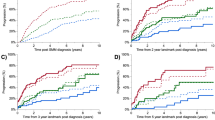
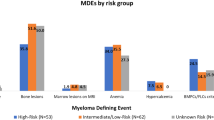
Abstract
New therapies have markedly improved survival outcomes for patients with multiple myeloma (MM). However, the onset of active disease, as defined by the 2014 International Myeloma Working Group SLiM–CRAB criteria, is often linked to substantial and irreversible morbidity. MM always develops from an asymptomatic precursor state called smouldering multiple myeloma (SMM). The clinical trajectory of SMM varies considerably; low-risk SMM often has an indolent course, similar to monoclonal gammopathy of undetermined significance, whereas nearly half of the subset of patients with high-risk SMM have progression to symptomatic MM within 2 years. Highly active treatments for MM, which remains an incurable disease, are being investigated for the management of SMM, with the aim of delaying or even preventing such progression. Both early therapeutic intervention and active surveillance are reasonable management options for patients with high-risk SMM, with decisions individualized through a detailed risk–benefit discussion with the patient. In this Review, we discuss current approaches for diagnostic evaluation, risk stratification and management of SMM, as well as future challenges and emerging opportunities in the field.
Key points
-
Within the first 5 years following diagnosis, smouldering multiple myeloma (SMM) has a tenfold higher risk of progression to MM compared with monoclonal gammopathy of undetermined significance.
-
The clinical course of SMM is heterogeneous; high-risk SMM is associated with a 40–50% risk of progression to MM within the first 2 years of diagnosis.
-
Advanced imaging, especially PET–CT and MRI, is crucial in the diagnosis of SMM, to rule out overt MM and enhance prognostication.
-
Current risk-stratification tools for SMM, although validated and reproducible, are suboptimal; new approaches based on the disease biology are needed to enhance prognostication.
-
For patients with high-risk SMM, both early therapeutic intervention and active surveillance are reasonable management options, with decisions individualized through a detailed risk–benefit discussion with the patient.
-
Rational end point selection, focused on improving long-term patient outcomes, is key for future clinical trial strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Joseph, N. S. et al. Quadruplet therapy for newly diagnosed myeloma: comparative analysis of sequential cohorts with triplet therapy lenalidomide, bortezomib and dexamethasone (RVd) versus daratumamab with RVD (DRVd) in transplant-eligible patients. Blood Cancer J. 14, 159 (2024).
Binder, M. et al. Mortality trends in multiple myeloma after the introduction of novel therapies in the United States. Leukemia 36, 801–808 (2022).
Huang, J. et al. The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 9, e670–e677 (2022).
Siegel, R. L., Kratzer, T. B., Giaquinto, A. N., Sung, H. & Jemal, A. Cancer statistics, 2025. CA Cancer J. Clin. 75, 10–45 (2025).
Cowan, A. J. et al. Global burden of multiple myeloma: a systematic analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 4, 1221–1227 (2018).
Kumar, S. K. et al. Multiple myeloma. Nat. Rev. Dis. Primers 3, 17046 (2017).
Landgren, O. et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood 113, 5412–5417 (2009).
Rajkumar, S. V. et al. International Myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 15, e538–e548 (2014).
Kyle, R. A. et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 378, 241–249 (2018).
Kyle, R. A. et al. Long-term follow-up of IgM monoclonal gammopathy of undetermined significance. Blood 102, 3759–3764 (2003).
Rajkumar, S. V., Larson, D. & Kyle, R. A. Diagnosis of smoldering multiple myeloma. N. Engl. J. Med. 365, 474–475 (2011).
Raje, N. & Yee, A. J. How we approach smoldering multiple myeloma. J. Clin. Oncol. 38, 1119–1125 (2020).
Thorsteinsdóttir, S. et al. Prevalence of smoldering multiple myeloma based on nationwide screening. Nat. Med. 29, 467–472 (2023).
Kyle, R. A. et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N. Engl. J. Med. 356, 2582–2590 (2007).
