Abstract
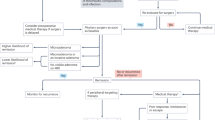
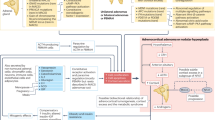
Cushing syndrome (CS) is a constellation of signs and symptoms caused by excessive exposure to exogenous or endogenous glucocorticoid hormones. Endogenous CS is caused by increased cortisol production by one or both adrenal glands (adrenal CS) or by elevated adrenocorticotropic hormone (ACTH) secretion from a pituitary tumour (Cushing disease (CD)) or non-pituitary tumour (ectopic ACTH secretion), which stimulates excessive cortisol production. CS is associated with severe multisystem morbidity, including impaired cardiovascular and metabolic function, infections and neuropsychiatric disorders, which notably reduce quality of life. Mortality is increased owing to pulmonary emboli, infection, myocardial infarction and cerebrovascular accidents. The clinical presentation is variable and because some CS signs and symptoms are common in the general population, the diagnosis might not be considered until many features have accumulated. Guidelines recommend screening patients with suspected CS with 24-h urine cortisol, bedtime salivary cortisol and/or 1 mg dexamethasone suppression test. Subsequently, determining the aetiology of CS is important as it affects management. The first-line therapy for all aetiologies of endogenous CS is surgical resection of the causal tissue, including corticotroph adenoma or ectopic tumour for ACTH-dependent CS or unilateral or bilateral adrenalectomy for adrenal CS. Second-line therapies include steroidogenesis inhibitors for any cause of CS, pituitary radiation (with or without steroidogenesis inhibitors) for CD, and bilateral adrenalectomy for ACTH-dependent causes of CS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lacroix, A., Feelders, R. A., Stratakis, C. A. & Nieman, L. K. Cushing’s syndrome. Lancet 386, 913–927 (2015).
Newell-Price, J., Trainer, P., Besser, M. & Grossman, A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr. Rev. 19, 647–672 (1998).
Nieman, L. K. et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 93, 1526–1540 (2008). These clinical practice guidelines outline an approach to the diagnosis of CS and suggest how to individualize the assessment based on the pitfalls of each test.
Tabarin, A. et al. Consensus statement by the French Society of Endocrinology (SFE) and French Society of Pediatric Endocrinology & Diabetology (SFEDP) on diagnosis of Cushing’s syndrome. Ann. Endocrinol. 83, 119–141 (2022).
Reincke, M. et al. Corticotroph tumor progression after bilateral adrenalectomy (Nelson’s syndrome): systematic review and expert consensus recommendations. Eur. J. Endocrinol. 184, P1–P16 (2021). This consensus paper reviews the presentation and management of corticotroph tumour progression.
Juhlin, C. C. et al. What did we learn from the molecular biology of adrenal cortical neoplasia? From histopathology to translational genomics. Endocr. Pathol. 32, 102–133 (2021).
Fassnacht, M. et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 189, G1–G42 (2023).
Alexandraki, K. I. & Grossman, A. B. The ectopic ACTH syndrome. Rev. Endocr. Metab. Disord. 11, 117–126 (2010). This paper provides an overview of the causes, evaluation and management of ectopic ACTH syndrome.
Hakami, O. A., Ahmed, S. & Karavitaki, N. Epidemiology and mortality of Cushing’s syndrome. Best. Pract. Res. Clin. Endocrinol. Metab. 35, 101521 (2021).
Giuffrida, G. et al. Global Cushing’s disease epidemiology: a systematic review and meta-analysis of observational studies. J. Endocrinol. Invest. 45, 1235–1246 (2022).
Broder, M. S., Neary, M. P., Chang, E., Cherepanov, D. & Ludlam, W. H. Incidence of Cushing’s syndrome and Cushing’s disease in commercially-insured patients <65 years old in the United States. Pituitary 18, 283–289 (2015).
Agustsson, T. T. et al. The epidemiology of pituitary adenomas in Iceland, 1955-2012: a nationwide population-based study. Eur. J. Endocrinol. 173, 655–664 (2015).
Ahn, C. H., Kim, J. H., Park, M. Y. & Kim, S. W. Epidemiology and comorbidity of adrenal Cushing syndrome: a nationwide cohort study. J. Clin. Endocrinol. Metab. 106, e1362–e1372 (2021).
Bolland, M. J. et al. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin. Endocrinol. 75, 436–442 (2011).
Etxabe, J. & Vazquez, J. A. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin. Endocrinol. 40, 479–484 (1994).
Lindholm, J. et al. Incidence and late prognosis of Cushing’s syndrome: a population-based study. J. Clin. Endocrinol. Metab. 86, 117–123 (2001).
Ragnarsson, O. et al. The incidence of Cushing’s disease: a nationwide Swedish study. Pituitary 22, 179–186 (2019).
Jing, Y. et al. Prevalence and characteristics of adrenal tumors in an unselected screening population : a cross-sectional study. Ann. Intern. Med. 175, 1383–1391 (2022).
Deutschbein, T. et al. Age-dependent and sex-dependent disparity in mortality in patients with adrenal incidentalomas and autonomous cortisol secretion: an international, retrospective, cohort study. Lancet Diabetes Endocrinol. 10, 499–508 (2022).
Kjellbom, A., Lindgren, O., Puvaneswaralingam, S., Londahl, M. & Olsen, H. Association between mortality and levels of autonomous cortisol secretion by adrenal incidentalomas: a cohort study. Ann. Intern. Med. 174, 1041–1049 (2021).
Prete, A. et al. Cardiometabolic disease burden and steroid excretion in benign adrenal tumors : a cross-sectional multicenter study. Ann. Intern. Med. 175, 325–334 (2022). This paper reviews data from more than 13,000 people with functioning and non-functioning adrenal masses and analyses the relationship between cortisol excess and cardiometabolic comorbidities.
Zavatta, G. et al. Mild autonomous cortisol secretion in adrenal incidentalomas and risk of fragility fractures: a large cross-sectional study. Eur. J. Endocrinol. 188, 343–352 (2023).
Society for Endocrinology. CRH stock availability update – Ferring Pharmaceuticals. Society for Endocrinology www.endocrinology.org/news/item/21098/crh-stock-availability-update-ferring-pharmaceuticals (2023).
Ntali, G., Hakami, O., Wattegama, M., Ahmed, S. & Karavitaki, N. Mortality of patients with Cushing’s disease. Exp. Clin. Endocrinol. Diabetes 129, 203–207 (2021).
Pivonello, R., De Martino, M. C., De Leo, M., Simeoli, C. & Colao, A. Cushing’s disease: the burden of illness. Endocrine 56, 10–18 (2017).
Dekkers, O. M. et al. Multisystem morbidity and mortality in Cushing’s syndrome: a cohort study. J. Clin. Endocrinol. Metab. 98, 2277–2284 (2013).
Valassi, E. et al. High mortality within 90 days of diagnosis in patients with Cushing’s syndrome: results from the ERCUSYN registry. Eur. J. Endocrinol. 181, 461–472 (2019).
Clayton, R. N. et al. Mortality in patients with Cushing’s disease more than 10 years after remission: a multicentre, multinational, retrospective cohort study. Lancet Diabetes Endocrinol. 4, 569–576 (2016).
Limumpornpetch, P. et al. The effect of endogenous Cushing syndrome on all-cause and cause-specific mortality. J. Clin. Endocrinol. Metab. 107, 2377–2388 (2022).
Yaneva, M., Kalinov, K. & Zacharieva, S. Mortality in Cushing’s syndrome: data from 386 patients from a single tertiary referral center. Eur. J. Endocrinol. 169, 621–627 (2013).
