Abstract
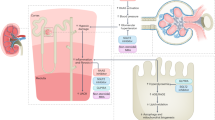
Kidney disease is one of the leading causes of mortality in persons with diabetes mellitus. Diabetic kidney disease (DKD) typically presents with a reduced estimated glomerular filtration rate and, in many but not all cases, with marked proteinuria. Strict glycaemic control and blood pressure control remain foundational in managing DKD, and advances in the understanding of disease mechanisms have redefined the therapeutic landscape. Large outcome trials, such as EMPA-KIDNEY, DAPA-CKD and CREDENCE, have demonstrated that sodium–glucose cotransporter 2 inhibitors slow chronic kidney disease progression and improve cardiovascular outcomes. Glucagon-like peptide 1 receptor agonists reduce albuminuria and preserve estimated glomerular filtration rate, as shown most recently in the FLOW trial. Finerenone, a non-steroidal mineralocorticoid receptor antagonist, lowered renal and cardiovascular risk in the FIDELIO-DKD and FIGARO-DKD trials. Combination approaches (for example, sodium–glucose cotransporter 2 inhibition plus endothelin receptor type A blockade in ZENITH-CKD), aldosterone synthase inhibition, and targeted anti-inflammatory or complement-modifying agents offer additional promise. We summarize the key pathophysiological drivers (glomerular hyperfiltration, podocyte injury, tubulointerstitial inflammation and fibrosis), review established treatments and highlight emerging strategies to prevent or halt DKD.
Key points
-
Diabetic kidney disease (DKD) is diagnosed by an estimated glomerular filtration rate <60 ml/min/1.73 m² for more than 3 months with or without albuminuria >300 mg/day or an albumin-to-creatinine ratio >0.3 in individuals with diabetes mellitus.
-
DKD is the leading cause of chronic kidney disease and end-stage kidney disease and is associated with high 10-year all-cause mortality among people with diabetes mellitus and chronic kidney disease.
-
Key mechanisms driving DKD include glomerular hyperfiltration, podocyte injury, tubulointerstitial inflammation and fibrosis.
-
Although landmark clinical trials have shaped current treatment guidelines, several potential therapeutic targets involved in DKD pathophysiology remain unexplored.
-
Established treatments include renin–angiotensin system blockers, sodium–glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists and non-steroidal mineralocorticoid receptor antagonists, which can slow disease progression; however, strict blood pressure control and glycaemic control remain essential for managing DKD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
10 October 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41574-025-01198-6
References
Centers for Disease Control and Prevention. National Diabetes Statistics Report https://www.cdc.gov/diabetes/php/data-research/index.html (2024).
Hussain, S. et al. Diabetic kidney disease: an overview of prevalence, risk factors, and biomarkers. Clin. Epidemiol. Glob. Health 9, 2–6 (2021).
Tuttle, K. R. et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care 37, 2864–2883 (2014).
Hoogeveen, E. K. The epidemiology of diabetic kidney disease. Kidney Dialysis 2, 433–442 (2022).
Afkarian, M. et al. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 24, 302–308 (2013).
González-Pérez, A., Saez, M., Vizcaya, D., Lind, M. & Garcia Rodriguez, L. Incidence and risk factors for mortality and end-stage renal disease in people with type 2 diabetes and diabetic kidney disease: a population-based cohort study in the UK. BMJ Open Diabetes Res. Care 9, e002146 (2021).
Liao, X. et al. Contribution of CKD to mortality in middle-aged and elderly people with diabetes: the China health and retirement longitudinal study. Diabetol. Metab. Syndr. 15, 122 (2023).
Reidy, K., Kang, H. M., Hostetter, T. & Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 124, 2333–2340 (2014).
Yamazaki, T., Mimura, I., Tanaka, T. & Nangaku, M. Treatment of diabetic kidney disease: current and future. Diabetes Metab. J. 45, 11–26 (2021).
Petrazzuolo, A. et al. Broadening horizons in mechanisms, management, and treatment of diabetic kidney disease. Pharmacol. Res. 190, 106710 (2023).
Winiarska, A., Knysak, M., Nabrdalik, K., Gumprecht, J. & Stompór, T. Inflammation and oxidative stress in diabetic kidney disease: the targets for SGLT2 inhibitors and GLP-1 receptor agonists. Int. J. Mol. Sci. 22, 10822 (2021).
Zinman, B. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 373, 2117–2128 (2015).
Marso, S. P. et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 375, 1834–1844 (2016).
Marso, S. P. et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 375, 311–322 (2016).
Persson, F. et al. Changes in albuminuria predict cardiovascular and renal outcomes in type 2 diabetes: a post hoc analysis of the LEADER trial. Diabetes Care 44, 1020–1026 (2021).
Tuttle, K. R. et al. Post hoc analysis of SUSTAIN 6 and PIONEER 6 trials suggests that people with type 2 diabetes at high cardiovascular risk treated with semaglutide experience more stable kidney function compared with placebo. Kidney Int. 103, 772–781 (2023).
Perkovic, V. et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N. Engl. J. Med. 391, 109–121 (2024).
Barrera-Chimal, J. & Jaisser, F. Pathophysiologic mechanisms in diabetic kidney disease: a focus on current and future therapeutic targets. Diabetes Obes. Metab. 22, 16–31 (2020).
Tervaert, T. W. et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 21, 556–563 (2010).