Rajkumar, S. V., Kumar, S., Lonial, S. & Mateos, M. V. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 12, 129 (2022).
Rajkumar, S. V., Bergsagel, P. L. & Kumar, S. Smoldering multiple myeloma: observation versus control versus cure. Hematol. Oncol. Clin. North Am. 38, 293–303 (2024).
Visram, A., Cook, J. & Warsame, R. Smoldering multiple myeloma: evolving diagnostic criteria and treatment strategies. Hematol. Am. Soc. Hematol. Educ. Program 2021, 673–681 (2021).
Lakshman, A. et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 8, 59 (2018).
Mateos, M.-V. et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 10, 102 (2020).
Visram, A. et al. Monoclonal proteinuria predicts progression risk in asymptomatic multiple myeloma with a free light chain ratio ≥100. Leukemia 36, 1429–1431 (2022).
Mangiacavalli, S. et al. Monoclonal gammopathy of undetermined significance: a new proposal of workup. Eur. J. Haematol. 91, 356–360 (2013).
Kyle, R. A. et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia 24, 1121–1127 (2010).
Zamagni, E., Cavo, M., Fakhri, B., Vij, R. & Roodman, D. Bones in multiple myeloma: imaging and therapy. Am. Soc. Clin. Oncol. Educ. Book 38, 638–646 (2018).
Merz, M. et al. Predictive value of longitudinal whole-body magnetic resonance imaging in patients with smoldering multiple myeloma. Leukemia 28, 1902–1908 (2014).
Zamagni, E. et al. Standardization of 18F-FDG–PET/CT according to Deauville criteria for metabolic complete response definition in newly diagnosed multiple myeloma. J. Clin. Oncol. 39, 116–125 (2021).
Hill, E. et al. Diagnostic performance of (18) F-FDG-PET/CT compared to standard skeletal survey for detecting bone destruction in smouldering multiple myeloma: time to move forward. Br. J. Haematol. 193, 125–128 (2021).
Zamagni, E. et al. 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia 30, 417–422 (2016).
Hillengass, J. et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J. Clin. Oncol. 28, 1606–1610 (2010).
Caers, J. et al. The role of positron emission tomography-computed tomography and magnetic resonance imaging in diagnosis and follow up of multiple myeloma. Haematologica 99, 629–637 (2014).
Ludwig, H., Kainz, S., Schreder, M., Zojer, N. & Hinke, A. SLiM CRAB criteria revisited: temporal trends in prognosis of patients with smoldering multiple myeloma who meet the definition of ‘biomarker-defined early multiple myeloma’ — a systematic review with meta-analysis. eClinicalMedicine 58, 101910 (2023).
Akhlaghi, T. et al. Evaluating serum free light chain ratio as a biomarker in multiple myeloma. Haematologica 110, 493–497 (2025).
Maura, F. et al. Genomics define malignant transformation in myeloma precursor conditions. J. Clin. Oncol. 8, Jco2501733 (2025).
Pérez-Persona, E. et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 110, 2586–2592 (2007).
Dispenzieri, A. et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood 111, 785–789 (2008).
Cowan, A. et al. Personalised progression prediction in patients with monoclonal gammopathy of undetermined significance or smouldering multiple myeloma (PANGEA): a retrospective, multicohort study. Lancet Haematol. 10, e203–e212 (2023).
Ravi, P. et al. Evolving changes in disease biomarkers and risk of early progression in smoldering multiple myeloma. Blood Cancer J. 6, e454 (2016).
Fernández de Larrea, C. et al. Evolving M-protein pattern in patients with smoldering multiple myeloma: impact on early progression. Leukemia 32, 1427–1434 (2018).
Visram, A. et al. Assessing the prognostic utility of smoldering multiple myeloma risk stratification scores applied serially post diagnosis. Blood Cancer J. 11, 186 (2021).
Bianchi, G. et al. High levels of peripheral blood circulating plasma cells as a specific risk factor for progression of smoldering multiple myeloma. Leukemia 27, 680–685 (2013).
