phase 2 healthcare delivery trial comparing quality of life during cancer surveillance with Xpert Bladder Cancer Monitor or Bladder EpiCheck Urine Testing Versus Frequent Cystoscopy. Eur. Urol. Open Sci. 63, 19–30 (2024).
Karmark, D. T. et al. The DaBlaCa-15 trial: the urinary biomarker test Xpert Bladder Cancer Monitor guiding cystoscopy in high-grade non-muscle-invasive bladder cancer: results from a randomized controlled trial. Eur. Urol. 88, 23–30 (2025).
Strandgaard, T. et al. Field cancerization is associated with tumor development, T-cell exhaustion, and clinical outcomes in bladder cancer. Eur. Urol. 85, 82–92 (2024).
St-Laurent, M.-P. et al. Correlation of urinary comprehensive genomic profile with risk of recurrence of BCG-unresponsive non-muscle invasive bladder cancer treated with atezolizumab in SWOG S1605. J. Clin. Oncol. 42, 529 (2024).
Narayan, V. M. et al. Minimal residual disease detection with urine-derived DNA is prognostic for recurrence-free survival in Bacillus Calmette–Guérin-unresponsive non-muscle-invasive bladder cancer treated with nadofaragene firadenovec. Eur. Urol. Oncol. https://doi.org/10.1016/j.euo.2024.09.016 (2024).
Catto, J. W. F. et al. Erdafitinib in BCG-treated high-risk non-muscle-invasive bladder cancer. Ann. Oncol. 35, 98–106 (2024).
Messing, E. M. et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. Jama 319, 1880–1888 (2018).
Sylvester, R. J., van der, M. A. & Lamm, D. L. Intravesical Bacillus Calmette–Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J. Urol. 168, 1964–1970 (2002).
Oddens, J. et al. Final results of an EORTC-GU cancers group randomized study of maintenance Bacillus Calmette–Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur. Urol. 63, 462–472 (2013).
Tabayoyong, W. B. et al. Systematic review on the utilization of maintenance intravesical chemotherapy in the management of non-muscle-invasive bladder cancer. Eur. Urol. Focus 4, 512–521 (2018).
Turker, P. et al. Upstaging of urothelial cancer at the time of radical cystectomy: factors associated with upstaging and its effect on outcome. BJU Int. 110, 804–811 (2012).
Shkolyar, E. et al. Optimizing cystoscopy and TURBT: enhanced imaging and artificial intelligence. Nat. Rev. Urol. 22, 46–54 (2025).
Lai, L. Y. et al. Narrow band imaging versus white light cystoscopy alone for transurethral resection of non-muscle invasive bladder cancer. Cochrane Database Syst. Rev. 4, CD014887 (2022).
Lange, N. et al. Photodetection of early human bladder cancer based on the fluorescence of 5-aminolaevulinic acid hexylester-induced protoporphyrin IX: a pilot study. Br. J. Cancer 80, 185–193 (1999).
Gono, K. et al. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J. Biomed. Opt. 9, 568–577 (2004).
Wu, S. et al. An artificial intelligence system for the detection of bladder cancer via cystoscopy: a multicenter diagnostic study. J. Natl Cancer Inst. 114, 220–227 (2022).
Chang, T. C. et al. Real-time detection of bladder cancer using augmented cystoscopy with deep learning: a pilot study. J. Endourol. https://doi.org/10.1089/end.2023.0056 (2023).
Yuan, H. et al. Therapeutic outcome of fluorescence cystoscopy guided transurethral resection in patients with non-muscle invasive bladder cancer: a meta-analysis of randomized controlled trials. PLoS ONE 8, e74142 (2013).
Veeratterapillay, R. et al. Time to turn on the blue lights: a systematic review and meta-analysis of photodynamic diagnosis for bladder cancer. Eur. Urol. Open Sci. 31, 17–27 (2021).
Heer, R. et al. A randomized trial of PHOTOdynamic surgery in non-muscle-invasive bladder cancer. NEJM Evid. 1, EVIDoa2200092 (2022).
St-Laurent, M. P., Suderman, J. & Black, P. C. Re: a randomized trial of PHOTOdynamic surgery in non-muscle-invasive bladder cancer. Eur. Urol. 83, 298–299 (2023).
Teoh, J. Y. et al. En bloc resection of bladder tumour: the rebirth of past through reminiscence. World J. Urol. 41, 2599–2606 (2023).
Teoh, J. Y. et al. Recurrence mechanisms of non-muscle-invasive bladder cancer — a clinical perspective. Nat. Rev. Urol. 19, 280–294 (2022).
Basile, G. et al. En bloc versus conventional transurethral resection of bladder tumors: a systematic review and meta-analysis of oncological, histopathological, and surgical outcomes. Eur. Urol. Oncol. https://doi.org/10.1016/j.euo.2024.10.004 (2024).
Yuen-Chun Teoh, J. et al. Transurethral en bloc resection versus standard resection of bladder tumour: a randomised, multicentre, phase 3 trial. Eur. Urol. 86, 103–111 (2024).
D’Andrea, D. et al. En bloc versus conventional resection of primary bladder tumor (eBLOC): a prospective, multicenter, open-label, phase 3 randomized controlled trial. Eur. Urol. Oncol. 6, 508–515 (2023).
