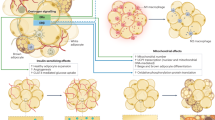
Abstract
Many diseases, including breast cancer, increase in women after menopause and with obesity. This Review addresses novel insights that link obesity, oestrogens, inflammation and breast cancer. Adipose tissue is chronically inflamed in obesity owing to pre-adipocyte expansion and activation of nuclear factor-κB (NF-κB), which upregulate pro-inflammatory cytokines. Obesity also impairs immunosurveillance. Emerging data indicate that the major oestrogens before and after menopause have opposing effects on inflammation. In contrast to the anti-inflammatory properties of premenopausal 17β-oestradiol, the dominant postmenopausal oestrogen, oestrone, is pro-inflammatory. Oestrone is synthesized in adipocytes, therefore the expanded adipose tissue biomass in obesity increases oestrone levels in both men and women, promoting NF-κB-driven inflammation. These pro-inflammatory effects of oestrone are also oncogenic, promoting breast cancer progression in laboratory models. The dominance of oestrone and loss of 17β-oestradiol might underlie the increased prevalence of hormone-responsive breast cancer after menopause, particularly in the context of obesity. Although oestrogens account for much of the excess breast cancer risk with obesity, data on 17β-oestradiol and oestrone levels in the breast and circulation in postmenopausal women, whether or not obesity is present, are limited. Weight loss is associated with reduced breast cancer risk and improved outcomes. The opportunity to use potent weight loss drugs as adjuncts to cancer therapy is discussed.
Key points
-
Increased oestrogen levels in obesity account for much of the increased breast cancer risk and mortality associated with obesity.
-
Activation of NF-κB contributes to oestrone-driven inflammation in adipose tissue and breast cancer.
-
The dominant premenopausal oestrogen, 17β-oestradiol, opposes NF-κB-driven inflammation in obesity, whereas oestrone, which dominates after menopause, promotes it.
-
Oestrone stimulates breast cancer stem cells, tumour growth and metastasis.
-
Oestrone and oestradiol levels in blood and in normal and malignant breast tissue, and their increase with weight gain after menopause need to be better characterized as they inform cancer risk.
-
Preclinical data support implementation of weight loss programmes as an adjuvant therapy for hormone-driven breast cancer and have implications for other cancers that have an increased risk and mortality in obesity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Lippman, M. E. & Allegra, J. C. Current concepts in cancer. Receptors in breast cancer. N. Engl. J. Med. 299, 930–933 (1978).
Lippman, M., Bolan, G. & Huff, K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 36, 4595–4601 (1976).
Lippman, M., Monaco, M. E. & Bolan, G. Effects of estrone, estradiol, and estriol on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 37, 1901–1907 (1977). This landmark study describes proliferative effects of oestradiol and oestrone on breast cancer growth in culture.
Clarke, R., Jones, B. C., Sevigny, C. M., Hilakivi-Clarke, L. A. & Sengupta, S. Experimental models of endocrine responsive breast cancer: strengths, limitations, and use. Cancer Drug Resist. 4, 762–783 (2021).
Jordan, V. C. Studies on the estrogen receptor in breast cancer — 20 years as a target for the treatment and prevention of cancer. Breast Cancer Res. Treat. 36, 267–285 (1995).
Santen, R. J., Brodie, H., Simpson, E. R., Siiteri, P. K. & Brodie, A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr. Rev. 30, 343–375 (2009).
Rudlowski, C. Male breast cancer. Breast Care 3, 183–189 (2008).
Li, N. H. Y. & Li, C. I. Incidence rate trends of breast cancer overall and by molecular subtype by race and ethnicity and age. JAMA Netw. Open 8, e2456142 (2025).
Koleckova, M., Kolar, Z., Ehrmann, J., Korinkova, G. & Trojanec, R. Age-associated prognostic and predictive biomarkers in patients with breast cancer. Oncol. Lett. 13, 4201–4207 (2017).