Sanoja-Flores, L. et al. Next generation flow for minimally-invasive blood characterization of MGUS and multiple myeloma at diagnosis based on circulating tumor plasma cells (CTPC). Blood Cancer J. 8, 117 (2018).
Uehara, A. et al. Different patterns of antigen expression in bone marrow and circulating tumor plasma cells in patients with MGUS and smoldering multiple myeloma. Blood 144, 4682 (2024).
Aljama, M. A. et al. Plasma cell proliferative index is an independent predictor of progression in smoldering multiple myeloma. Blood Adv. 2, 3149–3154 (2018).
Termini, R. et al. Circulating tumor and immune cells for minimally invasive risk stratification of smoldering multiple myeloma. Clin. Cancer Res. 28, 4771–4781 (2022).
Paiva, B. et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 122, 3591–3598 (2013).
Vigliotta, I. et al. Circulating multiple myeloma cells (CMMCs) as prognostic and predictive markers in multiple myeloma and smouldering MM patients. Cancers 16, 2929 (2024).
Bernhard, G. et al. Circulating tumor DNA as a liquid biopsy in plasma cell dyscrasias. Haematologica 103, e245–e248 (2018).
Martello, M. et al. High level of circulating cell-free tumor DNA at diagnosis correlates with disease spreading and defines multiple myeloma patients with poor prognosis. Blood Cancer J. 14, 208 (2024).
Kastritis, E. et al. Patterns of progression among 427 smoldering myeloma patients diagnosed after 2014: importance of monitoring. Blood Adv. https://doi.org/10.1182/bloodadvances.2025016083 (2025).
Bergsagel, P. L. & Kuehl, W. M. Molecular pathogenesis and a consequent classification of multiple myeloma. J. Clin. Oncol. 23, 6333–6338 (2005).
Dhodapkar, M. V. The immune system in multiple myeloma and precursor states: lessons and implications for immunotherapy and interception. Am. J. Hematol. 98, S4–S12 (2023).
Fonseca, R. et al. International Myeloma Working Group molecular classification of multiple myeloma: spotlight review. Leukemia 23, 2210–2221 (2009).
Alberge, J.-B. et al. Genomic landscape of multiple myeloma and its precursor conditions. Nat. Genet. 57, 1493–1503 (2025).
Maura, F. & Bergsagel, P. L. Targeting the tumor and the immune system in smoldering multiple myeloma. N. Engl. J. Med. 392, 1858–1860 (2025).
Aktas-Samur, A. et al. Differentiating between MM like smoldering myeloma (SMM) and non-progressor SMM. Blood 144, 1018 (2024).
Bolli, N. et al. Genomic patterns of progression in smoldering multiple myeloma. Nat. Commun. 9, 3363 (2018).
Bustoros, M. et al. Genomic profiling of smoldering multiple myeloma identifies patients at a high risk of disease progression. J. Clin. Oncol. 38, 2380–2389 (2020).
Bustoros, M. et al. Genetic subtypes of smoldering multiple myeloma are associated with distinct pathogenic phenotypes and clinical outcomes. Nat. Commun. 13, 3449 (2022).
Oben, B. et al. Whole-genome sequencing reveals progressive versus stable myeloma precursor conditions as two distinct entities. Nat. Commun. 12, 1861 (2021).
Boyle, E. M. et al. The molecular make up of smoldering myeloma highlights the evolutionary pathways leading to multiple myeloma. Nat. Commun. 12, 293 (2021).
Aktas Samur, A. et al. Genomically smoldering multiple myeloma is not a distinct entity but a collection of monoclonal gammopathy of undetermined significance or multiple myeloma. J. Clin. Oncol. https://doi.org/10.1200/JCO-25-00289 (2025).
Misund, K. et al. MYC dysregulation in the progression of multiple myeloma. Leukemia 34, 322–326 (2020).
Medina-Herrera, A. et al. The genomic profiling of high-risk smoldering myeloma patients treated with an intensive strategy unveils potential markers of resistance and progression. Blood Cancer J. 14, 74 (2024).
