Abstract
The role of physical activity and exercise training in preventing the development of chronic disease and reducing mortality is increasingly recognized. A substantial body of evidence indicates that exercise confers these benefits to subsequent generations. This Review examines the effects of parental exercise, focusing on findings published within the past 10 years and related to mechanisms of transmission and sex-specific differences in both parents and offspring. Epigenetic modifications, including DNA methylation, histone modification and small non-coding RNAs, are crucial in how parental exercise influences offspring development. Paternal exercise alters the small RNA pool and methylation profiles in sperm, whereas maternal exercise affects both the in utero environment and postnatal lactation factors. Notably, male offspring show enhanced metabolic benefits, whereas female offspring exhibit greater cardiac improvements than male offspring. These findings highlight the sex-dependent nature of the generational effects of exercise and emphasize the need for further research into the molecular underpinnings and long-term implications for both male and female offspring.
Key points
-
Over the past decade, maternal and paternal exercise have emerged as tools to prevent the increasing prevalence of cardiac and metabolic disease in offspring.
-
A number of studies investigating parental exercise have improved understanding of its tissue-specific and sex-specific effects on offspring.
-
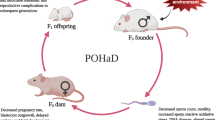
Studies have shown that beneficial effects of parental exercise extend to the second-generation offspring (F2) of dams that exercised in the founding generation (F0).
-
Signalling mechanisms that contribute to the effects of parental exercise have been described for proteins, fatty acids, oligosaccharides and exerkines in the placenta and breast milk of exercised dams.
-
Paternal exercise effects might be explained by epigenetic adaptations observed in sperm of sires and tissues of their offspring, and short non-coding RNAs are emerging as potential epigenetic mediators of paternal exercise effects.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Booth, F. W., Roberts, C. K. & Laye, M. J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2, 1143–1211 (2012).
Lee, D. C. et al. Leisure-time running reduces all-cause and cardiovascular mortality risk. J. Am. Coll. Cardiol. 64, 472–481 (2014).
Kusuyama, J., Alves-Wagner, A. B., Makarewicz, N. S. & Goodyear, L. J. Effects of maternal and paternal exercise on offspring metabolism. Nat. Metab. 2, 858–872 (2020).
Pinckard, K. M. et al. Maternal exercise preserves offspring cardiovascular health via oxidative regulation of the ryanodine receptor. Mol. Metab. 82, 101914 (2024). This study shows the effects of maternal exercise on the cardiovascular health of offspring as they age, demonstrating sex-specific effects in females mediated by calcium handling.
Wang, C. et al. Association of assisted reproductive technology, germline de novo mutations and congenital heart defects in a prospective birth cohort study. Cell Res. 31, 919–928 (2021). Through whole-genome sequencing of 407 offspring and their 365 families, the authors show that offspring of fathers who exercise carry less germline de novo mutations, a cause for congenital heart defects.
Claycombe-Larson, K. G., Bundy, A. N. & Roemmich, J. N. Paternal high-fat diet and exercise regulate sperm miRNA and histone methylation to modify placental inflammation, nutrient transporter mRNA expression and fetal weight in a sex-dependent manner. J. Nutr. Biochem. 81, 108373 (2020).
Costa-Júnior, J. M. et al. Paternal exercise improves the metabolic health of offspring via epigenetic modulation of the germline. Int. J. Mol. Sci. 23, 1 (2022). Methylation at CpG islands of main metabolic genes, including imprinting control regions, is replicated in sperm of exercised sires and muscle of their adult offspring, who show healthier muscle and whole-body metabolic phenotypes.
Freitas-Dias, R. et al. Offspring from trained male mice inherit improved muscle mitochondrial function through PPAR co-repressor modulation. Life Sci. 291, 120239 (2022).
Kusuyama, J. et al. Placental superoxide dismutase 3 mediates benefits of maternal exercise on offspring health. Cell Metab. 33, 939–956 (2021). This study describes a VDR–SOD3–AMPK–TET signalling mechanism that mediates the effects of maternal exercise on offspring liver through demethylation of glucose metabolism genes from embryonic stages to adulthood.
Kusuyama, J. et al. Maternal exercise-induced SOD3 reverses the deleterious effects of maternal high-fat diet on offspring metabolism through stabilization of H3K4me3 and protection against WDR82 carbonylation. Diabetes 71, 1170–1181 (2022).
Harris, J. E. et al. Exercise-induced 3′-sialyllactose in breast milk is a critical mediator to improve metabolic health and cardiac function in mouse offspring. Nat. Metab. 2, 678–687 (2020). The authors demonstrate a mechanism through which breast milk of exercised dams improves offspring metabolic and cardiovascular health through an oligosaccharide, 3′-sialyllactose.