Jenkins, E. O. et al. Age-specific changes in intrinsic breast cancer subtypes: a focus on older women. Oncologist 19, 1076–1083 (2014).
Chen, M. T. et al. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci. Rep. 7, 9254 (2017).
Siiteri, P. K. Review of studies on estrogen biosynthesis in the human. Cancer Res. 42, 3269s–3273s (1982). This is a landmark review of oestrogen biosynthetic pathways in humans.
Dandliker, W. B. et al. Investigation of hormone-receptor interactions by means of fluorescence labeling. Cancer Res. 38, 4212–4224 (1978).
Sasson, S. & Notides, A. C. Estriol and estrone interaction with the estrogen receptor. I. Temperature-induced modulation of the cooperative binding of [3H]estriol and [3H]estrone to the estrogen receptor. J. Biol. Chem. 258, 8113–8117 (1983).
Key, T. J. et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J. Natl Cancer Inst. 95, 1218–1226 (2003).
Koliaki, C., Dalamaga, M. & Liatis, S. Update on the obesity epidemic: after the sudden rise, is the upward trajectory beginning to flatten? Curr. Obes. Rep. 12, 514–527 (2023).
Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 360, 1–8 (2020).
Harborg, S. et al. New horizons: epidemiology of obesity, diabetes mellitus, and cancer prognosis. J. Clin. Endocrinol. Metab. 109, 924–935 (2024).
Ward, Z. J. et al. Projected US state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381, 2440–2450 (2019). This is a review of the prevalence of obesity in the USA and projected increases over the next decade.
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N. Engl. J. Med. 348, 1625–1638 (2003). This landmark prospective study identified relationships between obesity and excess cancer mortality.
Renehan, A. G., Tyson, M., Egger, M., Heller, R. F. & Zwahlen, M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371, 569–578 (2008).
Reeves, G. K. et al. Cancer incidence and mortality in relation to body mass index in the million women study: cohort study. BMJ 335, 1134 (2007).
World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. AICR https://www3.paho.org/hq/dmdocuments/2011/nutrition-AICR-WCR-food-physical-activ.pdf (2007).
Shi, X. et al. Role of body mass index and weight change in the risk of cancer: a systematic review and meta-analysis of 66 cohort studies. J. Glob. Health 14, 04067 (2024).
Petrelli, F. et al. Association of obesity with survival outcomes in patients with cancer: a systematic review and meta-analysis. JAMA Netw. Open 4, e213520 (2021).
World Cancer Research Fund/American Institute for Cancer Research. Diet, nutrition, physical activity and cancer: a global perspective. Continuous update project expert report 2018. AICR https://www.wcrf.org/wp-content/uploads/2024/11/Summary-of-Third-Expert-Report-2018.pdf (2018).
Ligibel, J. A. et al. American Society of Clinical Oncology position statement on obesity and cancer. J. Clin. Oncol. 32, 3568–3574 (2014).
Picon-Ruiz, M., Morata-Tarifa, C., Valle-Goffin, J. J., Friedman, E. R. & Slingerland, J. M. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J. Clin. 67, 378–397 (2017).
Suzuki, R., Orsini, N., Saji, S., Key, T. J. & Wolk, A. Body weight and incidence of breast cancer defined by estrogen and progesterone receptor status — a meta-analysis. Int. J. Cancer 124, 698–712 (2009).
Munsell, M. F., Sprague, B. L., Berry, D. A., Chisholm, G. & Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen–progestin use and hormone receptor status. Epidemiol. Rev. 36, 114–136 (2014). This is a large meta-analysis of 89 epidemiological reports of joint relationships among BMI, menopausal status and breast cancer.
Chan, D. S. et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 25, 1901–1914 (2014). This is a large meta-analysis of 82 studies demonstrating the relationship between BMI and breast cancer survival.
Schairer, C. et al. Risk factors for inflammatory breast cancer and other invasive breast cancers. J. Natl Cancer Inst. 105, 1373–1384 (2013).