Dang, M. et al. Single cell clonotypic and transcriptional evolution of multiple myeloma precursor disease. Cancer Cell 41, 1032–1047.e4 (2023).
Ledergor, G. et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 24, 1867–1876 (2018).
Dutta, A. K. et al. MinimuMM-seq: genome sequencing of circulating tumor cells for minimally invasive molecular characterization of multiple myeloma pathology. Cancer Discov. 13, 348–363 (2023).
Lightbody, E. D. et al. SWIFT-seq enables comprehensive single-cell transcriptomic profiling of circulating tumor cells in multiple myeloma and its precursors. Nat. Cancer 6, 1595–1611 (2025).
Walker, B. A. et al. Aberrant global methylation patterns affect the molecular pathogenesis and prognosis of multiple myeloma. Blood 117, 553–562 (2011).
Heuck, C. J. et al. Myeloma is characterized by stage-specific alterations in DNA methylation that occur early during myelomagenesis. J. Immunol. 190, 2966–2975 (2013).
Liu, R. et al. Co-evolution of tumor and immune cells during progression of multiple myeloma. Nat. Commun. 12, 2559 (2021).
Bailur, J. K. et al. Early alterations in stem-like/resident T cells, innate and myeloid cells in the bone marrow in preneoplastic gammopathy. JCI Insight 5, e127807 (2019).
Schinke, C. et al. Characterizing the role of the immune microenvironment in multiple myeloma progression at a single-cell level. Blood Adv. 6, 5873–5883 (2022).
Kini Bailur, J. et al. Changes in bone marrow innate lymphoid cell subsets in monoclonal gammopathy: target for IMiD therapy. Blood Adv. 1, 2343–2347 (2017).
Guillerey, C. et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J. Clin. Investig. 125, 2077–2089 (2015).
Paiva, B. et al. Immune status of high-risk smoldering multiple myeloma patients and its therapeutic modulation under LenDex: a longitudinal analysis. Blood 127, 1151–1162 (2016).
Zavidij, O. et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat. Cancer 1, 493–506 (2020).
Kawano, Y. et al. Blocking IFNAR1 inhibits multiple myeloma-driven Treg expansion and immunosuppression. J. Clin. Invest. 128, 2487–2499 (2018).
Baertsch, M.-A. et al. Spatial dissection of the bone marrow microenvironment in multiple myeloma by high dimensional multiplex tissue imaging. Blood 142, 85 (2023).
Kim, J. et al. Macrophages and mesenchymal stromal cells support survival and proliferation of multiple myeloma cells. Br. J. Haematol. 158, 336–346 (2012).
Scavelli, C. et al. Vasculogenic mimicry by bone marrow macrophages in patients with multiple myeloma. Oncogene 27, 663–674 (2008).
Sun, J. et al. Tumor-associated macrophages in multiple myeloma: advances in biology and therapy. J. Immunother. Cancer 10, e003975 (2022).
Costello, R. T. et al. Differential expression of natural killer cell activating receptors in blood versus bone marrow in patients with monoclonal gammopathy. Immunology 139, 338–341 (2013).
Bernal, M. et al. Changes in activatory and inhibitory natural killer (NK) receptors may induce progression to multiple myeloma: implications for tumor evasion of T and NK cells. Hum. Immunol. 70, 854–857 (2009).
Abe, M. et al. Osteoclasts enhance myeloma cell growth and survival via cell–cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood 104, 2484–2491 (2004).
An, G. et al. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: therapeutic implication. Blood 128, 1590–1603 (2016).
Jafari, A., Fairfield, H., Andersen, T. L. & Reagan, M. R. Myeloma–bone marrow adipocyte axis in tumour survival and treatment response. Br. J. Cancer 125, 775–777 (2021).
Hernandez, M., Shin, S., Muller, C. & Attané, C. The role of bone marrow adipocytes in cancer progression: the impact of obesity. Cancer Metastasis Rev. 41, 589–605 (2022).