Wolfs, D. et al. Brown fat-activating lipokine 12,13-diHOME in human milk is associated with infant adiposity. J. Clin. Endocrinol. Metab. 106, E943–E956 (2021). In humans, 12,13-diHOME increases in breast milk with an acute bout of exercise and is associated with a healthier body composition at birth and during the first months of life.
Son, J. S. et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci. Adv. 6, eaaz0359 (2020).
Sheldon, R. D. et al. Gestational exercise protects adult male offspring from high-fat diet-induced hepatic steatosis. J. Hepatol. 64, 171–178 (2016).
Alves-Wagner, A. B. et al. Grandmaternal exercise improves metabolic health of second-generation offspring. Mol. Metab. 60, 101490 (2022). This study provides the metabolic phenotype of male and female second-generation (F2) offspring of exercised grandmother mice (F0) born through first-generation (F1) males.
Martins Terra, M. et al. Multigenerational effects of chronic maternal exposure to a high sugar/fat diet and physical training. J. Dev. Orig. Health Dis. 11, 159–167 (2020).
Hardy, D. B., Mu, X., Marchiori, K. S. & Mottola, M. F. Exercise in pregnancy increases placental angiogenin without changes in oxidative or endoplasmic reticulum stress. Med. Sci. Sports Exerc. 53, 1846–1854 (2021).
Aparicio, V. A. et al. Influence of a concurrent exercise training program during pregnancy on the placenta mitochondrial DNA integrity and content of minerals with enzymatic relevance. The GESTAFIT project. Placenta 139, 19–24 (2023).
Baena-García, L. et al. A concurrent prenatal exercise program increases neonatal and placental weight and shortens labor: the GESTAFIT project. Scand. J. Med. Sci. Sports 33, 465–474 (2022).
Zhao, S. K. et al. Recreational physical activity before and during pregnancy and placental DNA methylation — an epigenome-wide association study. Am. J. Clin. Nutr. 116, 1168–1183 (2022).
Ruebel, M. L. et al. Maternal exercise prior to and during gestation induces sex-specific alterations in the mouse placenta. Int. J. Mol. Sci. 24, 16441 (2023).
Vinel, C. et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 24, 1360–1371 (2018).
Son, J. S. et al. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J. Physiol. 597, 3333–3347 (2019).
Son, J. S. et al. Maternal inactivity programs skeletal muscle dysfunction in offspring mice by attenuating apelin signaling and mitochondrial biogenesis. Cell Rep. 33, 108461 (2020).
Chae, S. A. et al. Exerkine apelin reverses obesity-associated placental dysfunction by accelerating mitochondrial biogenesis in mice. Am. J. Physiol. Endocrinol. Metab. 322, E467–E479 (2022).
Moholdt, T. & Stanford, K. I. Exercised breast milk: a kick-start to prevent childhood obesity? Trends Endocrinol. Metab. 35, 23–30 (2024).
Song, L. et al. Maternal exercise and high-fat diet affect hypothalamic neural projections in rat offspring in a sex-specific manner. J. Nutritional Biochem. 103, 108958 (2022).
Ribeiro, T. A. et al. Maternal low intensity physical exercise prevents obesity in offspring rats exposed to early overnutrition. Sci. Rep. 7, 7634 (2017).
Quiclet, C. et al. Maternal training during lactation modifies breast milk fatty acid composition and male offspring glucose homeostasis in rat. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1867, 159223 (2022).
Bopp, M., Lovelady, C., Hunter, C. & Kinsella, T. Maternal diet and exercise: effects on long-chain polyunsaturated fatty acid concentrations in breast milk. J. Am. Diet. Assoc. 105, 1098–1103 (2005).
Alexandre-Gouabau, M. C., David-Sochard, A., Royer, A. L., Parnet, P. & Paillé, V. Moderate high caloric maternal diet impacts dam breast milk metabotype and offspring lipidome in a sex-specific manner. Int. J. Mol. Sci. 21, 5428 (2020).
Castillo, P. et al. Reverting to a healthy diet during lactation normalizes maternal milk lipid content of diet-induced obese rats and prevents early alterations in the plasma lipidome of the offspring. Mol. Nutr. Food Res. 66, e2200204 (2022).
Martínez-Oca, P. et al. Maternal diet determines milk microbiome composition and offspring gut colonization in Wistar rats. Nutrients 15, 4322 (2023).