Ragnarsson, O. et al. Overall and disease-specific mortality in patients with Cushing disease: a Swedish nationwide study. J. Clin. Endocrinol. Metab. 104, 2375–2384 (2019).
Golounina, O. O. et al. Survival predictors in patients with ectopic ACTH syndrome [Russian]. Probl. Endokrinol. 68, 30–42 (2022).
Ilias, I. et al. Cushing’s syndrome due to ectopic corticotropin secretion: twenty years’ experience at the National Institutes of Health. J. Clin. Endocrinol. Metab. 90, 4955–4962 (2005).
Valassi, E. et al. The European Registry on Cushing’s syndrome: 2-year experience. Baseline demographic and clinical characteristics. Eur. J. Endocrinol. 165, 383–392 (2011).
Valassi, E. Clinical presentation and etiology of Cushing’s syndrome: data from ERCUSYN. J. Neuroendocrinol. 34, e13114 (2022).
Amodru, V. et al. Cushing’s syndrome in the elderly: data from the European Registry on Cushing’s syndrome. Eur. J. Endocrinol. 188, 395–406 (2023).
Frara, S. et al. Novel approaches to bone comorbidity in Cushing’s disease: an update. Pituitary 25, 754–759 (2022).
Guo, W. et al. Effect of hypercortisolism on bone mineral density and bone metabolism: a potential protective effect of adrenocorticotropic hormone in patients with Cushing’s disease. J. Int. Med. Res. 46, 492–503 (2018).
Isidori, A. M. et al. The ectopic adrenocorticotropin syndrome: clinical features, diagnosis, management, and long-term follow-up. J. Clin. Endocrinol. Metab. 91, 371–377 (2006).
Dogra, P. et al. High prevalence of frailty in patients with adrenal adenomas and adrenocortical hormone excess: a cross-sectional multi-centre study with prospective enrolment. Eur. J. Endocrinol. 189, 318–326 (2023).
Valassi, E. et al. Worse health-related quality of life at long-term follow-up in patients with Cushing’s disease than patients with cortisol producing adenoma. Data from the ERCUSYN. Clin. Endocrinol. 88, 787–798 (2018).
Beuschlein, F. et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N. Engl. J. Med. 370, 1019–1028 (2014).
Lodish, M. & Stratakis, C. A. A genetic and molecular update on adrenocortical causes of Cushing syndrome. Nat. Rev. Endocrinol. 12, 255–262 (2016).
Rege, J. et al. Targeted mutational analysis of cortisol-producing adenomas. J. Clin. Endocrinol. Metab. 107, e594–e603 (2022).
Wu, L. et al. Mutational landscape of non-functional adrenocortical adenomas. Endocr. Relat. Cancer 29, 521–532 (2022).
Kamilaris, C. D. C., Stratakis, C. A. & Hannah-Shmouni, F. Molecular genetic and genomic alterations in Cushing’s syndrome and primary aldosteronism. Front. Endocrinol. 12, 632543 (2021).
Bertherat, J. et al. Clinical, pathophysiologic, genetic, and therapeutic progress in primary bilateral macronodular adrenal hyperplasia. Endocr. Rev. 44, 567–628 (2023). This is a comprehensive review of PBMAH.
Lacroix, A. Extensive expertise in endocrinology: glucose-dependent insulinotropic peptide-dependent Cushing’s syndrome. Eur. J. Endocrinol. 188, R56–R72 (2023).
Lecoq, A. L. et al. Adrenal GIPR expression and chromosome 19q13 microduplications in GIP-dependent Cushing’s syndrome. JCI Insight 2, e92184 (2017).
St-Jean, M., Ghorayeb, N. E., Bourdeau, I. & Lacroix, A. Aberrant G-protein coupled hormone receptor in adrenal diseases. Best. Pract. Res. Clin. Endocrinol. Metab. 32, 165–187 (2018).
Cavalcante, I. P. et al. Primary bilateral macronodular adrenal hyperplasia: definitely a genetic disease. Nat. Rev. Endocrinol. 18, 699–711 (2022).
Assie, G. et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N. Engl. J. Med. 369, 2105–2114 (2013).
Lao, L. et al. ARMC5 is part of an RPB1-specific ubiquitin ligase implicated in adrenal hyperplasia. Nucleic Acids Res. 50, 6343–6367 (2022).
Okuno, Y., Fukuhara, A., Otsuki, M. & Shimomura, I. ARMC5-CUL3 E3 ligase targets full-length SREBF in adrenocortical tumors. JCI Insight 7, e151390 (2022).
Cavalcante, I. P. et al. Tumor suppressor gene ARMC5 controls adrenal redox state through NRF1 turnover. Endocr. Relat. Cancer 29, 615–624 (2022).
Chasseloup, F. et al. Loss of KDM1A in GIP-dependent primary bilateral macronodular adrenal hyperplasia with Cushing’s syndrome: a multicentre, retrospective, cohort study. Lancet Diabetes Endocrinol. 9, 813–824 (2021).
Larose, S. et al. Coexistence of myelolipoma and primary bilateral macronodular adrenal hyperplasia with GIP-dependent Cushing’s syndrome. Front. Endocrinol. 10, 618 (2019).
Vaczlavik, A. et al. KDM1A inactivation causes hereditary food-dependent Cushing syndrome. Genet. Med. 24, 374–383 (2022).
Bourdeau, I. et al. Primary pigmented nodular adrenocortical disease: paradoxical responses of cortisol secretion to dexamethasone occur in vitro and are associated with increased expression of the glucocorticoid receptor. J. Clin. Endocrinol. Metab. 88, 3931–3937 (2003).
Louiset, E. et al. The paradoxical increase in cortisol secretion induced by dexamethasone in primary pigmented nodular adrenocortical disease involves a glucocorticoid receptor-mediated effect of dexamethasone on protein kinase A catalytic subunits. J. Clin. Endocrinol. Metab. 94, 2406–2413 (2009).
Bram, Z. et al. PKA regulatory subunit 1A inactivating mutation induces serotonin signaling in primary pigmented nodular adrenal disease. JCI Insight 1, e87958 (2016).
Weigand, I. et al. Impact of USP8 gene mutations on protein deregulation in Cushing disease. J. Clin. Endocrinol. Metab. 104, 2535–2546 (2019).
Lidhar, K. et al. Low expression of the cell cycle inhibitor p27Kip1 in normal corticotroph cells, corticotroph tumors, and malignant pituitary tumors. J. Clin. Endocrinol. Metab. 84, 3823–3830 (1999).
Jordan, S., Lidhar, K., Korbonits, M., Lowe, D. G. & Grossman, A. B. Cyclin D and cyclin E expression in normal and adenomatous pituitary. Eur. J. Endocrinol. 143, R1–R6 (2000).
Armeni, E. & Grossman, A. Seliciclib: a new treatment for Cushing’s disease? touchREV Endocrinol. 20, 3–4 (2024).
Roussel-Gervais, A. et al. Cooperation between cyclin E and p27Kip1 in pituitary tumorigenesis. Mol. Endocrinol. 24, 1835–1845 (2010).
Reincke, M. et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat. Genet. 47, 31–38 (2015).
Theodoropoulou, M. & Reincke, M. Genetics of Cushing’s disease: from the lab to clinical practice. Pituitary 25, 689–692 (2022). This paper reviews genetic causes of CD.
Cohen, M. et al. Germline USP8 mutation associated with pediatric Cushing disease and other clinical features: a new syndrome. J. Clin. Endocrinol. Metab. 104, 4676–4682 (2019).
Theodoropoulou, M. et al. Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J. Endocrinol. 183, 385–394 (2004).