Robinson, M. H. et al. Regulation of antigen-specific T cell infiltration and spatial architecture in multiple myeloma and premalignancy. J. Clin. Invest. 133, e167629 (2023).
Abdallah, N. H. et al. Mode of progression in smoldering multiple myeloma: a study of 406 patients. Blood Cancer J. 14, 9 (2024).
Werly, A. et al. Patterns of progression in a contemporary cohort of 447 patients with smoldering multiple myeloma. Blood Cancer J. 14, 176 (2024).
Landgren, O. et al. EVIDENCE meta-analysis: evaluating minimal residual disease as an intermediate clinical end point for multiple myeloma. Blood 144, 359–367 (2024).
Mateos, M.-V. et al. Curative strategy (GEM-CESAR) for high-risk smoldering myeloma (SMM): post-hoc analysis of sustained undetectable measurable residual disease (MRD). Blood 140, 292–294 (2022).
Kumar, S. K. et al. Fixed duration therapy with daratumumab, carfilzomib, lenalidomide and dexamethasone for high risk smoldering multiple myeloma — results of the ascent trial. Blood 140, 1830–1832 (2022).
Nadeem, O. et al. Immuno-PRISM: a randomized phase II platform study of bispecific antibodies in high-risk smoldering myeloma. Blood 142, 206 (2023).
Kazandjian, D. et al. Carfilzomib, lenalidomide, and dexamethasone followed by lenalidomide maintenance for prevention of symptomatic multiple myeloma in patients with high-risk smoldering myeloma: a phase 2 nonrandomized controlled trial. JAMA Oncol. 7, 1678–1685 (2021).
Broijl, A. et al. Results of the phase II randomized trial EMN15/HOVON147: carfilzomib–lenalidomide–dexamethasone vs lenalidomide-dexamethasone in patients with high-risk smoldering multiple myeloma. Blood 144, 676 (2024).
Nadeem, O. et al. Deeper response predicts better outcomes in high-risk-smoldering-myeloma: results of the I-PRISM phase II clinical trial. Nat. Commun. 16, 358 (2025).
Landgren, O. et al. Efficacy and safety of daratumumab in intermediate/high-risk smoldering multiple myeloma: final analysis of CENTAURUS. Blood 145, 1658–1669 (2025).
Sklavenitis-Pistofidis, R. et al. Immune biomarkers of response to immunotherapy in patients with high-risk smoldering myeloma. Cancer Cell 40, 1358–1373.e8 (2022).
Rögnvaldsson, S. et al. Iceland screens, treats, or prevents multiple myeloma (iStopMM): a population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies. Blood Cancer J. 11, 94 (2021).
Mateos, M. V. et al. Lenalidomide–dexamethasone versus observation in high-risk smoldering myeloma after 12 years of median follow-up time: a randomized, open-label study. Eur. J. Cancer 174, 243–250 (2022).
Mateos, M. V. et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N. Engl. J. Med. 369, 438–447 (2013).
Lonial, S. et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma. J. Clin. Oncol. 38, 1126–1137 (2020).
Dimopoulos, M. A. et al. Daratumumab or active monitoring for high-risk smoldering multiple myeloma. N. Engl. J. Med. 392, 1777–1788 (2025).
Witzig, T. E. et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia 27, 220–225 (2013).
Musto, P. et al. A multicenter, randomized clinical trial comparing zoledronic acid versus observation in patients with asymptomatic myeloma. Cancer 113, 1588–1595 (2008).
D’Arena, G. et al. Pamidronate versus observation in asymptomatic myeloma: final results with long-term follow-up of a randomized study. Leukemia Lymphoma 52, 771–775 (2011).
Hjorth, M. et al. Initial versus deferred melphalan-prednisone therapy for asymptomatic multiple myeloma stage I-a randomized study. Myeloma Group of Western Sweden. Eur. J. Haematol. 50, 95–102 (1993).
Abdallah, N. et al. Phase III randomized trial of Thal+ZLD versus ZLD in patients with asymptomatic multiple myeloma — updated results after 18-year follow-up. Leukemia 38, 1169–1171 (2024).