Le Bourgot, C. et al. Maternal short chain fructo-oligosaccharides supplementation during late gestation and lactation influences milk components and offspring gut metabolome: a pilot study. Sci. Rep. 14, 4236 (2024).
Zamanillo, R., Sánchez, J., Serra, F. & Palou, A. Breast milk supply of microRNA associated with leptin and adiponectin is affected by maternal overweight/obesity and influences infancy BMI. Nutrients 11, 2589 (2019).
Shah, K. B. et al. Gestational diabetes mellitus is associated with altered abundance of exosomal microRNAs in human milk. Clin. Ther. 44, 172–185 (2022).
Lowry, D. E., Paul, H. A. & Reimer, R. A. Impact of maternal obesity and prebiotic supplementation on select maternal milk microRNA levels and correlation with offspring outcomes. Br. J. Nutr. 127, 335–343 (2022).
Matos, B., Howl, J., Ferreira, R. & Fardilha, M. Exploring the effect of exercise training on testicular function. Eur. J. Appl. Physiol. 119, 1–8 (2019).
McPherson, N. O., Fullston, T., Bakos, H. W., Setchell, B. P. & Lane, M. Obese father’s metabolic state, adiposity, and reproductive capacity indicate son’s reproductive health. Fertil. Steril. 101, 865–873 (2014).
Stanford, K. I. et al. Paternal exercise improves glucose metabolism in adult offspring. Diabetes 67, 2530–2540 (2018).
McPherson, N. O., Owens, J. A., Fullston, T. & Lane, M. Preconception diet or exercise intervention in obese fathers normalizes sperm microRNA profile and metabolic syndrome in female offspring. Am. J. Physiol. Endocrinol. Metab. 308, E805–E821 (2015).
Murashov, A. K. et al. Paternal long-term exercise programs offspring for low energy expenditure and increased risk for obesity in mice. FASEB J. 30, 775–784 (2016).
Bartel, D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004).
Ingerslev, L. R. et al. Endurance training remodels sperm-borne small RNA expression and methylation at neurological gene hotspots. Clin. Epigenetics 10, 12 (2018).
Lin, T. et al. Small RNA perspective of physical exercise-related improvement of male reproductive dysfunction due to obesity. Front. Endocrinol. 13, 1038449 (2022).
Short, A. K. et al. Exercise alters mouse sperm small noncoding RNAs and induces a transgenerational modification of male offspring conditioned fear and anxiety. Transl. Psychiatry 7, e1114 (2017).
Fullston, T. et al. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 27, 4226–4243 (2013).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Tomar, A. et al. Epigenetic inheritance of diet-induced and sperm-borne mitochondrial RNAs. Nature 630, 720–727 (2024).
Nätt, D. & Öst, A. Male reproductive health and intergenerational metabolic responses from a small RNA perspective. J. Intern. Med. 288, 305–320 (2020).
Wu, P. H. et al. The evolutionarily conserved piRNA-producing locus pi6 is required for male mouse fertility. Nat. Genet. 52, 728–739 (2020).
Hernández-Saavedra, D. et al. Maternal exercise and paternal exercise induce distinct metabolite signatures in offspring tissues. Diabetes 71, 2094–2105 (2022). Metabolomic analyses of offspring muscle, liver and heart describe the main tissue metabolic shifts in offspring in response to maternal exercise alone, paternal exercise alone, and maternal and paternal exercise together.
Stanford, K. I. et al. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 64, 427–433 (2015).
Stanford, K. I. et al. Maternal exercise improves glucose tolerance in female offspring. Diabetes 66, 2124–2136 (2017).
Liu, J. et al. Aerobic exercise preconception and during pregnancy enhances oxidative capacity in the hindlimb muscles of mice offspring. J. Strength. Cond. Res. 32, 1391–1403 (2018).
Laker, R. C. et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1α gene and age-dependent metabolic dysfunction in the offspring. Diabetes 63, 1605–1611 (2014).
Beleza, J. et al. Gestational exercise increases male offspring’s maximal workload capacity early in life. Int. J. Mol. Sci. 23, 3916 (2022).
Son, J. S. et al. Maternal exercise intergenerationally drives muscle-based thermogenesis via activation of apelin-AMPK signaling. EBioMedicine 76, 103842 (2022).
Quiclet, C. et al. Maternal exercise modifies body composition and energy substrates handling in male offspring fed a high-fat/high-sucrose diet. J. Physiol. 595, 7049–7062 (2017).
Jevtovic, F. et al. Myogenically differentiated mesenchymal stem cell insulin sensitivity is associated with infant adiposity at 1 and 6 months of age. Obesity 31, 2349–2358 (2023).