Tan, W. S. & Kelly, J. D. Intravesical device-assisted therapies for non-muscle-invasive bladder cancer. Nat. Rev. Urol. 15, 667–685 (2018).
Joice, G. A., Bivalacqua, T. J. & Kates, M. Optimizing pharmacokinetics of intravesical chemotherapy for bladder cancer. Nat. Rev. Urol. 16, 599–612 (2019).
Tan, W. S. et al. Adjuvant intravesical chemohyperthermia versus passive chemotherapy in patients with intermediate-risk non-muscle-invasive bladder cancer (HIVEC-II): a phase 2, open-label, randomised controlled trial. Eur. Urol. 83, 497–504 (2023).
Angulo, J. C. et al. Hyperthermic mitomycin C in intermediate-risk non-muscle-invasive bladder cancer: results of the HIVEC-1 trial. Eur. Urol. Oncol. 6, 58–66 (2023).
Guerrero-Ramos, F. et al. Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial. World J. Urol. 40, 999–1004 (2022).
Soloway, M. S., Bruck, D. S. & Kim, S. S. Expectant management of small, recurrent, noninvasive papillary bladder tumors. J. Urol. 170, 438–441 (2003).
Contieri, R. et al. Deintensification of treatment for low-grade bladder tumors: a collaborative review by the International Bladder Cancer Group (IBCG). Eur. Urol. Oncol. 8, 179–189 (2025).
Pedersen, G. L., Erikson, M. S., Mogensen, K., Rosthøj, S. & Hermann, G. G. Outpatient photodynamic diagnosis-guided laser destruction of bladder tumors is as good as conventional inpatient photodynamic diagnosis-guided transurethral tumor resection in patients with recurrent intermediate-risk low-grade Ta bladder tumors. A prospective randomized noninferiority clinical trial. Eur. Urol. 83, 125–130 (2023).
Contieri, R. et al. Long-term follow-up and factors associated with active surveillance failure for patients with non-muscle-invasive bladder cancer: the Bladder Cancer Italian Active Surveillance (BIAS) experience. Eur. Urol. Oncol. 5, 251–255 (2022).
Yanagisawa, T. et al. A systematic review and meta-analysis of chemoablation for non-muscle-invasive bladder cancer. Eur. Urol. Focus 9, 463–479 (2023).
Prasad, S. M. et al. Primary chemoablation of recurrent low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102: a single-arm, open-label, phase 3 trial (ENVISION). J. Urol. 213, 205–216 (2025).
Prasad, S. M. et al. Treatment of low-grade intermediate-risk nonmuscle-invasive bladder cancer with UGN-102 ± transurethral resection of bladder tumor compared to transurethral resection of bladder tumor monotherapy: a randomized, controlled, phase 3 trial (ATLAS). J. Urol. 210, 619–629 (2023).
Lindgren, M. S. et al. DaBlaCa-13 study: oncological outcome of short-term, intensive chemoresection with mitomycin in nonmuscle invasive bladder cancer: primary outcome of a randomized controlled trial. J. Clin. Oncol. 41, 206–211 (2023).
Racioppi, M. et al. Chemoablation with intensive intravesical mitomycin C treatment: a new approach for non-muscle-invasive bladder cancer. Eur. Urol. Oncol. 2, 576–583 (2019).
Mostafid, A. H. et al. CALIBER: a phase II randomized feasibility trial of chemoablation with mitomycin-C vs surgical management in low-risk non-muscle-invasive bladder cancer. BJU Int. 125, 817–826 (2020).
Malmström, P. U. et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus Bacillus Calmette–Guérin for non-muscle-invasive bladder cancer. Eur. Urol. 56, 247–256 (2009).
Ansari Djafari, A. et al. Intravesical gemcitabine versus intravesical Bacillus Calmette–Guerin for the treatment of intermediate-risk non-muscle invasive bladder cancer: a randomized controlled trial. Urol. J. 20, 123–128 (2023).
Böhle, A., Jocham, D. & Bock, P. R. Intravesical Bacillus Calmette–Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J. Urol. 169, 90–95 (2003).
Bhindi, B. et al. Canadian Urological Association guideline on the management of non-muscle-invasive bladder cancer — full-text. Can. Urol. Assoc. J. 15, E424–E460 (2021).
McElree, I. M., Steinberg, R. L., Mott, S. L., O’Donnell, M. A. & Packiam, V. T. Comparison of sequential intravesical gemcitabine and docetaxel vs Bacillus Calmette–Guérin for the treatment of patients with high-risk non-muscle-invasive bladder cancer. JAMA Netw. Open 6, e230849 (2023).
Patel, S. H. et al. A phase 2 trial of intravesical gemcitabine and docetaxel in the treatment of Bacillus Calmette–Guérin-naive nonmuscle-invasive urothelial carcinoma of the bladder. J. Urol. 212, 95–103 (2024).
FDA (ed. U.S. Department of Health and Human Services Food and Drug Administration). https://www.fda.gov/media/101468/download (2024).
Shore, N. D. et al. Sasanlimab plus BCG in BCG-naive, high-risk non-muscle invasive bladder cancer: the randomized phase 3 CREST trial. Nat. Med. https://doi.org/10.1038/s41591-025-03738-z (2025).
Shore, N. D. et al. Sasanlimab in combination with Bacillus Calmette–Guérin improves event-free survival versus Bacillus Calmette–Guérin as standard of care in high-risk non-muscle-invasive bladder cancer: phase 3 crest study results. J. Urol. 213, e1 (2025).