Scilletta, S. et al. Update on diabetic kidney disease (DKD): focus on non-albuminuric DKD and cardiovascular risk. Biomolecules 13, 752 (2023).
Qi, C., Mao, X., Zhang, Z. & Wu, H. Classification and differential diagnosis of diabetic nephropathy. J. Diabetes Res. 2017, 1–7 (2017).
Oshima, M. et al. Trajectories of kidney function in diabetes: a clinicopathological update. Nat. Rev. Nephrol. 17, 740–750 (2021).
Alicic, R. Z. R., Michele, T. & Tuttle, K. R. Diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 12, 2032–2045 (2017).
Umanath, K. & Lewis, J. B. Update on diabetic nephropathy: core curriculum 2018. Am. J. Kidney Dis. 71, 884–895 (2018).
Park, C. W. Diabetic kidney disease: from epidemiology to clinical perspectives. Diabetes Metab. J. 38, 252 (2014).
Palmer, M. B. et al. The role of glomerular epithelial injury in kidney function decline in patients with diabetic kidney disease in the TRIDENT Cohort. Kidney Int. Rep. 6, 1066–1080 (2021).
Coresh, J. et al. Change in albuminuria and subsequent risk of end-stage kidney disease: an individual participant-level consortium meta-analysis of observational studies. Lancet Diabetes Endocrinol. 7, 115–127 (2019).
Di Pino, A. et al. High glomerular filtration rate is associated with impaired arterial stiffness and subendocardial viability ratio in prediabetic subjects. Nutr. Metab. Cardiovasc. Dis. 31, 3393–3400 (2021).
Mogensen, C. E. & Christensen, C. K. Predicting diabetic nephropathy in insulin-dependent patients. N. Engl. J. Med. 311, 89–93 (1984).
Mogensen, C. E. & Christensen, C. K. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N. Engl. J. Med. 310, 356–360 (1984).
Pasternak, M. et al. Association of albuminuria and regression of chronic kidney disease in adults with newly diagnosed moderate to severe chronic kidney disease. JAMA Netw. Open 5, e2225821 (2022).
Ninomiya, T. et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 20, 1813–1821 (2009).
Drexler, Y. et al. Associations between albuminuria and mortality among US adults by demographic and comorbidity factors. J. Am. Heart Assoc. 12, e030773 (2023).
Thomas, M. C. et al. Nonalbuminuric renal impairment in type 2 diabetic patients and in the general population (National Evaluation of the Frequency of Renal impairment cO-existing with NIDDM [NEFRON] 11). Diabetes Care 32, 1497–1502 (2009).
Yamanouchi, M., Furuichi, K., Hoshino, J., Ubara, Y. & Wada, T. Nonproteinuric diabetic kidney disease. Clin. Exp. Nephrol. 24, 573–581 (2020).
Porrini, E. et al. Non-proteinuric pathways in loss of renal function in patients with type 2 diabetes. Lancet Diabetes Endocrinol. 3, 382–391 (2015).
Bolignano, D. & Zoccali, C. Non-proteinuric rather than proteinuric renal diseases are the leading cause of end-stage kidney disease. Nephrol. Dialysis Transplant. 32, ii194–ii199 (2017).
El-Achkar, T. M. et al. A multimodal and integrated approach to interrogate human kidney biopsies with rigor and reproducibility: guidelines from the kidney precision medicine project. Physiol. Genomics 53, 1–11 (2021).
Patel, J. et al. Molecular signatures of diabetic kidney disease hiding in a patient with hypertension-related kidney disease: a clinical pathologic molecular correlation. Clin. J. Am. Soc. Nephrol. 17, 594–601 (2022).
Ferkowicz, M. J. et al. Molecular signatures of glomerular neovascularization in a patient with diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 19, 266–275 (2024).
Townsend, R. R. et al. Rationale and design of the transformative research in diabetic nephropathy (TRIDENT) study. Kidney Int. 97, 10–13 (2020).
Mohandes, S. et al. An interim analysis of clinical and histopathological results of the transformative research in diabetic nephropathy (TRIDENT) study. J. Am. Soc. Nephrol. 33, 119–119 (2022).
Liu, H. et al. Kidney multiome-based genetic scorecard reveals convergent coding and regulatory variants. Science 387, eadp4753 (2025).
Gu, X. et al. Kidney disease genetic risk variants alter lysosomal beta-mannosidase (MANBA) expression and disease severity. Sci. Transl. Med. 13, eaaz1458 (2021).
Wuttke, M. et al. A catalog of genetic loci associated with kidney function from analyses of a million individuals. Nat. Genet. 51, 957–972 (2019).
Stanzick, K. J. et al. Discovery and prioritization of variants and genes for kidney function in <1.2 million individuals. Nat. Commun. 12, 4350 (2021).
Winkler, T. W. et al. Differential and shared genetic effects on kidney function between diabetic and non-diabetic individuals. Commun. Biol. 5, 580 (2022).
Sandholm, N. et al. New susceptibility loci associated with kidney disease in type 1 diabetes. PLoS Genet. 8, e1002921 (2012).
Hanson, R. L. et al. Identification of PVT1 as a candidate gene for end-stage renal disease in type 2 diabetes using a pooling-based genome-wide single nucleotide polymorphism association study. Diabetes 56, 975–983 (2007).