Jevtovic, F. et al. Effects of maternal exercise on infant mesenchymal stem cell mitochondrial function, insulin action, and body composition in infancy. Physiol. Rep. 12, e16028 (2024). Complementing their previous study (ref. 60), Jevtovic et al. investigate metabolic substrate preference, insulin sensitivity and its association with later-in-life adiposity in myogenic, blood cord-derived mesenchymal stem cells of mothers engaging in an exercise programme.
Krout, D. et al. Paternal exercise protects mouse offspring from high-fat-diet-induced type 2 diabetes risk by increasing skeletal muscle insulin signaling. J. Nutritional Biochem. 57, 35–44 (2018).
Hinrichs, H., Faerber, A., Young, M., Ballentine, S. J. & Thompson, M. D. Maternal exercise protects male offspring from maternal diet–programmed nonalcoholic fatty liver disease progression. Endocrinology 164, bqad010 (2023).
Bae-Gartz, I. et al. Maternal exercise conveys protection against NAFLD in the offspring via hepatic metabolic programming. Sci. Rep. 10, 15424 (2020).
Kasper, P. et al. Maternal exercise mediates hepatic metabolic programming via activation of AMPK-PGC1 Axis in the offspring of obese mothers. Cells 10, 1247 (2021).
Cunningham, R. P. et al. Maternal physical activity and sex impact markers of hepatic mitochondrial health. Med. Sci. Sports Exerc. 50, 2040–2048 (2018).
Stevanović-Silva, J. et al. Exercise performed during pregnancy positively modulates liver metabolism and promotes mitochondrial biogenesis of female offspring in a rat model of diet-induced gestational diabetes. Biochim. Biophys. Acta Mol. Basis Dis. 1868, 166526 (2022).
Stevanović-Silva, J. et al. Maternal high-fat high-sucrose diet and gestational exercise modulate hepatic fat accumulation and liver mitochondrial respiratory capacity in mothers and male offspring. Metabolism 116, 154704 (2021).
Siti, F. et al. Maternal exercise before and during gestation modifies liver and muscle mitochondria in rat offspring. J. Exp. Biol. 222, jeb194969 (2019).
Batista, R. O. et al. Paternal exercise protects against liver steatosis in the male offspring of mice submitted to high fat diet. Life Sci. 263, 118583 (2020).
Lefebvre, P. & Staels, B. Hepatic sexual dimorphism — implications for non-alcoholic fatty liver disease. Nat. Rev. Endocrinol. 17, 662–670 (2021).
Chung, E. et al. Maternal exercise upregulates mitochondrial gene expression and increases enzyme activity of fetal mouse hearts. Physiol. Rep. 5, e13184 (2017).
Saiyin, T. et al. Maternal voluntary exercise mitigates oxidative stress and incidence of congenital heart defects in pre-gestational diabetes. J. Cell Mol. Med. 23, 5553–5565 (2019).
Ye, F., Lu, X., van Neck, R., Jones, D. L. & Feng, Q. Novel circRNA-miRNA-mRNA networks regulated by maternal exercise in fetal hearts of pregestational diabetes. Life Sci. 314, 121308 (2023).
Schulkey, C. E. et al. The maternal-age-associated risk of congenital heart disease is modifiable. Nature 520, 230–233 (2015).
Beeson, J. H. et al. Maternal exercise intervention in obese pregnancy improves the cardiovascular health of the adult male offspring. Mol. Metab. 16, 35–44 (2018).
De Sousa Neto, I. V. et al. Paternal resistance training induced modifications in the left ventricle proteome independent of offspring diet. Oxid. Med. Cell Longev. 2020, 5603580 (2020).
Schellong, K. et al. Maternal but not paternal high-fat diet (HFD) exposure at conception predisposes for ‘diabesity’ in offspring generations. Int. J. Env. Res. Public Health 17, 4229 (2020).
Takahashi, Y. et al. Transgenerational inheritance of acquired epigenetic signatures at CpG islands in mice. Cell 186, 715–731 (2023).
Acknowledgements
K.I.S. is supported by National Institutes of Health (NIH) Grants R01DK133859-01A1 to K.I.S. and the American Heart Association Grant AHA 23SFRNPCS1067042. E.F.-S. is supported by AHAPOST906327.
Author information
Authors and Affiliations
Contributions
E.F.-S. researched data for the article. The authors contributed equally to all other aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Susana Pereira, who co-reviewed with Carolina Tocantins; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Félix-Soriano, E., Stanford, K.I. Parental exercise mediates fetal metabolic and cardiac programming. Nat Rev Endocrinol (2025). https://doi.org/10.1038/s41574-025-01207-8
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41574-025-01207-8