Lonial, S. et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 387, 1551–1560 (2016).
Landgren, C. O. et al. Daratumumab monotherapy for patients with intermediate-risk or high-risk smoldering multiple myeloma: a randomized, open-label, multicenter, phase 2 study (CENTAURUS). Leukemia 34, 1840–1852 (2020).
Meeting of the Oncologic Drugs Advisory Committee (ODAC). FDA. youtube.com https://www.youtube.com/live/iSGFdhMgh1E (2025).
US Food and Drug Administration. FDA approves daratumumab and hyaluronidase-fihj for high-risk smoldering multiple myeloma. fda.gov https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-daratumumab-and-hyaluronidase-fihj-high-risk-smoldering-multiple-myeloma (2025).
European Medicines Agency. Darzalex. ema.europa.eu https://www.ema.europa.eu/en/medicines/human/variation/darzalex (2025).
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Multiple Myeloma, version 1 (2026). nccn.org https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf. (accessed 1 July 2025).
Dimopoulos, M. A., Voorhees, P. M. & Rajkumar, S. V. Daratumumab for high-risk smoldering multiple myeloma. N. Engl. J. Med. 393, 616–617 (2025).
Sonneveld, P. et al. Daratumumab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N. Engl. J. Med. 390, 301–313 (2023).
Usmani, S. Z. et al. Final analysis of carfilzomib, dexamethasone, and daratumumab vs carfilzomib and dexamethasone in the CANDOR study. Blood Adv. 7, 3739–3748 (2023).
Dimopoulos, M. A., Voorhees, P. M. & Rajkumar, S. V. Daratumumab for high-risk smoldering multiple myeloma. N. Engl. J. Med. 393, 617 (2025).
Kazandjian, D. et al. Genomic profiling to contextualize the results of intervention for smoldering multiple myeloma. Clin. Cancer Res. 30, 4482–4490 (2024).
Diamond, B. et al. Biallelic antigen escape is a mechanism of resistance to anti-CD38 antibodies in multiple myeloma. Blood 146, 1575–1585 (2025).
Landgren, O. et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12 482 persons from the National Health and Nutritional Examination Survey. Leukemia 28, 1537–1542 (2014).
Kazandjian, D. Multiple myeloma epidemiology and survival: a unique malignancy. Semin. Oncol. 43, 676–681 (2016).
Bertamini, L. et al. Serum free light chains in a racially diverse population including African Americans and populations from South Africa. Blood 145, 840–849 (2025).
Mateos, M.-V. et al. Curativestategy (GEM-CESAR) for high-risk smoldering myeloma (SMM): carfilzomib, lenalidomide and dexamethasone (KRd) as induction followed by HDT-ASCT, consolidation with Krd and maintenance with Rd. Blood 132, 2142 (2018).
Mateos, M.-V. et al. Curative strategy for high-risk smoldering myeloma: carfilzomib, lenalidomide, and dexamethasone (KRd) followed by transplant, KRd consolidation, and Rd maintenance. J. Clin. Oncol. 42, 3247–3256 (2024).
Nadeem, O. et al. Phase II trial of daratumumab, bortezomib, lenalidomide and dexamethasone in high-risk smoldering multiple myeloma. Blood 144, 3360 (2024).
Cohen, A. D. et al. How to train your T cells: overcoming immune dysfunction in multiple myeloma. Clin. Cancer Res. 26, 1541–1554 (2020).
Raab, M. S. et al. Phase 2 study of teclistamab-based induction regimens in patients with transplant-eligible (TE) newly diagnosed multiple myeloma (NDMM): results from the GMMG-HD10/DSMM-XX (MajesTEC-5) trial. Blood 144, 493 (2024).
Paul, B. et al. Idecabtagene vicleucel (Ide-cel) in patients (Pts) with newly diagnosed multiple myeloma (NDMM) with an inadequate response to front-line autologous stem cell transplantation (ASCT): KarMMa-2 cohort 2c extended follow-up. Blood 144, 3388 (2024).