Thameem, F. et al. A genome-wide search for linkage of estimated glomerular filtration rate (eGFR) in the family investigation of nephropathy and diabetes (FIND). PLoS ONE 8, e81888 (2013).
Palmer, N. D. et al. Evaluation of candidate nephropathy susceptibility genes in a genome-wide association study of African American diabetic kidney disease. PLoS ONE 9, e88273 (2014).
Igo, J. R. P. et al. Genomewide linkage scan for diabetic renal failure and albuminuria: the FIND study. Am. J. Nephrol. 33, 381–389 (2011).
Miao, Z. et al. Single cell regulatory landscape of the mouse kidney highlights cellular differentiation programs and disease targets. Nat. Commun. 12, 2277 (2021).
[No authors listed]. Diabetes control and complications trial (DCCT): results of feasibility study. The DCCT research group. Diabetes Care 10, 1–19 (1987).
King, P., Peacock, I. & Donnelly, R. The UK prospective diabetes study (UKPDS): clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 48, 643–648 (1999).
Dluhy, R. G. M. & Graham, T. Intensive glycemic control in the ACCORD and ADVANCE trials. N. Engl. J. Med. 358, 2630–2633 (2008).
Patel, A. et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008).
Zoungas, S. et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N. Engl. J. Med. 371, 1392–1406 (2014).
Gerstein, H. C. et al. Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N. Engl. J. Med. 358, 2545–2559 (2008).
Duckworth, W. et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 360, 129–139 (2009).
Rossing, P. et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 102, S1–S127 (2022).
De Boer, I. H. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the diabetes control and complications trial/epidemiology of diabetes interventions and complications cohort. Arch. Intern. Med. 171, 412 (2011).
Natarajan, R. Epigenetic mechanisms in diabetic vascular complications and metabolic memory: the 2020 Edwin Bierman award lecture. Diabetes 70, 328–337 (2021).
Lachin, J. M. & Nathan, D. M. Understanding metabolic memory: the prolonged influence of glycemia during the diabetes control and complications trial (DCCT) on future risks of complications during the study of the epidemiology of diabetes interventions and complications (EDIC). Diabetes Care 44, 2216–2224 (2021).
Zheng, W., Guo, J. & Liu, Z.-S. Effects of metabolic memory on inflammation and fibrosis associated with diabetic kidney disease: an epigenetic perspective. Clin. Epigenetics 13, 87 (2021).
VanderJagt, T. A. Epigenetic profiles of pre-diabetes transitioning to type 2 diabetes and nephropathy. World J. Diabetes 6, 1113 (2015).
Chen, Z. et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc. Natl Acad. Sci. USA 113, E3002–E3011 (2016).
Mai, V. Q. & Meere, M. Modelling the phosphorylation of glucose by human hexokinase I. Mathematics 9, 2315 (2021).
Ighodaro, O. M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomedicine Pharmacother. 108, 656–662 (2018).
Wu, T., Ding, L., Andoh, V., Zhang, J. & Chen, L. The mechanism of hyperglycemia-induced renal cell injury in diabetic nephropathy disease: an update. Life 13, 539 (2023).
Ma, X. et al. Advances in oxidative stress in pathogenesis of diabetic kidney disease and efficacy of TCM intervention. Ren. Fail. 45, 2146512 (2023).
Sugahara, M., Pak, W. L. W., Tanaka, T., Tang, S. C. W. & Nangaku, M. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology 26, 491–500 (2021).
Lazarev, V. F., Guzhova, I. V. & Margulis, B. A. Glyceraldehyde-3-phosphate dehydrogenase is a multifaceted therapeutic target. Pharmaceutics 12, 416 (2020).
Boulom, V. L. et al. Poly ADP-ribose polymerase (PARP) inhibition modulates glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity in a diabetic mouse model of hind limb ischemia reperfusion. J. Am. Coll. Surg. 215, S159 (2012).
Zhang, B., Yang, Y., Yi, J., Zhao, Z. & Ye, R. Hyperglycemia modulates M1/M2 macrophage polarization via reactive oxygen species overproduction in ligature-induced periodontitis. J. Periodontal Res. 56, 991–1005 (2021).
Braithwaite, A. T. et al. Multi-organ single-cell RNA sequencing in mice reveals early hyperglycemia responses that converge on fibroblast dysregulation. FASEB J. 38, e23448 (2024).
Edgar, L. et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 144, 961–982 (2021).
Tonneijck, L. M. et al. Glomerular hyperfiltration in diabetes: mechanisms, clinical significance, and treatment. J. Am. Soc. Nephrol. 28, 1023–1039 (2017).
Premaratne, E. et al. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab. J. 41, 5–17 (2015).
Kanai, Y., Lee, W. S., You, G., Brown, D. & Hediger, M. A. The human kidney low affinity Na+/glucose cotransporter SGLT2. Delineation of the major renal reabsorptive mechanism for D-glucose. J. Clin. Investig. 93, 397–404 (1994).
Yang, Y. & Xu, G. Update on pathogenesis of glomerular hyperfiltration in early diabetic kidney disease. Front. Endocrinol. 13, 872918 (2022).
Console, L. et al. The link between the mitochondrial fatty acid oxidation derangement and kidney injury. Front. Physiol. 11, 794 (2020).
Li, S. Y. & Susztak, K. The role of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) in kidney disease. Semin. Nephrol. 38, 121–126 (2018).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13, 629–646 (2017).