De Santis, M. et al. Durvalumab in combination with BCG for BCG-naive, high-risk, non-muscle-invasive bladder cancer (POTOMAC): final analysis of a randomised, open-label, phase 3 trial. Lancet https://doi.org/10.1016/S0140-6736(25)01897-5 (2025).
Bowden, M. Imfinzi regimen demonstrated statistically significant and clinically meaningful improvement in disease-free survival for high-risk non-muscle-invasive bladder cancer in POTOMAC Phase III trial. https://www.astrazeneca.com/media-centre/press-releases/2025/imfinzi-improved-dfs-in-early-bladder-cancer.html (2025).
Roupret, M. et al. ALBAN (GETUG-AFU 37): a phase 3, randomized, open-label, international trial of intravenous atezolizumab and intravesical Bacillus Calmette–Guérin (BCG) versus BCG alone in BCG-naive high-risk, non-muscle invasive bladder cancer (NMIBC). Ann. Oncol. https://doi.org/10.1016/j.annonc.2025.09.017 (2025).
Roumiguié, M. et al. International Bladder Cancer Group consensus statement on clinical trial design for patients with Bacillus Calmette–Guérin-exposed high-risk non-muscle-invasive bladder cancer. Eur. Urol. 82, 34–46 (2022).
Flaig, T. W. et al. NCCN guidelines® insights: bladder cancer, version 3.2024. J. Natl Compr. Canc. Netw. 22, 216–225 (2024).
Herr, H. W. & Sogani, P. C. Does early cystectomy improve the survival of patients with high risk superficial bladder tumors? J. Urol. 166, 1296–1299 (2001).
Hautmann, R. E., Gschwend, J. E., de Petriconi, R. C., Kron, M. & Volkmer, B. G. Cystectomy for transitional cell carcinoma of the bladder: results of a surgery only series in the neobladder era. J. Urol. 176, 486–492 (2006).
Sobhani, S. et al. Perioperative mortality for radical cystectomy in the modern era: experience from a tertiary referral center. Int. Braz. J. Urol. 49, 351–358 (2023).
Maibom, S. L. et al. Short-term morbidity and mortality following radical cystectomy: a systematic review. BMJ Open 11, e043266 (2021).
Mossanen, M. et al. Examining the relationship between complications and perioperative mortality following radical cystectomy: a population-based analysis. BJU Int. 124, 40–46 (2019).
Tan, W. S. et al. Bladder-sparing treatment in patients with Bacillus Calmette-Guérin-unresponsive non-muscle-invasive bladder cancer: an analysis of long-term survival outcomes. Eur. Urol. Open Sci. 53, 16–22 (2023).
Taylor, J. I. et al. Long-term outcomes of bladder-sparing therapy vs radical cystectomy in BCG-unresponsive non-muscle-invasive bladder cancer. BJU Int. 135, 260–268 (2025).
Gore, J. L. et al. Radical cystectomy versus bladder-sparing therapy for recurrent high-grade non-muscle invasive bladder cancer: results from the Comparison of Intravesical Therapy and Surgery as Treatment Options (CISTO) study. J. Urol. 213, e3–e4 (2025).
Dinney, C. P., Greenberg, R. E. & Steinberg, G. D. Intravesical valrubicin in patients with bladder carcinoma in situ and contraindication to or failure after Bacillus Calmette–Guérin. Urol. Oncol. 31, 1635–1642 (2013).
Steinberg, G. et al. Efficacy and safety of valrubicin for the treatment of bacillus Calmette–Guerin refractory carcinoma in situ of the bladder. The Valrubicin Study Group. J. Urol. 163, 761–767 (2000).
NCC Network. NCCN Clinical Practice Guidelines in Oncology (Bladder Cancer). https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (2025).
Balar, A. V. et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 22, 919–930 (2021).
Black, P. C. et al. Phase 2 trial of atezolizumab in Bacillus Calmette–Guerin-unresponsive high-risk non-muscle-invasive bladder cancer: SWOG S1605. Eur. Urol. https://doi.org/10.1016/j.eururo.2023.08.004 (2023).
Li, R. et al. A phase II study of durvalumab for Bacillus Calmette–Guerin (BCG) unresponsive urothelial carcinoma in situ of the bladder. Clin. Cancer Res. 29, 3875–3881 (2023).
Necchi, A. et al. Pembrolizumab monotherapy for high-risk non-muscle-invasive bladder cancer without carcinoma in situ and unresponsive to BCG (KEYNOTE-057): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 25, 720–730 (2024).
Boorjian, S. A. et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 22, 107–117 (2021).
FDA. in 761336Orig1s000 (ed. Center for Drug Evaluation and Research). https://www.accessdata.fda.gov/drugsatfda_docs/nda/2024/761336Orig1s000MultidisciplineR.pdf (2024).
Dubois, S., Patel, H. J., Zhang, M., Waldmann, T. A. & Müller, J. R. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 180, 2099–2106 (2008).
Rubinstein, M. P. et al. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha}. Proc. Natl Acad. Sci. USA 103, 9166–9171 (2006).
Chamie, K. et al. IL-15 superagonist NAI in BCG-unresponsive non-muscle-invasive bladder cancer. NEJM Evid. 2, EVIDoa2200167 (2023).