Fukuoka, H., Shichi, H., Yamamoto, M. & Takahashi, Y. The mechanisms underlying autonomous adrenocorticotropic hormone secretion in Cushing’s disease. Int. J. Mol. Sci. 21, 9132 (2020).
Pekul, M. et al. Relevance of mutations in protein deubiquitinases genes and TP53 in corticotroph pituitary tumors. Front. Endocrinol. 15, 1302667 (2024).
Perez-Rivas, L. G. et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J. Clin. Endocrinol. Metab. 100, E997–E1004 (2015).
Albani, A. et al. The USP8 mutational status may predict long-term remission in patients with Cushing’s disease. Clin. Endocrinol. https://doi.org/10.1111/cen.13802 (2018).
Sbiera, S. et al. Driver mutations in USP8 wild-type Cushing’s disease. Neuro Oncol. 21, 1273–1283 (2019).
Vila, G. et al. Sonic hedgehog regulates CRH signal transduction in the adult pituitary. FASEB J. 19, 281–283 (2005).
Abraham, A. P. et al. USP8, USP48, and BRAF mutations differ in their genotype-phenotype correlation in Asian Indian patients with Cushing’s disease. Endocrine 75, 549–559 (2022).
Chen, J. et al. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat. Commun. 9, 3171 (2018).
Uzilov, A. V. et al. USP8 and TP53 drivers are associated with CNV in a corticotroph adenoma cohort enriched for aggressive tumors. J. Clin. Endocrinol. Metab. 106, 826–842 (2021).
Hernández-Ramírez, L. C. et al. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease. Endocr. Relat. Cancer 24, 379–392 (2017).
Roussel-Gervais, A. et al. The Cables1 gene in glucocorticoid regulation of pituitary corticotrope growth and Cushing disease. J. Clin. Endocrinol. Metab. 102, 513–522 (2016).
Araki, T. et al. Two distinctive POMC promoters modify gene expression in Cushing disease. J. Clin. Endocrinol. Metab. 106, e3346–e3363 (2021).
Clark, A. J. L., Lavender, P. M., Coates, P., Johnson, M. R. & Rees, L. H. In vitro and in vivo analysis of the processing and fate of the peptide products of the short proopiomelanocortin mRNA. Mol. Endocrinol. 4, 1737–1743 (1990).
Jeannotte, L., Burbach, J. P. H. & Drouin, J. Unusual proopiomelanocortin ribonucleic acids in extrapituitary tissues: intronless transcripts in testes and long poly(A) tails in hypothalamus. Mol. Endocrinol. 1, 749–757 (1987).
Lacaze-Masmonteil, T., de Keyzer, Y., Luton, J. P., Kahn, A. & Bertagna, X. Characterization of pro-opiomelanocortin transcription in human non-pituitary tissues. Proc. Natl Acad. Sci. USA 84, 7261–7265 (1987).
Korbonits, M. et al. Expression of 11β-hydroxysteroid dehydrogenase isoenzymes in the human pituitary: induction of the type 2 enzyme in corticotropinomas and other pituitary tumors. J. Clin. Endocrinol. Metab. 86, 2728–2733 (2001).
Riebold, M. et al. A C-terminal HSP90 inhibitor restores glucocorticoid sensitivity and relieves a mouse allograft model of Cushing disease. Nat. Med. 21, 276–280 (2015).
Ciato, D. et al. Inhibition of heat shock factor 1 enhances repressive molecular mechanisms on the POMC promoter. Neuroendocrinology 109, 362–373 (2019).
Du, L. et al. Evidence for orphan nuclear receptor TR4 in the etiology of Cushing disease. Proc. Natl Acad. Sci. USA 110, 8555–8560 (2013).
Philips, A. et al. Novel dimeric Nur77 signaling mechanisms in endocrine and lymphoid cells. Mol. Cell. Biol. 17, 5946–5951 (1997).
Boutillier, A. L. et al. Corticotropin-releasing hormone stimulates proopiomelanocortin transcription by cFos-dependent and -independent pathways: characterization of an AP1 site in exon 1. Mol. Endocrinol. 9, 745–755 (1995).
Ray, D. W., Ren, S. G. & Melmed, S. Leukemia inhibitory factor (LIF) stimulates proopiomelanocortin (POMC) expression in a corticotroph cell line. Role of STAT pathway. J. Clin. Invest. 97, 1852–1859 (1996).
Takayasu, S. et al. Involvement of nuclear factor-kB and Nurr-1 in cytokine-induced transcription of proopiomelanocortin gene in AtT20 corticotroph cells. Neuroimmunomodulation 17, 88–96 (2010).
Bilodeau, S. et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes. Dev. 20, 2871–2886 (2006).
Araki, T. et al. E2F1-mediated human POMC expression in ectopic Cushing’s syndrome. Endocr. Relat. Cancer 23, 857–870 (2016).
Picon, A., Bertagna, X. & de Keyzer, Y. Analysis of the human proopiomelanocortin gene promoter in a small cell lung carcinoma cell line reveals an unusual role for E2F transcription factors. Oncogene 18, 2627–2633 (1999).
Ray, D. W., Littlewood, A. C., Clark, A. J., Davis, J. R. & White, A. Human small cell lung cancer cell lines expressing the proopiomelanocortin gene have aberrant glucocorticoid receptor function. J. Clin. Invest. 93, 1625–1630 (1994).
Suda, T. et al. Corticotropin-releasing hormone, proopiomelanocortin, and glucocorticoid receptor gene expression in adrenocorticotropin-producing tumors in vitro. J. Clin. Invest. 92, 2790–2795 (1993).
Elamin, M. B. et al. Accuracy of diagnostic tests for Cushing’s syndrome: a systematic review and metaanalyses. J. Clin. Endocrinol. Metab. 93, 1553–1562 (2008).
Findling, J. W. & Raff, H. Diagnosis of endocrine disease: differentiation of pathologic/neoplastic hypercortisolism (Cushing’s syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-Cushing’s syndrome). Eur. J. Endocrinol. 176, R205–R216 (2017). This paper discusses how to discriminate between CS and physiological causes of hypercortisolism.
Ceccato, F. et al. Dexamethasone measurement during low-dose suppression test for suspected hypercortisolism: threshold development with and validation. J. Endocrinol. Invest. 43, 1105–1113 (2020).
Nowak, E. et al. Diagnostic challenges in cyclic Cushing’s syndrome: a systematic review. Lancet Diabetes Endocrinol. 11, 593–606 (2023).
Braun, L. T. et al. Whom should we screen for Cushing syndrome? The Endocrine Society Practice guideline recommendations 2008 revisited. J. Clin. Endocrinol. Metab. 107, e3723–e3730 (2022).
Findling, J. W. & Raff, H. Differentiation of pathologic/neoplastic hypercortisolism (Cushing syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-Cushing syndrome): response to Letter to the Editor. Eur. J. Endocrinol. 178, L3 (2018).
Pecori Giraldi, F. et al. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test and the desmopressin test to distinguish Cushing’s syndrome from pseudo-Cushing’s states. Clin. Endocrinol. 66, 251–257 (2007).
Wehbeh, L. et al. The addition of corticotropin-releasing hormone to 2-day low dose dexamethasone suppression test provides additional case detection. Endocrine 80, 425–432 (2023).
Mondin, A. et al. Second-line tests in the differential diagnosis of neoplastic and non-neoplastic hypercortisolism: a systematic review and meta-analysis. J. Endocrinol. Invest. 46, 1947–1959 (2023).
Page-Wilson, G. et al. Evaluating the burden of endogenous Cushing’s syndrome using a web-based questionnaire and validated patient-reported outcome measures. Pituitary 26, 364–374 (2023).