Huynh, C., Ryu, J., Lee, J., Inoki, A. & Inoki, K. Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases. Nat. Rev. Nephrol. 19, 102–122 (2023).
Liu, H., Li, Y. & Xiong, J. The role of hypoxia-inducible factor-1 alpha in renal disease. Molecules 27, 7318 (2022).
Sugahara, M., Tanaka, T. & Nangaku, M. Hypoxia-inducible factor and oxygen biology in the kidney. Kidney360 1, 1021–1031 (2020).
Stanigut, A. M. et al. Hypoxia-inducible factors and diabetic kidney disease — how deep can we go? Int. J. Mol. Sci. 23, 10413 (2022).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Heerspink, H. J. L. et al. Dapagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 383, 1436–1446 (2020).
The EMPA-KIDNEY Collaborative Group. Empagliflozin in patients with chronic kidney disease. N. Engl. J. Med. 388, 117–127 (2023).
Cherney, D. Z. I. et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129, 587–597 (2014).
Kidokoro, K. et al. Evaluation of glomerular hemodynamic function by empagliflozin in diabetic mice using in vivo imaging. Circulation 140, 303–315 (2019).
Van Bommel, E. J. M. et al. Renal hemodynamic effects of sodium-glucose cotransporter 2 inhibitors in hyperfiltering people with type 1 diabetes and people with type 2 diabetes and normal kidney function. Kidney Int. 97, 631–635 (2020).
Zeni, L., Norden, A. G. W., Cancarini, G. & Unwin, R. J. A more tubulocentric view of diabetic kidney disease. J. Nephrol. 30, 701–717 (2017).
Vallon, V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu. Rev. Med. 66, 255–270 (2015).
Ingelfinger, J. R. A. et al. Nephron protection in diabetic kidney disease. N. Engl. J. Med. 375, 2096–2098 (2016).
Zaman, M. A., Oparil, S. & Calhoun, D. A. Drugs targeting the renin–angiotensin–aldosterone system. Nat. Rev. Drug Discov. 1, 621–636 (2002).
Burns, K. D. Angiotensin II and its receptors in the diabetic kidney. Am. J. Kidney Dis. 36, 449–467 (2000).
Helal, I., Fick-Brosnahan, G. M., Reed-Gitomer, B. & Schrier, R. W. Glomerular hyperfiltration: definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 8, 293–300 (2012).
Cortinovis, M., Perico, N., Ruggenenti, P., Remuzzi, A. & Remuzzi, G. Glomerular hyperfiltration. Nat. Rev. Nephrol. 18, 435–451 (2022).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N. Engl. J. Med. 329, 1456–1462 (1993).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Sawaf, H. et al. Therapeutic advances in diabetic nephropathy. J. Clin. Med. 11, 378 (2022).
Yusuf, S. et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 358, 1547–1559 (2008).
Parving, H.-H. et al. Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N. Engl. J. Med. 367, 2204–2213 (2012).
Stockand, J. D. New ideas about aldosterone signaling in epithelia. Am. J. Physiol. Ren. Physiol. 282, F559–F576 (2002).
Connell, J. M. C. & Davies, E. The new biology of aldosterone. J. Endocrinol. 186, 1–20 (2005).
Agarwal, R. et al. A comparative post hoc analysis of finerenone and spironolactone in resistant hypertension in moderate-to-advanced chronic kidney disease. Clin. Kidney J. 16, 293–302 (2023).
Viengchareun, S. et al. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl. Recept. Signal. 5, e012 (2007).
Barrera-Chimal, J., Jaisser, F. & Anders, H. J. The mineralocorticoid receptor in chronic kidney disease. Br. J. Pharmacol. 179, 3152–3164 (2022).
Yang, P., Huang, T. & Xu, G. The novel mineralocorticoid receptor antagonist finerenone in diabetic kidney disease: progress and challenges. Metab. Clin. Exp. 65, 1342–1349 (2016).
Bakris, G. L. et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N. Engl. J. Med. 383, 2219–2229 (2020).
Pitt, B. et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N. Engl. J. Med. 385, 2252–2263 (2021).
Agarwal, R. et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur. Heart J. 43, 474–484 (2022).
Tuttle, K. R. et al. Efficacy and safety of aldosterone synthase inhibition with and without empagliflozin for chronic kidney disease: a randomised, controlled, phase 2 trial. Lancet 403, 379–390 (2024).
Kriz, W., Hähnel, B., Hosser, H., Rösener, S. & Waldherr, R. D. Structural analysis of how podocytes detach from the glomerular basement membrane under hypertrophic stress. Front. Endocrinol. 5, 207 (2014).
Chen, J. K., Chen, J., Thomas, G., Kozma, S. C. & Harris, R. C. S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy. Am. J. Physiol. 297, F585–F593 (2009).
Vallon, V. & Thomson, S. C. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat. Rev. Nephrol. 16, 317–336 (2020).
Sever, S. Role of actin cytoskeleton in podocytes. Pediatr. Nephrol. 36, 2607–2614 (2021).
Advani, A. et al. Inhibition of the epidermal growth factor receptor preserves podocytes and attenuates albuminuria in experimental diabetic nephropathy. Nephrology 16, 573–581 (2011).