Ramesh, N. et al. CG0070, a conditionally replicating granulocyte macrophage colony-stimulating factor — armed oncolytic adenovirus for the treatment of bladder cancer. Clin. Cancer Res. 12, 305–313 (2006).
Tyson, M. D. et al. Final results: BOND-003 cohort C-phase 3, single-arm study of intravesical cretostimogene grenadenorepvec for high-risk BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Urol. 213, e1 (2025).
Li, R. et al. Oncolytic adenoviral therapy plus pembrolizumab in BCG-unresponsive non-muscle-invasive bladder cancer: the phase 2 CORE-001 trial. Nat. Med. 30, 2216–2223 (2024).
Daneshmand, S. et al. TAR-200 for Bacillus Calmette–Guérin-unresponsive high-risk non-muscle-invasive bladder cancer: results from the phase IIb SunRISe-1 study. J. Clin. Oncol. https://doi.org/10.1200/jco-25-01651 (2025).
Steinberg, R. L. et al. Intravesical sequential gemcitabine and docetaxel versus Bacillus Calmette–Guerin (BCG) plus interferon in patients with recurrent non-muscle invasive bladder cancer following a single induction course of BCG. Urol. Oncol. 40, 9.e1–9.e7 (2022).
Scilipoti, P. et al. Gemcitabine and docetaxel for high-risk non-muscle-invasive bladder cancer: EuroGemDoce group results. BJU Int. https://doi.org/10.1111/bju.16645 (2025).
Taylor, J. et al. Oncologic outcomes of sequential intravesical gemcitabine and docetaxel compared with Bacillus Calmette–Guérin in patients with Bacillus Calmette–Guérin-unresponsive non-muscle invasive bladder cancer. Eur. Urol. Oncol. 8, 469–476 (2025).
Bivalacqua, T., Daneshmand, S., Shore, N. D., Joshi, S. & Dinney, C. P. N. CORE-008: a phase 2, multi-arm, multi-cohort, open-label study to evaluate intravesical cretostimogene grenadenorepvec in participants with high-risk non-muscle invasive bladder cancer. J. Clin. Oncol. 43, TPS901 (2025).
Porten, S. et al. SunRISe-5: a phase 3, randomized, open-label study of TAR-200 compared with intravesical chemotherapy after Bacillus Calmette–Guérin in recurrent high-risk non-muscle-invasive bladder cancer. Urol. Oncol. Semin. Original Investig. 43, 54–55 (2025).
Alam, S. M. et al. Abstract PR005: final results from a phase I trial of intravesical chemoimmunotherapy with gemcitabine and Bacillus Calmette–Guérin (BCG) for patients with BCG-exposed high-grade non-muscle invasive bladder cancer. Clin. Cancer Res. 30, PR005 (2024).
Panebianco, V. et al. Multiparametric magnetic resonance imaging for bladder cancer: development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur. Urol. 74, 294–306 (2018).
Carando, R. et al. The effectiveness of multiparametric magnetic resonance imaging in bladder cancer (Vesical Imaging-Reporting and Data System): a systematic review. Arab. J. Urol. 18, 67–71 (2020).
Kazan, O. et al. Diagnostic validity of the vesical imaging-reporting and data system (VI-RADS): a real-world study. Actas Urol. Esp. 47, 638–644 (2023).
Bryan, R. T. et al. Randomized comparison of magnetic resonance imaging versus transurethral resection for staging new bladder cancers: results from the prospective BladderPath trial. J. Clin. Oncol. 43, 1417–1428 (2025).
James, N. D. et al. Randomized comparison of upfront magnetic resonance imaging versus transurethral resection for staging new bladder cancers: Final survival analysis from the BladderPath trial. Ann. Oncol. https://doi.org/10.1016/j.annonc.2025.09.130 (2025).
Pecoraro, M. et al. Vesical Imaging-Reporting and Data System (VI-RADS) for assessment of response to systemic therapy for bladder cancer: preliminary report. Abdom. Radiol. 47, 763–770 (2022).
Necchi, A. et al. VI-RADS use predicting the outcome of neoadjuvant pembrolizumab in muscle-invasive bladder cancer. BJU Int. https://doi.org/10.1111/bju.16191 (2023).
Wang, N., Jiang, P. & Lu, Y. Is fluorine-18 fluorodeoxyglucose positron emission tomography useful for detecting bladder lesions? A meta-analysis of the literature. Urol. Int. 92, 143–149 (2014).
Bouchelouche, K. PET/CT in bladder cancer: an update. Semin. Nucl. Med. 52, 475–485 (2022).
Li, T. et al. Performance of (18)F-FDG PET/MRI and its parameters in staging and neoadjuvant therapy response evaluation in bladder cancer. iScience 27, 109657 (2024).
Cigliola, A. et al. Utility of fluoro-deoxy-glucose positron emission tomography (FDG-PET) to predict a pelvic lymph node involvement (LNI) in patients (pts) with muscle-invasive bladder cancer (MIBC) enrolled in neoadjuvant therapy trials. J. Clin. Oncol. 42, 527 (2024).
Bezzi, C. et al. Staging and neoadjuvant response evaluation in patients with muscle-invasive bladder cancer (MIBC) using [18F]F-FDG PET/MRI. J. Nucl. Med. 65, 241941 (2024).