Rubinstein, G. et al. Time to diagnosis in Cushing’s syndrome: a meta-analysis based on 5367 patients. J. Clin. Endocrinol. Metab. 105, dgz136 (2020).
Nieman, L. K. et al. Treatment of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 100, 2807–2831 (2015). These clinical practice guidelines review the treatment options for CS and factors to consider in developing an individualized plan based on available options and patient values and preferences.
Ciftci, S. et al. The importance of DHEA-S levels in Cushing’s syndrome; is there a cut-off value in the differential diagnosis? Horm. Metab. Res. 54, 232–237 (2022).
Di Dalmazi, G. et al. Steroid profiling by LC-MS/MS in nonsecreting and subclinical cortisol-secreting adrenocortical adenomas. J. Clin. Endocrinol. Metab. 100, 3529–3538 (2015).
Fleseriu, M. et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 9, 847–875 (2021).
Frete, C. et al. Non-invasive diagnostic strategy in ACTH-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 105, dgaa409 (2020).
Elenius, H., McGlotten, R. & Nieman, L. K. Ovine CRH stimulation and 8 mg dexamethasone suppression tests in 323 patients with ACTH-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 109, e182–e189 (2023).
Bonneville, J. F., Potorac, I., Petrossians, P., Tshibanda, L. & Beckers, A. Pituitary MRI in Cushing’s disease – an update. J. Neuroendocrinol. 34, e13123 (2022).
Zemskova, M. S. et al. Utility of various functional and anatomic imaging modalities for detection of ectopic adrenocorticotropin-secreting tumors. J. Clin. Endocrinol. Metab. 95, 1207–1219 (2010).
Chabot, V. et al. Ectopic ACTH Cushing’s syndrome: V3 vasopressin receptor but not CRH receptor gene expression in a pulmonary carcinoid tumor. Horm. Res. 50, 226–231 (1998).
Wang, F. F. et al. Plasma corticotrophin response to desmopressin in patients with Cushing’s disease correlates with the expression of vasopressin receptor 2, but not with that of vasopressin receptor 1 or 3, in their pituitary tumours. Clin. Endocrinol. 76, 253–263 (2012).
Newell-Price, J. et al. Optimal response criteria for the human CRH test in the differential diagnosis of ACTH-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 87, 1640–1645 (2002).
Nieman, L. K. et al. A simplified morning ovine corticotropin-releasing hormone stimulation test for the differential diagnosis of adrenocorticotropin-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 77, 1308–1312 (1993).
Ceccato, F. & Di Dalmazi, G. Shortage of hCRH for the diagnosis of endogenous CS: the end of an era or the beginning of a new journey? J. Endocrinol. Invest 46, 2189–2191 (2023).
Vassiliadi, D. A. & Tsagarakis, S. Diagnosis of endocrine disease: the role of the desmopressin test in the diagnosis and follow-up of Cushing’s syndrome. Eur. J. Endocrinol. 178, R201–R214 (2018).
Castinetti, F. & Lacroix, A. Is desmopressin useful in the evaluation of Cushing syndrome? J. Clin. Endocrinol. Metab. 107, e4295–e4301 (2022). This paper provides an overview of the desmopressin stimulation test and how to interpret it.
Qiao, J. et al. The usefulness of the combined high-dose dexamethasone suppression test and desmopressin stimulation test in establishing the source of ACTH secretion in ACTH-dependent Cushing’s syndrome. Endocr. J. 68, 839–848 (2021).
Barbot, M. et al. Second-line tests in the differential diagnosis of ACTH-dependent Cushing’s syndrome. Pituitary 19, 488–495 (2016).
Ceccato, F. et al. Dynamic testing for differential diagnosis of ACTH-dependent Cushing syndrome: a systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 108, e178–e188 (2023).
Akbari, H. et al. Usefulness of prolactin measurement in inferior petrosal sinus sampling with desmopressin for Cushing’s syndrome. Br. J. Neurosurg. 34, 253–257 (2020).
Wind, J. J. et al. The lateralization accuracy of inferior petrosal sinus sampling in 501 patients with Cushing’s disease. J. Clin. Endocrinol. Metab. 98, 2285–2293 (2013). This paper compares the efficacy of inferior petrosal sinus sampling and pituitary MRI for localization of corticotroph tumours.
Detomas, M. et al. Bilateral inferior petrosal sinus sampling with human CRH stimulation in ACTH-dependent Cushing’s syndrome: results from a retrospective multicenter study. Eur. J. Endocrinol. https://doi.org/10.1093/ejendo/lvad050 (2023).
Valizadeh, M., Ahmadi, A. R., Ebadinejad, A., Rahmani, F. & Abiri, B. Diagnostic accuracy of bilateral inferior petrosal sinus sampling using desmopressin or corticotropic-releasing hormone in ACTH-dependent Cushing’s syndrome: a systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 23, 881–892 (2022).
Chen, S. et al. The optimal cut-off of BIPSS in differential diagnosis of ACTH-dependent Cushing’s syndrome: is stimulation necessary? J. Clin. Endocrinol. Metab. 105, dgz194 (2020).
Deipolyi, A. R., Alexander, B., Rho, J., Hirsch, J. A. & Oklu, R. Bilateral inferior petrosal sinus sampling using desmopressin or corticotropic-releasing hormone: a single-center experience. J. Neurointerv. Surg. 7, 690–693 (2015).
Gandhi, C. D., Meyer, S. A., Patel, A. B., Johnson, D. M. & Post, K. D. Neurologic complications of inferior petrosal sinus sampling. AJNR Am. J. Neuroradiol. 29, 760–765 (2008).
Young, J. et al. Management of endocrine disease: Cushing’s syndrome due to ectopic ACTH secretion: an expert operational opinion. Eur. J. Endocrinol. 182, R29–R58 (2020). This paper provides a perspective on management options for ectopic ACTH secretion.
Hayes, A. R. & Grossman, A. B. Distinguishing Cushing’s disease from the ectopic ACTH syndrome: needles in a haystack or hiding in plain sight? J. Neuroendocrinol. 34, e13137 (2022).
Isidori, A. M., Kaltsas, G. A. & Grossman, A. B. Ectopic ACTH syndrome. Front. Horm. Res. 35, 143–156 (2006).
Wannachalee, T. et al. The clinical impact of [68Ga]-DOTATATE PET/CT for the diagnosis and management of ectopic adrenocorticotropic hormone-secreting tumours. Clin. Endocrinol. 91, 288–294 (2019).
Kamilaris, C. D. C., Faucz, F. R., Voutetakis, A. & Stratakis, C. A. Carney complex. Exp. Clin. Endocrinol. Diabetes 127, 156–164 (2019).
Athimulam, S., Grebe, S. & Bancos, I. Steroid profiling in the diagnosis of mild and overt Cushing’s syndrome. Best. Pract. Res. Clin. Endocrinol. Metab. 35, 101488 (2021).
Braun, L. T. et al. Delineating endogenous Cushing’s syndrome by GC-MS urinary steroid metabotyping. eBioMedicine 99, 104907 (2024).
Senanayake, R. et al. New types of localization methods for adrenocorticotropic hormone-dependent Cushing’s syndrome. Best. Pract. Res. Clin. Endocrinol. Metab. 35, 101513 (2021).
Brossaud, J. et al. Hair cortisol and cortisone measurements for the diagnosis of overt and mild Cushing’s syndrome. Eur. J. Endocrinol. 184, 445–454 (2021).
Greff, M. J. E. et al. Hair cortisol analysis: an update on methodological considerations and clinical applications. Clin. Biochem. 63, 1–9 (2019).