Tufro, A. & Veron, D. VEGF and podocytes in diabetic nephropathy. Semin. Nephrol. 32, 385–393 (2012).
Nakagawa, T. Uncoupling of the VEGF-endothelial nitric oxide axis in diabetic nephropathy: an explanation for the paradoxical effects of VEGF in renal disease. Am. J. Physiol. 292, F1665–F1672 (2007).
Jiang, S. et al. Cellular crosstalk of glomerular endothelial cells and podocytes in diabetic kidney disease. J. Cell Commun. Signal. 16, 313–331 (2022).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Investig. 111, 707–716 (2003).
Veron, D. et al. Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts alphaVbeta3 integrin signaling in the glomerulus. PLoS ONE 7, e40589 (2012).
Fu, J., Lee, K., Chuang, P. Y., Liu, Z. & He, J. C. Glomerular endothelial cell injury and cross talk in diabetic kidney disease. Am. J. Physiol. Ren. Physiol. 308, F287–F297 (2015).
Tanabe, K., Maeshima, Y., Sato, Y. & Wada, J. Antiangiogenic therapy for diabetic nephropathy. BioMed. Res. Int. 2017, 5724069 (2017).
Cheng, H., Wang, H., Fan, X., Paueksakon, P. & Harris, R. C. Improvement of endothelial nitric oxide synthase activity retards the progression of diabetic nephropathy in db/db mice. Kidney Int. 82, 1176–1183 (2012).
Flyvbjerg, A. et al. The role of growth hormone, insulin-like growth factors (IGFs), and IGF-binding proteins in experimental diabetic kidney disease. Metab. Clin. Exp. 44, 67–71 (1995).
Petrosyan, A. et al. A glomerulus-on-a-chip to recapitulate the human glomerular filtration barrier. Nat. Commun. 10, 3656 (2019).
Lassén, E. & Daehn, I. S. Molecular mechanisms in early diabetic kidney disease: glomerular endothelial cell dysfunction. Int. J. Mol. Sci. 21, 9456 (2020).
Murray, I. V. & Paolini, M. A. Histology, Kidney and Glomerulus (StatPearls Publishing, 2024).
Ebefors, K., Lassén, E., Anandakrishnan, N., Azeloglu, E. U. & Daehn, I. S. Modeling the glomerular filtration barrier and intercellular crosstalk. Front. Physiol. 12, 689083 (2021).
Yu, H., Song, Y. Y. & Li, X. H. Early diabetic kidney disease: focus on the glycocalyx. World J. Diabetes 14, 460–480 (2023).
Garsen, M., Rops, A. L. W. M. M., Rabelink, T. J., Berden, J. H. M. & Van Der Vlag, J. The role of heparanase and the endothelial glycocalyx in the development of proteinuria. Nephrol. Dialysis Transplant. 29, 49–55 (2014).
Lipowsky, H. H. & Lescanic, A. The effect of doxycycline on shedding of the glycocalyx due to reactive oxygen species. Microvascular Res. 90, 80–85 (2013).
van Golen, R. F., van Gulik, T. M. & Heger, M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic. Biol. Med. 52, 1382–1402 (2012).
Jeansson, M. & Haraldsson, B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am. J. Physiol. 290, F111–F116 (2006).
Salmon, A. H. & Satchell, S. C. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J. Pathol. 226, 562–574 (2012).
Lewis, E. J. et al. Sulodexide for kidney protection in type 2 diabetes patients with microalbuminuria: a randomized controlled trial. Am. J. Kidney Dis. 58, 729–736 (2011).
Isermann, B. et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat. Med. 13, 1349–1358 (2007).
Madhusudhan, T., Kerlin, B. A. & Isermann, B. The emerging role of coagulation proteases in kidney disease. Nat. Rev. Nephrol. 12, 94–109 (2016).
Suzuki, K. Thrombomodulin: a key regulator of intravascular blood coagulation, fibrinolysis, and inflammation, and a treatment for disseminated intravascular coagulation. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 101, 75–97 (2025).
Boron, M., Hauzer-Martin, T., Keil, J. & Sun, X.-L. Circulating thrombomodulin: release mechanisms, measurements, and levels in diseases and medical procedures. TH Open 6, e194–e212 (2022).
Mormile, A. et al. Physiological inhibitors of blood coagulation and prothrombin fragment F 1+2 in type 2 diabetic patients with normoalbuminuria and incipient nephropathy. Acta Diabetologica 33, 241–245 (1996).
Matsumoto, K. et al. Inverse correlation between activated protein C generation and carotid atherosclerosis in type 2 diabetic patients. Diabet. Med. 24, 1322–1328 (2007).
Gil-Bernabe, P. et al. Exogenous activated protein C inhibits the progression of diabetic nephropathy. J. Thrombosis Haemost. 10, 337–346 (2012).
Chung, E. Y. M., Badve, S. V., Heerspink, H. J. L. & Wong, M. G. Endothelin receptor antagonists in kidney protection for diabetic kidney disease and beyond? Nephrology 28, 97–108 (2023).
Martínez-Díaz, I. et al. Endothelin receptor antagonists in kidney disease. Int. J. Mol. Sci. 24, 3427 (2023).
Anguiano, L., Riera, M., Pascual, J. & Soler, M. Endothelin blockade in diabetic kidney disease. J. Clin. Med. 4, 1171–1192 (2015).