Xu, X. et al. Quantitative identification of nonmuscle-invasive and muscle-invasive bladder carcinomas: a multiparametric MRI radiomics analysis. J. Magn. Reson. Imaging 49, 1489–1498 (2019).
He, K. et al. Progress of multiparameter magnetic resonance imaging in bladder cancer: a comprehensive literature review. Diagnostics (Basel) https://doi.org/10.3390/diagnostics14040442 (2024).
Arita, Y. et al. Multiparametric MRI and artificial intelligence in predicting and monitoring treatment response in bladder cancer. Insights Imaging 16, 7 (2025).
Nix, J. et al. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur. Urol. 57, 196–201 (2010).
Parekh, D. J. et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 391, 2525–2536 (2018).
Bochner, B. H. et al. Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy: a randomized clinical trial. Eur. Urol. 67, 1042–1050 (2015).
Khan, M. S. et al. A single-centre early phase randomised Controlled Three-Arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). Eur. Urol. 69, 613–621 (2016).
Catto, J. W. F. et al. Effect of robot-assisted radical cystectomy with intracorporeal urinary diversion vs open radical cystectomy on 90-day morbidity and mortality among patients with bladder cancer: a randomized clinical trial. JAMA 327, 2092–2103 (2022).
Rai, B. P. et al. Robotic versus open radical cystectomy for bladder cancer in adults. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD011903.pub2 (2019).
Khetrapal, P. et al. Robot-assisted radical cystectomy versus open radical cystectomy: a systematic review and meta-analysis of perioperative, oncological, and quality of life outcomes using randomized controlled trials. Eur. Urol. 84, 393–405 (2023).
Wang, Y.-C. et al. Extended versus non-extended lymphadenectomy during radical cystectomy for patients with bladder cancer: a meta-analysis of the effect on long-term and short-term outcomes. World J. Surg. Oncol. 17, 225 (2019).
van der Heijden, A. G. et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2025 guidelines. Eur. Urol. 87, 582–600 (2025).
Lerner, S. P. et al. Standard or extended lymphadenectomy for muscle-invasive bladder cancer. N. Engl. J. Med. 391, 1206–1216 (2024).
Gschwend, J. E. et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur. Urol. 75, 604–611 (2019).
Ślusarczyk, A. et al. Comparison of extended and standard lymph node dissection in radical cystectomy for urothelial bladder cancer: a systematic review and meta-analysis. World J. Urol. 43, 181 (2025).
Grossman, H. B. et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N. Engl. J. Med. 349, 859–866 (2003).
Griffiths, G., Hall, R., Sylvester, R., Raghavan, D. & Parmar, M. K. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J. Clin. Oncol. 29, 2171–2177 (2011).
Vale, C. L. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202 (2005).
von der Maase, H. et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J. Clin. Oncol. 18, 3068–3077 (2000).
Pfister, C. et al. Perioperative dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin in muscle-invasive bladder cancer (VESPER): survival endpoints at 5 years in an open-label, randomised, phase 3 study. Lancet Oncol. 25, 255–264 (2024).
Powles, T. et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N. Engl. J. Med. 390, 875–888 (2024).
van der Heijden, M. S. et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N. Engl. J. Med. 389, 1778–1789 (2023).
Galsky, M. D. et al. Perioperative pembrolizumab therapy in muscle-invasive bladder cancer: phase III KEYNOTE-866 and KEYNOTE-905/EV-303. Future Oncol. 17, 3137–3150 (2021).
Sonpavde, G. et al. ENERGIZE: a phase III study of neoadjuvant chemotherapy alone or with nivolumab with/without linrodostat mesylate for muscle-invasive bladder cancer. Future Oncol. 16, 4359–4368 (2020).
Powles, T. et al. Perioperative durvalumab with neoadjuvant chemotherapy in operable bladder cancer. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2408154 (2024).
Gupta, S. et al. Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. J. Clin. Oncol. 38, 439 (2020).
Kim, H. et al. Neoadjuvant nivolumab plus gemcitabine/cisplatin chemotherapy in muscle-invasive urothelial carcinoma of the bladder. Cancer Res. Treat. 55, 636–642 (2023).
Vulsteke, C. et al. Perioperative (periop) enfortumab vedotin (EV) plus pembrolizumab (pembro) in participants (pts) with muscle-invasive bladder cancer (MIBC) who are cisplatin-ineligible: The phase III KEYNOTE-905 study. Ann. Oncol. https://doi.org/10.1016/j.annonc.2025.09.124 (2025).
James, N. D. et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N. Engl. J. Med. 366, 1477–1488 (2012).
Hall, E. et al. Chemoradiotherapy in muscle-invasive bladder cancer: 10-yr follow-up of the phase 3 randomised controlled BC2001 trial. Eur. Urol. 82, 273–279 (2022).
Softness, K. et al. Radical cystectomy versus trimodality therapy for muscle-invasive urothelial carcinoma of the bladder. Urol. Oncol. 40, 272.e271–272.e279 (2022).
Zlotta, A. R. et al. Radical cystectomy versus trimodality therapy for muscle-invasive bladder cancer: a multi-institutional propensity score matched and weighted analysis. Lancet Oncol. 24, 669–681 (2023).
Giacalone, N. J. et al. Long-term outcomes after bladder-preserving tri-modality therapy for patients with muscle-invasive bladder cancer: an updated analysis of the Massachusetts General Hospital Experience. Eur. Urol. 71, 952–960 (2017).