Graham, U. M., Hunter, S. J., McDonnell, M., Mullan, K. R. & Atkinson, A. B. A comparison of the use of urinary cortisol to creatinine ratios and nocturnal salivary cortisol in the evaluation of cyclicity in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 98, E72–E76 (2013).
Couselo, M., Frara, S., Giustina, A. & Casanueva, F. F. Pituitary tumor centers of excellence for Cushing’s disease. Pituitary 25, 772–775 (2022).
Alexandraki, K. I. et al. Long-term remission and recurrence rates in Cushing’s disease: predictive factors in a single-centre study. Eur. J. Endocrinol. 168, 639–648 (2013).
Petersenn, S. et al. Therapy of endocrine disease: outcomes in patients with Cushing’s disease undergoing transsphenoidal surgery: systematic review assessing criteria used to define remission and recurrence. Eur. J. Endocrinol. 172, R227–R239 (2015).
Ciric, I. et al. Transsphenoidal surgery for Cushing disease: experience with 136 patients. Neurosurgery 70, 70–81 (2012).
Jagannathan, J. et al. Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease. J. Neurosurg. 111, 531–539 (2009).
Fassnacht, M. et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 175, G1–G34 (2016).
Fassnacht, M. et al. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 179, G1–G46 (2018).
Fassnacht, M. et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 31, 1476–1490 (2020).
Meloche-Dumas, L., Mercier, F. & Lacroix, A. Role of unilateral adrenalectomy in bilateral adrenal hyperplasias with Cushing’s syndrome. Best. Pract. Res. Clin. Endocrinol. Metab. 35, 101486 (2021).
Smith, P. W. et al. Bilateral adrenalectomy for refractory Cushing disease: a safe and definitive therapy. J. Am. Coll. Surg. 208, 1059–1064 (2009).
Reincke, M. et al. A critical reappraisal of bilateral adrenalectomy for ACTH-dependent Cushing’s syndrome. Eur. J. Endocrinol. 173, M23–M32 (2015).
Sarkis, P. et al. Bilateral adrenalectomy in Cushing’s disease: altered long-term quality of life compared to other treatment options. Ann. Endocrinol. 80, 32–37 (2019).
Katznelson, L. Role of radiation in the treatment of Cushing disease. Pituitary 25, 740–742 (2022).
Minniti, G. et al. Long-term follow-up results of postoperative radiation therapy for Cushing’s disease. J. Neurooncol. 84, 79–84 (2007).
Gheorghiu, M. L. Updates in the outcomes of radiation therapy for Cushing’s disease. Best. Pract. Res. Clin. Endocrinol. Metab. 35, 101514 (2021).
Graffeo, C. S. et al. Biological effective dose as a predictor of hypopituitarism after single-fraction pituitary adenoma radiosurgery: dosimetric analysis and cohort study of patients treated using contemporary techniques. Neurosurgery 88, E330–E335 (2021).
Castinetti, F. Medical management of Cushing’s disease: when and how? J. Neuroendocrinol. 34, e13120 (2022).
Feelders, R. A. et al. Advances in the medical treatment of Cushing’s syndrome. Lancet Diabetes Endocrinol. 7, 300–312 (2019).
Castinetti, F. How best to monitor the specific side effects of medical treatments of Cushing’s disease. Best. Pract. Res. Clin. Endocrinol. Metab. 36, 101718 (2022).
Gadelha, M. R., Wildemberg, L. E. & Shimon, I. Pituitary acting drugs: cabergoline and pasireotide. Pituitary 25, 722–725 (2022).
Bolanowski, M., Kaluzny, M., Witek, P. & Jawiarczyk-Przybylowska, A. Pasireotide – a novel somatostatin receptor ligand after 20 years of use. Rev. Endocr. Metab. Disord. 23, 601–620 (2022).
Lacroix, A. et al. Efficacy and safety of once-monthly pasireotide in Cushing’s disease: a 12 month clinical trial. Lancet Diabetes Endocrinol. 6, 17–26 (2018).
Ferriere, A. et al. Cabergoline for Cushing’s disease: a large retrospective multicenter study. Eur. J. Endocrinol. 176, 305–314 (2017).
Castinetti, F., Nieman, L. K., Reincke, M. & Newell-Price, J. Approach to the patient treated with steroidogenesis inhibitors. J. Clin. Endocrinol. Metab. 106, 2114–2123 (2021).
Kamenicky, P. et al. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing’s syndrome. J. Clin. Endocrinol. Metab. 96, 2796–2804 (2011).
Corcuff, J. B. et al. Rapid control of severe neoplastic hypercortisolism with metyrapone and ketoconazole. Eur. J. Endocrinol. 172, 473–481 (2015).
Feelders, R. A. et al. Pasireotide alone or with cabergoline and ketoconazole in Cushing’s disease. N. Engl. J. Med. 362, 1846–1848 (2010).
Puglisi, S. et al. New perspectives for mitotane treatment of adrenocortical carcinoma. Best. Pract. Res. Clin. Endocrinol. Metab. 34, 101415 (2020).
Poirier, J. et al. Recovery of adrenal insufficiency is frequent after adjuvant mitotane therapy in patients with adrenocortical carcinoma. Cancers 12, 639 (2020).
Pivonello, R. et al. Medical treatment of Cushing’s disease: an overview of the current and recent clinical trials. Front. Endocrinol. 11, 648 (2020).
Poirier, J., Bonnet-Serrano, F., Thomeret, L., Bouys, L. & Bertherat, J. Prolonged adrenocortical blockade following discontinuation of osilodrostat. Eur. J. Endocrinol. 188, K29–K32 (2023).
Ferriere, A., Salenave, S., Puerto, M., Young, J. & Tabarin, A. Prolonged adrenal insufficiency following discontinuation of osilodrostat treatment for intense hypercortisolism. Eur. J. Endocrinol. 190, L1–L3 (2024).
Castinetti, F. et al. Ketoconazole in Cushing’s disease: is it worth a try? J. Clin. Endocrinol. Metab. 99, 1623–1630 (2014).
Constantinescu, S. M. et al. Etomidate infusion at low doses is an effective and safe treatment for severe Cushing’s syndrome outside intensive care. Eur. J. Endocrinol. 183, 161–167 (2020).
Fleseriu, M. & Petersenn, S. Medical therapy for Cushing’s disease: adrenal steroidogenesis inhibitors and glucocorticoid receptor blockers. Pituitary 18, 245–252 (2015).
Newell-Price, J. et al. Use of late-night salivary cortisol to monitor response to medical treatment in Cushing’s disease. Eur. J. Endocrinol. 182, 207–217 (2020).
Pivonello, R. et al. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J. Clin. Endocrinol. Metab. 94, 223–230 (2009).
Barroso-Sousa, R. et al. Complete resolution of hypercortisolism with sorafenib in a patient with advanced medullary thyroid carcinoma and ectopic ACTH (adrenocorticotropic hormone) syndrome. Thyroid 24, 1062–1066 (2014).
Valassi, E. et al. Delayed remission after transsphenoidal surgery in patients with Cushing’s disease. J. Clin. Endocrinol. Metab. 95, 601–610 (2010).
Ironside, N. et al. Earlier post-operative hypocortisolemia may predict durable remission from Cushing’s disease. Eur. J. Endocrinol. 178, 255–263 (2018).
He, X., Findling, J. W. & Auchus, R. J. Glucocorticoid withdrawal syndrome following treatment of endogenous Cushing syndrome. Pituitary 25, 393–403 (2022).
Balasko, A., Zibar Tomsic, K., Kastelan, D. & Dusek, T. Hypothalamic-pituitary-adrenal axis recovery after treatment of Cushing’s syndrome. J. Neuroendocrinol. 34, e13172 (2022).