Barton, M., Shaw, S., d’Uscio, L. V., Moreau, P. & Lüscher, T. F. Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation. Biochem. Biophys. Res. Commun. 238, 861–865 (1997).
Saleh, M. A., Boesen, E. I., Pollock, J. S., Savin, V. J. & Pollock, D. M. Endothelin receptor A-specific stimulation of glomerular inflammation and injury in a streptozotocin-induced rat model of diabetes. Diabetologia 54, 979–988 (2011).
Haruhara, K., Kanzaki, G. & Tsuboi, N. Nephrons, podocytes and chronic kidney disease: strategic antihypertensive therapy for renoprotection. Hypertension Res. 46, 299–310 (2023).
Kelly, D. J. et al. Effects of endothelin or angiotensin II receptor blockade on diabetes in the transgenic (mRen-2)27 rat. Kidney Int. 57, 1882–1894 (2000).
Cosenzi, A. et al. Nephroprotective effect of bosentan in diabetic rats. J. Cardiovasc. Pharmacol. 42, 752–756 (2003).
Mann, J. F. et al. Avosentan for overt diabetic nephropathy. J. Am. Soc. Nephrol. 21, 527–535 (2010).
de Zeeuw, D. et al. The endothelin antagonist atrasentan lowers residual albuminuria in patients with type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 25, 1083–1093 (2014).
Schievink, B. et al. Prediction of the effect of atrasentan on renal and heart failure outcomes based on short-term changes in multiple risk markers. Eur. J. Prevent. Cardiol. 23, 758–768 (2016).
Heerspink, H. J. L. et al. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet 393, 1937–1947 (2019).
Ahmad, N. et al. Endothelin receptor antagonists as a potential treatment of diabetic nephropathy: a systematic review. Cureus https://doi.org/10.7759/cureus.19325 (2021).
Heerspink, H. J. L. et al. Zibotentan in combination with dapagliflozin compared with dapagliflozin in patients with chronic kidney disease (ZENITH-CKD): a multicentre, randomised, active-controlled, phase 2b, clinical trial. Lancet 402, 2004–2017 (2023).
Tsai, Y.-C. et al. Angiopoietin-2, angiopoietin-1 and subclinical cardiovascular disease in chronic kidney disease. Sci. Rep. 6, 39400 (2016).
Kim, W. The role of angiopoietin-1 in kidney disease. Electrolyte Blood Press. 6, 22 (2008).
Gnudi, L. Angiopoietins and diabetic nephropathy. Diabetologia 59, 1616–1620 (2016).
Davis, B. et al. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J. Am. Soc. Nephrol. 18, 2320–2329 (2007).
Lim, H. S., Blann, A. D., Chong, A. Y., Freestone, B. & Lip, G. Y. H. Plasma vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in diabetes. Diabetes Care 27, 2918–2924 (2004).
Iseki, K., Ikemiya, Y., Iseki, C. & Takishita, S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int. 63, 1468–1474 (2003).
Cravedi, P. & Remuzzi, G. Pathophysiology of proteinuria and its value as an outcome measure in chronic kidney disease. Br. J. Clin. Pharmacol. 76, 516–523 (2013).
Kuusniemi, A.-M. et al. Kidneys with heavy proteinuria show fibrosis, inflammation, and oxidative stress, but no tubular phenotypic change. Kidney Int. 68, 121–132 (2005).
Hung, P.-H., Hsu, Y.-C., Chen, T.-H. & Lin, C.-L. Recent advances in diabetic kidney diseases: from kidney injury to kidney fibrosis. Int. J. Mol. Sci. 22, 11857 (2021).
Stadler, K., Goldberg, I. J. & Susztak, K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr. Diabetes Rep. 15, 40 (2015).
Birn, H. et al. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J. Clin. Investig. 105, 1353–1361 (2000).
Humphreys, B. D. et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J. Clin. Investig. 123, 4023–4035 (2013).
Kang, H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015).
Han, S. H. et al. PGC-1α protects from notch-induced kidney fibrosis development. J. Am. Soc. Nephrol. 28, 3312–3322 (2017).
Proctor, G. et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55, 2502–2509 (2006).
Chau, B. N. et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 4, 121ra118 (2012).
Davis, T. M. E. et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the fenofibrate intervention and event lowering in diabetes (FIELD) study. Diabetologia 54, 280–290 (2011).
Ginsberg, H. N. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1563–1574 (2010).
Forsblom, C. et al. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes. Diabetes Care 33, 215–220 (2010).
Hyun, Y. Y., Kim, K. S., Hong, S., Han, K. & Park, C. Y. Fenofibrate and risk of end-stage renal disease: a nationwide cohort study. Diabetes Obes. Metab. 26, 4583–4590 (2024).
Jenkins, A. J. et al. Not enough known about fenofibrate’s kidney effects in people with type 2 diabetes. Diabetes Res. Clin. Pract. 210, 111612 (2024).
Kim, J., Kim, H.-S. & Chung, J. H. Molecular mechanisms of mitochondrial DNA release and activation of the cGAS-STING pathway. Exp. Mol. Med. 55, 510–519 (2023).
Qi, H. et al. Glomerular endothelial mitochondrial dysfunction is essential and characteristic of diabetic kidney disease susceptibility. Diabetes 66, 763–778 (2017).