Mazza, P. et al. Conservative management following complete clinical response to neoadjuvant chemotherapy of muscle invasive bladder cancer: contemporary outcomes of a multi-institutional cohort study. J. Urol. 200, 1005–1013 (2018).
Geynisman, D. M. et al. Phase II trial of risk-enabled therapy after neoadjuvant chemotherapy for muscle-invasive bladder cancer (RETAIN 1). J. Clin. Oncol. https://doi.org/10.1200/jco-24-01214 (2024).
Culine, S. et al. Refining the characterization and outcome of pathological complete responders after neoadjuvant chemotherapy for muscle-invasive bladder cancer: lessons from the randomized phase III VESPER (GETUG-AFU V05) trial. Cancers (Basel) https://doi.org/10.3390/cancers15061742 (2023).
Ghatalia, P. et al. A phase 2 trial of risk enabled therapy after neoadjuvant chemo-immunotherapy for muscle-invasive bladder cancer (RETAIN-2). J. Clin. Oncol. 43, 815 (2025).
Balli, S., Bolek, H. & Ürün, Y. Emerging strategies in adjuvant immunotherapy: a comparative review of bladder cancer and renal cell carcinoma treatments. Clin. Med. Insights Oncol. 18, 11795549241257238 (2024).
Advanced Bladder Cancer (ABC) Meta-analysis Collaborators Group. Adjuvant chemotherapy for muscle-invasive bladder cancer: a systematic review and meta-analysis of individual participant data from randomised controlled trials. Eur. Urol. 81, 50–61 (2022).
Leow, J. J. et al. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur. Urol. 66, 42–54 (2014).
Galsky, M. D. et al. Adjuvant nivolumab in high-risk muscle-invasive urothelial carcinoma: expanded efficacy from CheckMate 274. J. Clin. Oncol. 43, 15–21 (2024).
Apolo Andrea, B. et al. Adjuvant pembrolizumab versus observation in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 392, 45–55 (2025).
Bellmunt, J. et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 22, 525–537 (2021).
Lindskrog, S. V. et al. Circulating tumor DNA analysis in advanced urothelial carcinoma: insights from biological analysis and extended clinical follow-up. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.Ccr-23-1860 (2023).
Ben-David, R. et al. Longitudinal tumor-informed circulating tumor DNA status predicts disease upstaging and poor prognosis for patients undergoing radical cystectomy. Eur. Urol. Oncol. https://doi.org/10.1016/j.euo.2024.03.002 (2024).
Christensen, E. et al. Early detection of metastatic relapse and monitoring of therapeutic efficacy by ultra-deep sequencing of plasma cell-free DNA in patients with urothelial bladder carcinoma. J. Clin. Oncol. 37, 1547–1557 (2019).
van Dorp, J. et al. High- or low-dose preoperative ipilimumab plus nivolumab in stage III urothelial cancer: the phase 1B NABUCCO trial. Nat. Med. 29, 588–592 (2023).
Powles, T. et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 25, 1706–1714 (2019).
Powles, T. et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature 595, 432–437 (2021).
Powles, T. et al. Updated overall survival by circulating tumor DNA status from the phase 3 IMvigor010 trial: adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma. Eur. Urol. 85, 114–122 (2024).
Galsky, M. D. et al. Adjuvant nivolumab versus placebo for high-risk muscle-invasive urothelial carcinoma: 5-year efficacy and ctDNA results from CheckMate 274. Ann. Oncol. https://doi.org/10.1016/j.annonc.2025.09.139 (2025).
Bjerggaard Jensen, J. et al. 1960O Identification of bladder cancer patients that could benefit from early post-cystectomy immunotherapy based on serial circulating tumour DNA (ctDNA) testing: preliminary results from the TOMBOLA trial. Ann. Oncol. 35, S1133 (2024).
Dyrskjøt, L. et al. Abstract CT101: ctDNA-guided immunotherapy following cystectomy in patients with urothelial carcinoma: preliminary results from the TOMBOLA trial. Cancer Res. 85, CT101 (2025).
Powles, T. et al. ctDNA-guided adjuvant atezolizumab in muscle-invasive bladder cancer. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa2511885 (2025).
Bajorin, D. F. et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 384, 2102–2114 (2021).
Bellmunt, J. et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N. Engl. J. Med. 376, 1015–1026 (2017).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492 (2017).
Szabados, B. et al. Final results of neoadjuvant atezolizumab in cisplatin-ineligible patients with muscle-invasive urothelial cancer of the bladder. Eur. Urol. 82, 212–222 (2022).
Rosenberg, J. E. et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387, 1909–1920 (2016).
Necchi, A. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36, 3353–3360 (2018).
van Dijk, N. et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat. Med. 26, 1839–1844 (2020).
Plimack, E. R. et al. Correlative analysis of ATM, RB1, ERCC2, and FANCC mutations and pathologic complete response after neoadjuvant chemotherapy in patients with muscle-invasive bladder cancer: results from the SWOG S1314 trial. Eur. Urol. 86, 297–300 (2024).
Seiler, R. et al. Impact of molecular subtypes in muscle-invasive bladder cancer on predicting response and survival after neoadjuvant chemotherapy. Eur. Urol. 72, 544–554 (2017).