Cui, Q. et al. The recovery time of hypothalamic-pituitary-adrenal axis after curative surgery in Cushing’s disease and its related factor. Endocrine 81, 349–356 (2023).
Doherty, G. M., Nieman, L. K., Cutler, G. B. Jr, Chrousos, G. P. & Norton, J. A. Time to recovery of the hypothalamic-pituitary-adrenal axis after curative resection of adrenal tumors in patients with Cushing’s syndrome. Surgery 108, 1085–1090 (1990).
Husebye, E. S., Pearce, S. H., Krone, N. P. & Kampe, O. Adrenal insufficiency. Lancet 397, 613–629 (2021).
Mah, P. M. et al. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clin. Endocrinol. 61, 367–375 (2004).
Javorsky, B. R. et al. New cutoffs for the biochemical diagnosis of adrenal insufficiency after ACTH stimulation using specific cortisol assays. J. Endocr. Soc. 5, bvab022 (2021). This paper calls attention to the need for a lower cortisol criterion for the diagnosis of adrenal insufficiency when more specific immunoassays with less cross-reactivity with non-cortisol steroids are used.
Husni, H. et al. Cortisol values during the standard-dose cosyntropin stimulation test: personal experience with Elecsys cortisol II assay. Front. Endocrinol. 13, 978238 (2022).
Ramadoss, V. et al. Improving the interpretation of afternoon cortisol levels and SSTs to prevent misdiagnosis of adrenal insufficiency. J. Endocr. Soc. 5, bvab147 (2021).
Zha, L. et al. New diagnostic cutoffs for adrenal insufficiency after cosyntropin stimulation using Abbott Architect cortisol immunoassay. Endocr. Pract. 28, 684–689 (2022).
Hameed, N. et al. Remission rate after transsphenoidal surgery in patients with pathologically confirmed Cushing’s disease, the role of cortisol, ACTH assessment and immediate reoperation: a large single center experience. Pituitary 16, 452–458 (2013).
Friedman, R. B. et al. Repeat transsphenoidal surgery for Cushing’s disease. J. Neurosurg. 71, 520–527 (1989).
de Macedo Filho, L. J. M. et al. Endoscopic endonasal resection of the medial wall of the cavernous sinus and its impact on outcomes of pituitary surgery: a systematic review and meta-analysis. Brain Sci. 12, 1354 (2022).
Seastedt, K. P. et al. Characterization of outcomes by surgical management of lung neuroendocrine tumors associated with Cushing syndrome. JAMA Netw. Open. 4, e2124739 (2021).
Cambos, S. et al. Persistent cortisol response to desmopressin predicts recurrence of Cushing’s disease in patients with post-operative corticotropic insufficiency. Eur. J. Endocrinol. 182, 489–498 (2020).
Zhang, C. D. et al. Glucocorticoid withdrawal syndrome following surgical remission of endogenous hypercortisolism: a longitudinal observational study. Eur. J. Endocrinol. 188, 592–602 (2023).
Hochberg, Z., Pacak, K. & Chrousos, G. P. Endocrine withdrawal syndromes. Endocr. Rev. 24, 523–538 (2003).
Chrousos, G. P. & Gold, P. W. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA 267, 1244–1252 (1992).
Papanicolaou, D. A., Tsigos, C., Oldfield, E. H. & Chrousos, G. P. Acute glucocorticoid deficiency is associated with plasma elevations of interleukin-6: does the latter participate in the symptomatology of the steroid withdrawal syndrome and adrenal insufficiency? J. Clin. Endocrinol. Metab. 81, 2303–2306 (1996).
Aranda, G. et al. Long-term remission and recurrence rate in a cohort of Cushing’s disease: the need for long-term follow-up. Pituitary 18, 142–149 (2015).
Patil, C. G. et al. Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J. Clin. Endocrinol. Metab. 93, 358–362 (2008).
Bansal, P. et al. Duration of post-operative hypocortisolism predicts sustained remission after pituitary surgery for Cushing’s disease. Endocr. Connect. 6, 625–636 (2017).
Losa, M., Bianchi, R., Barzaghi, R., Giovanelli, M. & Mortini, P. Persistent adrenocorticotropin response to desmopressin in the early postoperative period predicts recurrence of Cushing’s disease. J. Clin. Endocrinol. Metab. 94, 3322–3328 (2009).
Catalino, M. P. et al. Postoperative serum cortisol and Cushing disease recurrence in patients with corticotroph adenomas. J. Clin. Endocrinol. Metab. 108, 3287–3294 (2023).
Amlashi, F. G. et al. Accuracy of late-night salivary cortisol in evaluating postoperative remission and recurrence in Cushing’s disease. J. Clin. Endocrinol. Metab. 100, 3770–3777 (2015).
Danet-Lamasou, M. et al. Accuracy of repeated measurements of late-night salivary cortisol to screen for early-stage recurrence of Cushing’s disease following pituitary surgery. Clin. Endocrinol. 82, 260–266 (2015).
Braun, L. T. et al. Signs, symptoms and biochemistry in recurrent Cushing disease: a prospective pilot study. Endocrine 73, 762–766 (2021).
Ram, Z. et al. Early repeat surgery for persistent Cushing’s disease. J. Neurosurg. 80, 37–45 (1994).
Alexandraki, K. I. & Grossman, A. B. Current strategies for the treatment of severe Cushing’s syndrome. Expert. Rev. Endocrinol. Metab. 11, 65–79 (2016).
Sarlis, N. J., Chanock, S. J. & Nieman, L. K. Cortisolemic indices predict severe infections in Cushing syndrome due to ectopic production of adrenocorticotropin. J. Clin. Endocrinol. Metab. 85, 42–47 (2000).
Pence, A., McGrath, M., Lee, S. L. & Raines, D. E. Pharmacological management of severe Cushing’s syndrome: the role of etomidate. Ther. Adv. Endocrinol. Metab. 13, 20420188211058583 (2022).
Bessiene, L. et al. Rapid control of severe ectopic Cushing’s syndrome by oral osilodrostat monotherapy. Eur. J. Endocrinol. 184, L13–L15 (2021).
Dormoy, A. et al. Efficacy and safety of osilodrostat in paraneoplastic Cushing syndrome: a real-world multicenter study in France. J. Clin. Endocrinol. Metab. 108, 1475–1487 (2023).
Fontaine-Sylvestre, C., Letourneau-Guillon, L., Moumdjian, R. A., Berthelet, F. & Lacroix, A. Corticotroph tumor progression during long-term therapy with osilodrostat in a patient with persistent Cushing’s disease. Pituitary 24, 207–215 (2021).
Valassi, E. et al. Corticotroph tumor progression after bilateral adrenalectomy: data from ERCUSYN. Endocr. Relat. Cancer 29, 681–691 (2022).
Papakokkinou, E. et al. Prevalence of Nelson’s syndrome after bilateral adrenalectomy in patients with cushing’s disease: a systematic review and meta-analysis. Pituitary 24, 797–809 (2021).
Fountas, A. & Karavitaki, N. Nelson’s syndrome: an update. Endocrinol. Metab. Clin. North. Am. 49, 413–432 (2020).
Bessiene, L. et al. Corticotroph tumor progression speed after adrenalectomy. Eur. J. Endocrinol. 187, 797–807 (2022).
Raverot, G. et al. Aggressive pituitary tumours and pituitary carcinomas. Nat. Rev. Endocrinol. 17, 671–684 (2021).
Fountas, A. et al. Outcomes of patients with Nelson’s syndrome after primary treatment: a multicenter study from 13 UK pituitary centers. J. Clin. Endocrinol. Metab. 105, dgz200 (2020).