Martín, A. et al. Pamidronate induces bone formation in patients with smouldering or indolent myeloma, with no significant anti-tumour effect. Br. J. Haematol. 118, 239–242 (2002).
Lipof, J., Baran, A. M., Passero, F. C., Burrows, K. & Lipe, B. Denosumab for smoldering multiple myeloma: rates of osteoporosis and change in lowest T-score after 12 months of treatment. J. Clin. Oncol. 41, e20057 (2023).
Roy, S. & Trinchieri, G. Microbiota: a key orchestrator of cancer therapy. Nat. Rev. Cancer 17, 271–285 (2017).
Scher, J. U. & Abramson, S. B. The microbiome and rheumatoid arthritis. Nat. Rev. Rheumatol. 7, 569–578 (2011).
Halfvarson, J. et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2, 17004 (2017).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Peng, Z. et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 8, 1251–1261 (2020).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
Baruch, E. N. et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 371, 602–609 (2021).
Spencer, J. & Sollid, L. M. The human intestinal B-cell response. Mucosal Immunol. 9, 1113–1124 (2016).
Jian, X. et al. Alterations of gut microbiome accelerate multiple myeloma progression by increasing the relative abundances of nitrogen-recycling bacteria. Microbiome 8, 74 (2020).
Pianko, M. J. et al. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 3, 2040–2044 (2019).
Calcinotto, A. et al. Microbiota-driven interleukin-17-producing cells and eosinophils synergize to accelerate multiple myeloma progression. Nat. Commun. 9, 4832 (2018).
Shah, U. A. et al. A high-fiber dietary intervention (NUTRIVENTION) in precursor plasma cell disorders improves biomarkers of disease and may delay progression to myeloma. Blood 144, 671 (2024).
Joseph, J. M. et al. Dietary risk factors for monoclonal gammopathy of undetermined significance in a racially diverse population. Blood Adv. 8, 538–548 (2024).
Ghobrial, I. M. et al. Round table discussion on optimal clinical trial design in precursor multiple myeloma. Blood Cancer Discov. 5, 146–152 (2024).
Rajkumar, S. V., Landgren, O. & Mateos, M. V. Smoldering multiple myeloma. Blood 125, 3069–3075 (2015).
Rodriguez Otero, P. et al. Trial in progress: a phase 2 study of linvoseltamab for the treatment of high-risk smoldering multiple myeloma (LINKER-SMM1). Blood 142, 3393 (2023).
Acknowledgements
The work of S.V.R. is supported by the NIH (R01 and SPORE).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
S.K. has acted as a consultant or adviser (with all payments made to his institution) for AbbVie, Bristol Myers Squibb/Celgene, Genentech/Roche, Janssen Oncology/Johnson & Johnson, K36, Pfizer, Regeneron, Sanofi and Takeda; declares research funding (to his institution) from AbbVie, Allogene Therapeutics, Bristol Myers Squibb/Celgene, CARsgen Therapeutics, GlaxoSmithKline, Janssen Oncology/Johnson & Johnson, MedImmune, Novartis, Regeneron, Roche/Genentech, Sanofi and Takeda; and has received reimbursement for travel, accommodation and expenses from AbbVie, Beigene, Janssen/Johnson & Johnson and Pfizer. S.V.R. is Chair of the International Myeloma Foundation Board of Directors; has received honoraria for CME Lectures for Medscape; declares intellectual property and royalties as an author and section editor for UpToDate (covering topics on plasma cell disorders); and served as principal investigator of the AQUILA trial, and in that capacity has also presented on behalf of Johnson & Johnson to the FDA ODAC in support of the approval of daratumumab for high-risk smouldering multiple myeloma. S.Z. declares no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks O. Landgren and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zanwar, S., Kumar, S. & Rajkumar, S.V. Diagnosis, risk stratification and management of smouldering multiple myeloma. Nat Rev Clin Oncol (2026). https://doi.org/10.1038/s41571-026-01119-0
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41571-026-01119-0