Holm, J. B. et al. The association between body mass index and neoadjuvant chemotherapy response in patients with breast cancer. Breast Cancer Res. 27, 130 (2025).
LeVee, A. & Mortimer, J. The challenges of treating patients with breast cancer and obesity. Cancers 15, 2526 (2023).
Warner, E. T. et al. Impact of race, ethnicity, and BMI on achievement of pathologic complete response following neoadjuvant chemotherapy for breast cancer: a pooled analysis of four prospective alliance clinical trials (A151426). Breast Cancer Res. Treat. 159, 109–118 (2016).
Barone, I. et al. Obesity and endocrine therapy resistance in breast cancer: mechanistic insights and perspectives. Obes. Rev. 23, e13358 (2022).
Qureshi, R. et al. The major pre- and postmenopausal estrogens play opposing roles in obesity-driven mammary inflammation and breast cancer development. Cell Metab. 31, 1154–1172 (2020). This is the first report showing that oestrone activates and oestradiol opposes NF-κB-driven inflammation in vivo, and that oestrone-bound ER cooperates with NF-κB to induce pro-inflammatory cytokine genes, increase cancer stem cells and ER+ breast cancer growth.
Frasor, J., El-Shennawy, L., Stender, J. D. & Kastrati, I. NF-kB affects estrogen receptor expression and activity in breast cancer through multiple mechanisms. Mol. Cell Endocrinol. 418 (Pt 3), 235–239 (2015).
Crozier, J. A. et al. Effect of body mass index on tumor characteristics and disease-free survival in patients from the HER2-positive adjuvant trastuzumab trial N9831. Cancer 119, 2447–2454 (2013).
Ligorio, F. et al. Prognostic impact of body mass index (BMI) in HER2+ breast cancer treated with anti-HER2 therapies: from preclinical rationale to clinical implications. Ther. Adv. Med. Oncol. 14, 17588359221079123 (2022).
Martel, S. et al. Body mass index and weight change in patients with HER2-positive early breast cancer: exploratory analysis of the ALTTO BIG 2-06 trial. J. Natl Compr. Cancer Netw. 19, 181–189 (2021).
Liedtke, S. et al. Postmenopausal sex hormones in relation to body fat distribution. Obesity 20, 1088–1095 (2012).
Key, T. J. et al. Steroid hormone measurements from different types of assays in relation to body mass index and breast cancer risk in postmenopausal women: reanalysis of eighteen prospective studies. Steroids 99, 49–55 (2015). This is a meta-analysis of 18 prospective studies reporting on the relationships between BMI and breast cancer risk as a follow-up of earlier reports by Key et al. published by the Journal of the National Cancer Institute in 2002 and 2003 from International Endogenous Hormones and Breast Cancer Collaborative Group data.
Grodin, J. M., Siiteri, P. K. & MacDonald, P. C. Source of estrogen production in postmenopausal women. J. Clin. Endocrinol. Metab. 36, 207–214 (1973).
Siiteri, P. K., Ashby, R., Schwarz, B. & MacDonald, P. C. Mechanism of estrogen action studies in the human. J. Steroid Biochem. 3, 459–470 (1972).
Nimrod, A. & Ryan, K. J. Aromatization of androgens by human abdominal and breast fat tissue. J. Clin. Endocrinol. Metab. 40, 367–372 (1975).
MacDonald, P. C., Edman, C. D., Hemsell, D. L., Porter, J. C. & Siiteri, P. K. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am. J. Obstet. Gynecol. 130, 448–455 (1978). This landmark paper showed that obesity potentiates oestrone production in extraglandular sites more in postmenopausal than in premenopausal women and increases postmenopausal endometrial cancer risk.
Yu, H. et al. Plasma sex steroid hormones and breast cancer risk in Chinese women. Int. J. Cancer 105, 92–97 (2003).
Adly, L. et al. Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. Int. J. Cancer 119, 2402–2407 (2006).
Miyoshi, Y., Tanji, Y., Taguchi, T., Tamaki, Y. & Noguchi, S. Association of serum estrone levels with estrogen receptor-positive breast cancer risk in postmenopausal Japanese women. Clin. Cancer Res. 9, 2229–2233 (2003).