Brue, T., Amodru, V. & Castinetti, F. Management of endocrine disease: management of Cushing’s syndrome during pregnancy: solved and unsolved questions. Eur. J. Endocrinol. 178, R259–R266 (2018).
van der Pas, R., Leebeek, F. W., Hofland, L. J., de Herder, W. W. & Feelders, R. A. Hypercoagulability in Cushing’s syndrome: prevalence, pathogenesis and treatment. Clin. Endocrinol. 78, 481–488 (2013).
Feelders, R. A. & Nieman, L. K. Hypercoagulability in Cushing’s syndrome: incidence, pathogenesis and need for thromboprophylaxis protocols. Pituitary 25, 746–749 (2022).
Caimari, F. et al. Cushing’s syndrome and pregnancy outcomes: a systematic review of published cases. Endocrine 55, 555–563 (2017).
Webb, S. M. & Valassi, E. Quality of life impairment after a diagnosis of Cushing’s syndrome. Pituitary 25, 768–771 (2022).
Webb, S. M. & Valassi, E. Morbidity of Cushing’s syndrome and impact of treatment. Endocrinol. Metab. Clin. North. Am. 47, 299–311 (2018).
Puglisi, S., Perini, A. M. E., Botto, C., Oliva, F. & Terzolo, M. Long-term consequences of Cushing syndrome: a systematic literature review. J. Clin. Endocrinol. Metab. 109, e901–e919 (2024).
Clayton, R. N. Cardiovascular complications of Cushings syndrome: impact on morbidity and mortality. J. Neuroendocrinol. 34, e13175 (2022).
Papakokkinou, E. et al. Excess morbidity persists in patients with Cushing’s disease during long-term remission: a Swedish nationwide study. J. Clin. Endocrinol. Metab. 105, dgaa291 (2020).
Ragnarsson, O., Berglund, P., Eder, D. N. & Johannsson, G. Long-term cognitive impairments and attentional deficits in patients with Cushing’s disease and cortisol-producing adrenal adenoma in remission. J. Clin. Endocrinol. Metab. 97, E1640–E1648 (2012).
Tiemensma, J. et al. Subtle cognitive impairments in patients with long-term cure of Cushing’s disease. J. Clin. Endocrinol. Metab. 95, 2699–2714 (2010).
Piasecka, M. et al. Psychiatric and neurocognitive consequences of endogenous hypercortisolism. J. Intern. Med. 288, 168–182 (2020).
Ebbehoj, A. et al. The socioeconomic consequences of Cushing’s syndrome: a nationwide cohort study. J. Clin. Endocrinol. Metab. 107, e2921–e2929 (2022).
Tiemensma, J. et al. Increased prevalence of psychopathology and maladaptive personality traits after long-term cure of Cushing’s disease. J. Clin. Endocrinol. Metab. 95, E129–E141 (2010).
Bengtsson, D. et al. Psychotropic drugs in patients with Cushing’s disease before diagnosis and at long-term follow-up: a nationwide study. J. Clin. Endocrinol. Metab. 106, 1750–1760 (2021).
Martel-Duguech, L. et al. Prevalence of sarcopenia after remission of hypercortisolism and its impact on HRQoL. Clin. Endocrinol. 95, 735–743 (2021).
Li, D. et al. Determinants of muscle function and health-related quality of life in patients with endogenous hypercortisolism: a cross-sectional study. Eur. J. Endocrinol. 188, 603–612 (2023).
Vogel, F. et al. Persisting muscle dysfunction in Cushing’s syndrome despite biochemical remission. J. Clin. Endocrinol. Metab. 105, e4490–e4498 (2020).
Kreitschmann-Andermahr, I. et al. Support needs of patients with Cushing’s disease and Cushing’s syndrome: results of a survey conducted in Germany and the USA. Int. J. Endocrinol. 2018, 9014768 (2018).
Valassi, E. et al. Unmet needs in Cushing’s syndrome: the patients’ perspective. Endocr. Connect. 11, e220027 (2022).
Acree, R. et al. Patient and provider perspectives on postsurgical recovery of Cushing syndrome. J. Endocr. Soc. 5, bvab109 (2021).
Neou, M. et al. Pangenomic classification of pituitary neuroendocrine tumors. Cancer Cell 37, 123–134.e5 (2020).
Manenschijn, L., van den Akker, E. L., Lamberts, S. W. & van Rossum, E. F. Clinical features associated with glucocorticoid receptor polymorphisms. An overview. Ann. N. Y. Acad. Sci. 1179, 179–198 (2009).
Ragnarsson, O. et al. Common genetic variants in the glucocorticoid receptor and the 11β-hydroxysteroid dehydrogenase type 1 genes influence long-term cognitive impairments in patients with Cushing’s syndrome in remission. J. Clin. Endocrinol. Metab. 99, E1803–E1807 (2014).
Regazzo, D., Mondin, A., Scaroni, C., Occhi, G. & Barbot, M. The role of glucocorticoid receptor in the pathophysiology of pituitary corticotroph adenomas. Int. J. Mol. Sci. 23, 6469 (2022).
Devine, K. et al. The ATP-binding cassette proteins ABCB1 and ABCC1 as modulators of glucocorticoid action. Nat. Rev. Endocrinol. 19, 112–124 (2023).
Lam-Chung, C. E. & Cuevas-Ramos, D. The promising role of risk scoring system for Cushing syndrome: time to reconsider current screening recommendations. Front. Endocrinol. 13, 1075785 (2022).
Mohammedi, K. et al. Evidence of persistent mild hypercortisolism in patients medically treated for Cushing disease: the Haircush study. J. Clin. Endocrinol. Metab. 108, e963–e970 (2023).
Kosilek, R. P. et al. Diagnostic use of facial image analysis software in endocrine and genetic disorders: review, current results and future perspectives. Eur. J. Endocrinol. 173, M39–M44 (2015).
Wei, R. et al. Deep-learning approach to automatic identification of facial anomalies in endocrine disorders. Neuroendocrinology 110, 328–337 (2020).
Upton, T. J. et al. High-resolution daily profiles of tissue adrenal steroids by portable automated collection. Sci. Transl. Med. 15, eadg8464 (2023).
Zhang, M. et al. Cushing’s syndrome is associated with gut microbial dysbiosis and cortisol-degrading bacteria. J. Clin. Endocrinol. Metab. 109, 1474–1484 (2023).
Valassi, E. et al. Gut microbial dysbiosis in patients with Cushing’s disease in long-term remission. Relationship with cardiometabolic risk. Front. Endocrinol. 14, 1074757 (2023).
Debono, M. et al. Resetting the abnormal circadian cortisol rhythm in adrenal incidentaloma patients with mild autonomous cortisol secretion. J. Clin. Endocrinol. Metab. 102, 3461–3469 (2017).
Zhou, J. et al. Demographic characteristics, etiology, and comorbidities of patients with Cushing’s syndrome: a 10-year retrospective study at a large general hospital in China. Int. J. Endocrinol. 2019, 7159696 (2019).
Bertherat, J. et al. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J. Clin. Endocrinol. Metab. 94, 2085–2091 (2009).
Wasserman, J. D., Zambetti, G. P. & Malkin, D. Towards an understanding of the role of p53 in adrenocortical carcinogenesis. Mol. Cell Endocrinol. 351, 101–110 (2012).
Fenske, M. How much “urinary free cortisol” is really cortisol during water diuresis in healthy individuals? Clin. Chem. 50, 1102–1104 (2004).
Nieman, L. K. Diagnosis of Cushing’s syndrome in the modern era. Endocrinol. Metab. Clin. North. Am. 47, 259–273 (2018).