Sjödahl, G. et al. Different responses to neoadjuvant chemotherapy in urothelial carcinoma molecular subtypes. Eur. Urol. 81, 523–532 (2022).
Taber, A. et al. Molecular correlates of cisplatin-based chemotherapy response in muscle invasive bladder cancer by integrated multi-omics analysis. Nat. Commun. 11, 4858 (2020).
Groeneveld, C. S. et al. Basal/squamous and mixed subtype bladder cancers present poor outcomes after neoadjuvant chemotherapy in the VESPER trial. Ann. Oncol. 36, 89–98 (2025).
Crabb, S. J. et al. Use of gene expression patterns to identify unique molecular subtypes in muscle invasive bladder cancer: GUSTO. J. Clin. Oncol. 42, TPS4621 (2024).
Horgan, D. et al. From theory to practice: implementing next-generation sequencing and public health genomics in healthcare systems. Crit. Rev. Oncol./Hematol. 201, 104433 (2024).
Vandekerkhove, G. et al. Toward informed selection and interpretation of clinical genomic tests in prostate cancer. JCO Precis. Oncol. 8, e2300654 (2024).
Miyake, M., Fujimoto, K. & Hirao, Y. Active surveillance for nonmuscle invasive bladder cancer. Investig. Clin. Urol. 57, S4–S13 (2016).
Hurle, R. et al. Active surveillance for low risk nonmuscle invasive bladder cancer: a confirmatory and resource consumption study from the BIAS project. J. Urol. 199, 401–406 (2018).
Linscott, J. et al. Tumor fraction and copy number burden from urinary cell-free tumor DNA (utDNA) to predict minimal residual disease prior to repeat-transurethral resection in high-risk non-muscle invasive bladder cancer (HR-NMIBC). J. Clin. Oncol. 42, 4600 (2024).
Linscott, J. A. et al. From detection to cure — emerging roles for urinary tumor DNA (utDNA) in bladder cancer. Curr. Oncol. Rep. 26, 945–958 (2024).
de Jong, F. C. et al. Non-muscle-invasive bladder cancer molecular subtypes predict differential response to intravesical Bacillus Calmette–Guérin. Sci. Transl. Med. 15, eabn4118 (2023).
Ferro, M. et al. Artificial intelligence in the advanced diagnosis of bladder cancer — comprehensive literature review and future advancement. Diagnostics (Basel) https://doi.org/10.3390/diagnostics13132308 (2023).
Packiam, V. T. et al. Presence of an artificial intelligence-powered predictive biomarker is associated with a poor response to intravesical Bacillus Calmette–Guerin but not to intravesical sequential gemcitabine/docetaxel in patients with high-grade non-muscle-invasive bladder cancer. Eur. Urol. Oncol. https://doi.org/10.1016/j.euo.2025.04.006 (2025).
Khoraminia, F. et al. Artificial intelligence in digital pathology for bladder cancer: hype or hope? A systematic review. Cancers (Basel). https://doi.org/10.3390/cancers15184518 (2023).
St-Laurent, M.-P. et al. Increasing life expectancy in patients with genitourinary malignancies: impact of treatment burden on disease management and quality of life. Eur. Urol. https://doi.org/10.1016/j.eururo.2024.11.026 (2024).
Casilla-Lennon, M. M. et al. Financial toxicity among patients with bladder cancer: reasons for delay in care and effect on quality of life. J. Urol. 199, 1166–1173 (2018).
Contieri, R. et al. The financial burden of guideline-recommended cancer medications for metastatic urothelial carcinoma. Eur. Urol. Focus 10, 662–665 (2024).
Li, A. et al. Cost-effectiveness of first-line enfortumab vedotin in addition to pembrolizumab for metastatic urothelial carcinoma in the United States. Front. Immunol. 15, 1464092 (2024).
Scilipoti, P. et al. The financial burden of localized and metastatic bladder cancer. Eur. Urol. 87, 536–550 (2025).
Irani, J. et al. BTA stat and BTA TRAK: a comparative evaluation of urine testing for the diagnosis of transitional cell carcinoma of the bladder. Eur. Urol. 35, 89–92 (1999).
Mian, C. et al. Immunocyt: a new tool for detecting transitional cell cancer of the urinary tract. J. Urol. 161, 1486–1489 (1999).
Têtu, B., Tiguert, R., Harel, F. & Fradet, Y. ImmunoCyt/uCyt+ improves the sensitivity of urine cytology in patients followed for urothelial carcinoma. Mod. Pathol. 18, 83–89 (2005).
Lotan, Y. & Shariat, S. F. Impact of risk factors on the performance of the nuclear matrix protein 22 point-of-care test for bladder cancer detection. BJU Int. 101, 1362–1367 (2008).
Dudderidge, T. et al. A novel, non-invasive test enabling bladder cancer detection in urine sediment of patients presenting with haematuria — a prospective multicentre performance evaluation of ADXBLADDER. Eur. Urol. Oncol. 3, 42–46 (2020).
Roupret, M. et al. Diagnostic accuracy of MCM5 for the detection of recurrence in nonmuscle invasive bladder cancer followup: a blinded, prospective cohort, multicenter European study. J. Urol. 204, 685–690 (2020).
Hirasawa, Y. et al. Diagnostic performance of Oncuria™, a urinalysis test for bladder cancer. J. Transl. Med. 19, 141 (2021).