Kakimoto, M. et al. Accumulation of 8-hydroxy-2′-deoxyguanosine and mitochondrial DNA deletion in kidney of diabetic rats. Diabetes 51, 1588–1595 (2002).
Al-Kafaji, G., Aljadaan, A., Kamal, A. & Bakhiet, M. Peripheral blood mitochondrial DNA copy number as a novel potential biomarker for diabetic nephropathy in type 2 diabetes patients. Exp. Therapeutic Med. 16, 1483–1492 (2018).
Cao, H. et al. Urinary mitochondrial DNA: a potential early biomarker of diabetic nephropathy. Diabetes Metab. Res. Rev. 35, e3131 (2019).
Sun, C. et al. The activation of cGAS-STING in acute kidney injury. J. Inflamm. Res. 16, 4461–4470 (2023).
Doke, T. et al. NAD+ precursor supplementation prevents mtRNA/RIG-I-dependent inflammation during kidney injury. Nat. Metab. 5, 414–430 (2023).
Mitrofanova, A., Fontanella, A. M., Burke, G. W., Merscher, S. & Fornoni, A. Mitochondrial contribution to inflammation in diabetic kidney disease. Cells 11, 3635 (2022).
Chung, K. W. et al. Mitochondrial damage and activation of the STING pathway lead to renal inflammation and fibrosis. Cell Metab. 30, 784–799.e5 (2019).
Mitrofanova, A. et al. Activation of stimulator of IFN genes (STING) causes proteinuria and contributes to glomerular diseases. J. Am. Soc. Nephrol. 33, 2153–2173 (2022).
Wan, J., Liu, D., Pan, S., Zhou, S. & Liu, Z. NLRP3-mediated pyroptosis in diabetic nephropathy. Front. Pharmacol. 13, 998574 (2022).
Feng, X. et al. Ferroptosis enhanced diabetic renal tubular injury via HIF-1α/HO-1 pathway in db/db mice. Front. Endocrinol. 12, 626390 (2021).
Xu, Y. et al. High glucose-induced apoptosis and necroptosis in podocytes is regulated by UCHL1 via RIPK1/RIPK3 pathway. Exp. Cell Res. 382, 111463 (2019).
Wang, Y. et al. TLR4/NF-κB signaling induces GSDMD-related pyroptosis in tubular cells in diabetic kidney disease. Front. Endocrinol. 10, 603 (2019).
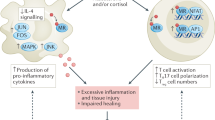
Jung, S. W. & Moon, J.-Y. The role of inflammation in diabetic kidney disease. Korean J. Intern. Med. 36, 753–766 (2021).
Pérez-Morales, R. E. et al. Inflammation in diabetic kidney disease. Nephron 143, 12–16 (2019).
Matoba, K. et al. Unraveling the role of inflammation in the pathogenesis of diabetic kidney disease. Int. J. Mol. Sci. 20, 3393 (2019).
Coca, S. G. et al. Plasma biomarkers and kidney function decline in early and established diabetic kidney disease. J. Am. Soc. Nephrol. 28, 2786–2793 (2017).
Park, J. et al. Functional methylome analysis of human diabetic kidney disease. JCI Insight 4, e128886 (2019).
Menne, J. et al. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol. Dialysis Transplant. 32, 307–315 (2016).
de Zeeuw, D. et al. The effect of CCR2 inhibitor CCX140-B on residual albuminuria in patients with type 2 diabetes and nephropathy: a randomised trial. Lancet Diabetes Endocrinol. 3, 687–696 (2015).
Heerspink, H. J. L. & De Zeeuw, D. Novel anti-inflammatory drugs for the treatment of diabetic kidney disease. Diabetologia 59, 1621–1623 (2016).
Kim, D. I. & Park, S. H. Sequential signaling cascade of IL-6 and PGC-1α is involved in high glucose-induced podocyte loss and growth arrest. Biochem. Biophys. Res. Commun. 435, 702–707 (2013).
Zhang, L., Xu, F. & Hou, L. IL-6 and diabetic kidney disease. Front. Immunol. 15, 1465625 (2024).
Moriyama, T. et al. Angiotensin II stimulates interleukin-6 release from cultured mouse mesangial cells. J. Am. Soc. Nephrol. 6, 95–101 (1995).
Sanchez-Alamo, B., Shabaka, A., Cachofeiro, V., Cases-Corona, C. & Fernandez-Juarez, G. Serum interleukin-6 levels predict kidney disease progression in diabetic nephropathy. Clin. Nephrol. 97, 1–9 (2022).
Koshino, A. et al. Interleukin-6 and cardiovascular and kidney outcomes in patients with type 2 diabetes: new insights from CANVAS. Diabetes Care 45, 2644–2652 (2022).
Ridker, P. M. et al. IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet 397, 2060–2069 (2021).
Liu, Y. et al. The alternative crosstalk between RAGE and nitrative thioredoxin inactivation during diabetic myocardial ischemia-reperfusion injury. Am. J. Physiol. 303, E841–E852 (2012).
Lee, D.-Y. et al. Nox4 NADPH oxidase mediates peroxynitrite-dependent uncoupling of endothelial nitric-oxide synthase and fibronectin expression in response to angiotensin II. J. Biol. Chem. 288, 28668–28686 (2013).
Pichler, R., Afkarian, M., Dieter, B. P. & Tuttle, K. R. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am. J. Physiol. Ren. Physiol. 312, F716–F731 (2017).