Chan, K. C. et al. Diminished urinary free cortisol excretion in patients with moderate and severe renal impairment. Clin. Chem. 50, 757–759 (2004).
Chen, A. X., Haas, A. V., Williams, G. H. & Vaidya, A. Dietary sodium intake and cortisol measurements. Clin. Endocrinol. 93, 539–545 (2020).
Baudrand, R. et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin. Endocrinol. 80, 677–684 (2014).
Findling, J. W., Raff, H. & Aron, D. C. The low-dose dexamethasone suppression test: a reevaluation in patients with Cushing’s syndrome. J. Clin. Endocrinol. Metab. 89, 1222–1226 (2004).
Vastbinder, M., Kuindersma, M., Mulder, A. H., Schuijt, M. P. & Mudde, A. H. The influence of oral contraceptives on overnight 1 mg dexamethasone suppression test. Neth. J. Med. 74, 158–161 (2016).
Baid, S. K. et al. Specificity of screening tests for Cushing’s syndrome in an overweight and obese population. J. Clin. Endocrinol. Metab. 94, 3857–3864 (2009).
Valassi, E. et al. Concomitant medication use can confound interpretation of the combined dexamethasone-corticotropin releasing hormone test in Cushing’s syndrome. J. Clin. Endocrinol. Metab. 94, 4851–4859 (2009). This paper highlights the increased risk of a falsely abnormal response to a low-dose dexamethasone suppression test in patients taking medications that interact with CYP3A4.
Liu, H., Bravata, D. M., Cabaccan, J., Raff, H. & Ryzen, E. Elevated late-night salivary cortisol levels in elderly male type 2 diabetic veterans. Clin. Endocrinol. 63, 642–649 (2005).
Carev, M. et al. Blood pressure dipping and salivary cortisol as markers of fatigue and sleep deprivation in staff anesthesiologists. Coll. Antropol. 35, 133–138 (2011).
Nieman, L. K. Molecular derangements and the diagnosis of ACTH-dependent Cushing’s syndrome. Endocr. Rev. 43, 852–877 (2022).
Alwani, R. A. et al. Differentiating between Cushing’s disease and pseudo-Cushing’s syndrome: comparison of four tests. Eur. J. Endocrinol. 170, 477–486 (2014).
Nugent, C. A., Warner, H. R., Dunn, J. T. & Tyler, F. H. Probability theory in the diagnosis of Cushing’s syndrome. J. Clin. Endocrinol. Metab. 24, 621–627 (1964).
Plotz, C. M., Knowlton, A. I. & Ragan, C. The natural history of Cushing’s syndrome. Am. J. Med. 13, 597–614 (1952).
Starkman, M. N. Neuropsychiatric findings in Cushing syndrome and exogenous glucocorticoid administration. Endocrinol. Metab. Clin. North. Am. 42, 477–488 (2013).
Van Staa, T. P., Abenhaim, L., Cooper, C., Zhang, B. & Leufkens, H. G. The use of a large pharmacoepidemiological database to study exposure to oral corticosteroids and risk of fractures: validation of study population and results. Pharmacoepidemiol. Drug. Saf. 9, 359–366 (2000).
Pofi, R., Caratti, G., Ray, D. W. & Tomlinson, J. W. Treating the side effects of exogenous glucocorticoids; can we separate the good from the bad? Endocr. Rev. 44, 975–1011 (2023).
Hopkins, R. L. & Leinung, M. C. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol. Metab. Clin. North. Am. 34, 371–384 (2005).
Prete, A. & Bancos, I. Glucocorticoid induced adrenal insufficiency. BMJ 374, n1380 (2021).
Jung, C. et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J. Clin. Endocrinol. Metab. 96, 1533–1540 (2011).
Lopes, L. M., Francisco, R. P., Galletta, M. A. & Bronstein, M. D. Determination of nighttime salivary cortisol during pregnancy: comparison with values in non-pregnancy and Cushing’s disease. Pituitary 19, 30–38 (2016).
Parksook, W. W., Porntharukchareon, T. & Sunthornyothin, S. Desmopressin stimulation test in a pregnant patient with Cushing’s disease. AACE Clin. Case Rep. 8, 105–108 (2022).
Ragonese, M., Cotta, O. R., Ferrau, F., Trimarchi, F. & Cannavo, S. How to diagnose and manage Cushing’s disease during pregnancy, when hypercortisolism is mild? Gynecol. Endocrinol. 28, 637–639 (2012).
Hamblin, R., Coulden, A., Fountas, A. & Karavitaki, N. The diagnosis and management of Cushing’s syndrome in pregnancy. J. Neuroendocrinol. 34, e13118 (2022).
Lindsay, J. R. & Nieman, L. K. The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr. Rev. 26, 775–799 (2005).
Lindsay, J. R., Jonklaas, J., Oldfield, E. H. & Nieman, L. K. Cushing’s syndrome during pregnancy: personal experience and review of the literature. J. Clin. Endocrinol. Metab. 90, 3077–3083 (2005).
Author information
Authors and Affiliations
Contributions
Introduction (L.K.N., F.C., J.N.-P. and A.L.); Epidemiology (L.K.N., F.C., J.N.-P. and E.V.); Mechanisms/pathophysiology (L.K.N., J.D., Y.T. and A.L.); Diagnosis, screening and prevention (L.K.N., J.N.-P., E.V. and A.L.); Management (L.K.N., F.C., J.N.-P., E.V., Y.T. and A.L.); Quality of life (L.K.N. and E.V.); Outlook (L.K.N., F.C., J.N.-P. and A.L.); overview of Primer (L.K.N., A.L., F.C. and J.N.-P.).
Corresponding author
Ethics declarations
Competing interests
J.N.-P. has received research support and consultancy paid to his institution from Recordati Rare Diseases, Crinetics Pharmaceuticals and Sparrow Pharmaceuticals, and is President of the Endocrine Society. F.C. received research grants and honoraria for expert advice from Recordati Rare Diseases, HRA Pharma Rare Diseases and Lundbeck. Y.T. has received honoraria from Novo Nordisk, Recordati Rare Diseases, Otsuka Pharma and Ascendis. A.L. has received research grants from Pfizer Canada and Recordati Canada Rare Diseases, serves on an advisory board for Recordati Canada Rare Diseases, is on a speakers bureau for Medunik Canada, and has spoken on behalf of Recordati Canada Rare Diseases; he receives royalties for work as an editor of the adrenal section of UpToDate and Encyclopedia of Endocrine Diseases, and is an inventor on a patent for endocrine diseases related to KDM1A for work performed at the Universite Paris–Saclay. L.K.N. receives royalties as an author and editor for UpToDate and has received research support paid to her institution from Crinetics Pharmaceuticals. E.V. received honoraria for consulting, lectures and advisory boards from HRA Pharma and Recordati Rare Diseases. J.D. declares no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks I. Bancos, who co-reviewed this manuscript with S. R. Chacko; M. Fragoso; C. Scaroni, who co-reviewed this manuscript with A. Mondin; and K. C. J. Yuen, who co-reviewed this manuscript with A. Vincent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nieman, L.K., Castinetti, F., Newell-Price, J. et al. Cushing syndrome. Nat Rev Dis Primers 11, 4 (2025). https://doi.org/10.1038/s41572-024-00588-w
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41572-024-00588-w
This article is cited by
-
Update in the Management of ACTH-Secreting Gastroenteropancreatic and Thoracic Neuroendocrine Neoplasms
Current Treatment Options in Oncology (2025)
-
Efficacy and safety of osilodrostat in patients with ectopic Cushing´s syndrome. a real-world study in Spain
Journal of Endocrinological Investigation (2025)