Zhang, Z. et al. GG02-30 Oncuria-monitor, a non-invasive test for the detection of recurrent bladder cancer: cross sectional performance evaluation from a multicenter study. J. Urol. https://doi.org/10.1097/01.JU.0001109860.96928.64.30 (2025).
Dimashkieh, H. et al. Evaluation of urovysion and cytology for bladder cancer detection: a study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 121, 591–597 (2013).
Harvey, J. C. et al. Analytical validation of Cxbladder(®) detect, triage, and monitor: assays for detection and management of urothelial carcinoma. Diagnostics (Basel) https://doi.org/10.3390/diagnostics14182061 (2024).
Kavalieris, L. et al. Performance characteristics of a multigene urine biomarker test for monitoring for recurrent urothelial carcinoma in a multicenter study. J. Urol. 197, 1419–1426 (2017).
Fleshner, N. et al. Consistent performance of bladder epicheck for NMIBC surveillance in the USA and European pivotal studies and in real world studies. Urol. Oncol. Semin. Original Investig. 42, S63 (2024).
Batista, R. et al. Validation of a novel, sensitive, and specific urine-based test for recurrence surveillance of patients with non-muscle-invasive bladder cancer in a comprehensive multicenter study. Front. Genet. 10, 1237 (2019).
Piatti, P. et al. Clinical evaluation of Bladder CARE, a new epigenetic test for bladder cancer detection in urine samples. Clin. Epigenet. 13, 84 (2021).
Valenberg, F. J. P. V. et al. Prospective validation of an mRNA-based urine test for surveillance of patients with bladder cancer. Eur. Urol. 75, 853–860 (2019).
Valenberg, F. et al. Validation of an mRNA-based urine test for the detection of bladder cancer in patients with haematuria. Eur. Urol. Oncol. 4, 93–101 (2021).
van Kessel, K. E. et al. Validation of a DNA methylation-mutation urine assay to select patients with hematuria for cystoscopy. J. Urol. 197, 590–595 (2017).
Zeng, S. et al. Noninvasive detection of urothelial carcinoma by cost-effective low-coverage whole-genome sequencing from urine-exfoliated cell DNA. Clin. Cancer Res. 26, 5646–5654 (2020).
Springer, S. U. et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife 7, e32143 (2018).
Nikkola, J. et al. Sensitive detection of urothelial cancer via high-volume urine DNA analysis. Eur. Urol. 87, 86–88 (2025).
Salari, K. et al. Development and multicenter case–control validation of urinary comprehensive genomic profiling for urothelial carcinoma diagnosis, surveillance, and risk prediction. Clin. Cancer Res. https://doi.org/10.1158/1078-0432.CCR-23-0570 (2023).
Ward, D. G. et al. Highly sensitive and specific detection of bladder cancer via targeted ultra-deep sequencing of urinary DNA. Eur. Urol. Oncol. 6, 67–75 (2023).
US Food and Drug Administration. 761336Orig1s000 Multi-discipline review https://www.accessdata.fda.gov/drugsatfda_docs/nda/2024/761336Orig1s000MultidisciplineR.pdf (2024).
Jacob, J. M. et al. TAR-200 monotherapy in patients with Bacillus Calmette–Guérin-unresponsive high-risk non-muscle-invasive bladder cancer carcinoma in situ: 1-year durability and patient-reported outcomes from SunRISe-1. J. Urol. 213, e2 (2025).
van der Heijden, M. S. et al. LBA85 TAR-200 +/- cetrelimab (CET) and CET alone in patients (pts) with Bacillus Calmette–Guérin-unresponsive (BCG UR) high-risk non-muscle-invasive bladder cancer (HR NMIBC): updated results from SunRISe-1 (SR-1). Ann. Oncol. 35, S1272–S1273 (2024).
Tyson, M. D. et al. P2-02 pivotal results from BOND-003: a phase 3, single-arm study of intravesical cretostimogene grenadenorepvec for the treatment of high risk, BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Urol. 211, e1 (2024).
Uchio, E. M. et al. A phase 3, single-arm study of CG0070 in subjects with nonmuscle invasive bladder cancer (NMIBC) unresponsive to Bacillus Calmette–Guerin (BCG). J. Clin. Oncol. 40, TPS598 (2022).
Kulkarni, G. S. et al. A phase II clinical study of intravesical photo dynamic therapy in patients with BCG-unresponsive NMIBC (interim analysis). J. Clin. Oncol. 41, 528–528 (2023).
Bryce, R., Tosone, C., Sullivan, J. C., Linback, T. & Steinberg, G. D. A phase 1/2 study of EG-70 (detalimogene voraplasmid) intravesical monotherapy for patients with BCG-unresponsive non-muscle invasive bladder cancer with carcinoma in situ. J. Clin. Oncol. 42, TPS4626 (2024).
Sternberg, C. N. et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 16, 76–86 (2015).
Ben-David, R. et al. Undetectable pre-radical cystectomy circulating tumour DNA status predicts improved oncological outcomes. BJU Int. 135, 473–480 (2025).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks M. Moschini, who co-reviewed with P. Scilipoti; B.W.G. van Rhijn; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
St-Laurent, MP., Nikkola, J., Tomiyama, E. et al. Advances in the management of localized bladder cancers. Nat Rev Clin Oncol (2026). https://doi.org/10.1038/s41571-025-01104-z
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41571-025-01104-z