Anderberg, R. J. et al. Serum amyloid A and inflammation in diabetic kidney disease and podocytes. Lab. Investig. 95, 250–262 (2015).
Babaei-Jadidi, R., Karachalias, N., Ahmed, N., Battah, S. & Thornalley, P. J. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes 52, 2110–2120 (2003).
Alkhalaf, A. et al. A double-blind, randomized, placebo-controlled clinical trial on benfotiamine treatment in patients with diabetic nephropathy. Diabetes Care 33, 1598–1601 (2010).
Matsui, T. et al. RAGE-aptamer blocks the development and progression of experimental diabetic nephropathy. Diabetes 66, 1683–1695 (2017).
Alicic, R. Z., Cox, E. J., Neumiller, J. J. & Tuttle, K. R. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat. Rev. Nephrol. 17, 227–244 (2021).
Tuttle, K. R. et al. Indicators of kidney fibrosis in patients with type 2 diabetes and chronic kidney disease treated with dulaglutide. Am. J. Nephrol. 54, 74–82 (2023).
Sattar, N. et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabet. Endocrinol. 9, 653–662 (2021).
Bjornstad, P. et al. MO399: Remodel: a mechanistic trial evaluating the effects of semaglutide on the kidneys in people with type 2 diabetes and chronic kidney disease. Nephrol. Dialysis Transplant. 37, gfac070.013 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05536804 (2025).
Jiao, Y. et al. Activation of complement C1q and C3 in glomeruli might accelerate the progression of diabetic nephropathy: evidence from transcriptomic data and renal histopathology. J. Diabetes Investig. 13, 839–849 (2022).
Duan, S. et al. Association of glomerular complement C4c deposition with the progression of diabetic kidney disease in patients with type 2 diabetes. Front. Immunol. 11, 2073 (2020).
Jiang, S. et al. Complement deposition predicts worsening kidney function and underlines the clinical significance of the 2010 Renal Pathology Society classification of diabetic nephropathy. Front. Immunol. 13, 868127 (2022).
Woroniecka, K. I. et al. Transcriptome analysis of human diabetic kidney disease. Diabetes 60, 2354–2369 (2011).
Hansen, T. K. et al. Association between mannose-binding lectin, high-sensitivity C-reactive protein and the progression of diabetic nephropathy in type 1 diabetes. Diabetologia 53, 1517–1524 (2010).
Qin, X. et al. Glycation inactivation of the complement regulatory protein CD59. Diabetes 53, 2653–2661 (2004).
Petr, V. & Thurman, J. M. The role of complement in kidney disease. Nat. Rev. Nephrol. 19, 771–787 (2023).
Zuber, J., Fakhouri, F., Roumenina, L. T., Loirat, C. & Frémeaux-Bacchi, V. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat. Rev. Nephrol. 8, 643–657 (2012).
Trambas, I. A., Coughlan, M. T. & Tan, S. M. Therapeutic potential of targeting complement C5a receptors in diabetic kidney disease. Int. J. Mol. Sci. 24, 8758 (2023).
Zhao, L., Zou, Y. & Liu, F. Transforming growth factor-Beta1 in diabetic kidney disease. Front. Cell Dev. Biol. 8, 187 (2020).
Wang, L., Wang, H.-L., Liu, T.-T. & Lan, H.-Y. TGF-Beta as a master regulator of diabetic nephropathy. Int. J. Mol. Sci. 22, 7881 (2021).
Kim, S. I. & Choi, M. E. TGF-β-activated kinase-1: new insights into the mechanism of TGF-β signaling and kidney disease. Kidney Res. Clin. Pract. 31, 94–105 (2012).
Wang, Y.-W. et al. siRNA-targeting transforming growth factor-β type I receptor reduces wound scarring and extracellular matrix deposition of scar tissue. J. Investig. Dermatol. 134, 2016–2025 (2014).
Kavsak, P. et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol. Cell 6, 1365–1375 (2000).
Chen, H. Y. et al. The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes 60, 590–601 (2011).
Petersen, M. et al. Oral administration of GW788388, an inhibitor of TGF-β type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 73, 705–715 (2008).
Voelker, J. et al. Anti–TGF-β1 antibody therapy in patients with diabetic nephropathy. J. Am. Soc. Nephrol. 28, 953–962 (2017).
Wang, N. & Zhang, C. Recent advances in the management of diabetic kidney disease: slowing progression. Int. J. Mol. Sci. 25, 3086 (2024).
Agarwal, R. et al. Finerenone with empagliflozin in chronic kidney disease and type 2 diabetes. N. Engl. J. Med. 393, 533–543 (2025).
Acknowledgements
K.S. is supported by the NIH grant numbers R01DK076077, R01DK087635, P50DK114786, R01DK105821 and R01DK132630. The Susztak lab is also supported by Boehringer Ingelheim, Bayer, Novo Nordisk, Novartis, Calico, Maze, Ventus, GSK, Gilead, Regeneron and ONO Pharma for studies not related to this manuscript.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. K.S. and V.M.L. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martinez Leon, V., Hilburg, R. & Susztak, K. Mechanisms of diabetic kidney disease and established and emerging treatments. Nat Rev Endocrinol 22, 21–35 (2026). https://doi.org/10.1038/s41574-025-01171-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41574-025-01171-3