Michels, K. B., Terry, K. L. & Willett, W. C. Longitudinal study on the role of body size in premenopausal breast cancer. Arch. Intern. Med. 166, 2395–2402 (2006).
Berstad, P. et al. A case–control study of body mass index and breast cancer risk in white and African–American women. Cancer Epidemiol. Biomark. Prev. 19, 1532–1544 (2010).
White, A. J., Nichols, H. B., Bradshaw, P. T. & Sandler, D. P. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer 121, 3700–3708 (2015).
Harris, H. R., Willett, W. C., Terry, K. L. & Michels, K. B. Body fat distribution and risk of premenopausal breast cancer in the Nurses’ Health study II. J. Natl Cancer Inst. 103, 273–278 (2011).
van den Brandt, P. A. et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am. J. Epidemiol. 152, 514–527 (2000).
Qureshi, R. et al. Estrone, the major postmenopausal estrogen, binds ERa to induce SNAI2, epithelial-to-mesenchymal transition, and ER+ breast cancer metastasis. Cell Rep. 41, 111672 (2022). This is the first demonstration that oestrone drives greater metastasis of ER+ breast cancer models than oestradiol and that oestrone-bound ER activates epithelial-to-mesenchymal transition drivers in breast cancer models.
Zhang, Q., Lenardo, M. J. & Baltimore, D. 30 years of NF-kB: a blossoming of relevance to human pathobiology. Cell 168, 37–57 (2017).
Mao, H., Zhao, X. & Sun, S. C. NF-kB in inflammation and cancer. Cell Mol. Immunol. 22, 811–839 (2025).
Rayet, B. & Gelinas, C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene 18, 6938–6947 (1999).
Nakshatri, H. et al. Constitutive activation of NF-kB during progression of breast cancer to hormone-independent growth. Mol. Cell Biol. 17, 3629–3639 (1997).
Sovak, M. A. et al. Aberrant nuclear factor-kB/Rel expression and the pathogenesis of breast cancer. J. Clin. Invest. 100, 2952–2960 (1997).
Romieu-Mourez, R. et al. Mouse mammary tumor virus c-rel transgenic mice develop mammary tumors. Mol. Cell Biol. 23, 5738–5754 (2003).
Hagberg, C. E. & Spalding, K. L. White adipocyte dysfunction and obesity-associated pathologies in humans. Nat. Rev. Mol. Cell Biol. 25, 270–289 (2024).
Simons, P. J., van den Pangaart, P. S., van Roomen, C. P., Aerts, J. M. & Boon, L. Cytokine-mediated modulation of leptin and adiponectin secretion during in vitro adipogenesis: evidence that tumor necrosis factor-α- and interleukin-1β-treated human preadipocytes are potent leptin producers. Cytokine 32, 94–103 (2005).
Vona-Davis, L. & Rose, D. P. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 20, 193–201 (2009).
Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017). This is an excellent review of the links between obesity, inflammation and metabolic disease.
McLaughlin, T., Ackerman, S. E., Shen, L. & Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Invest. 127, 5–13 (2017).
Brown, K. A. Metabolic pathways in obesity-related breast cancer. Nat. Rev. Endocrinol. 17, 350–363 (2021).
Taranto, D., Kloosterman, D. J. & Akkari, L. Macrophages and T cells in metabolic disorder-associated cancers. Nat. Rev. Cancer 24, 744–767 (2024). This is an excellent review of the links between macrophage and T cell dysfunction in metabolic disorders.
Chakarov, S., Bleriot, C. & Ginhoux, F. Role of adipose tissue macrophages in obesity-related disorders. J. Exp. Med. 219, e20211948 (2022).
Quail, D. F. & Dannenberg, A. J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 15, 139–154 (2019).
Iyengar, N. M. et al. Menopause is a determinant of breast adipose inflammation. Cancer Prev. Res. 8, 349–358 (2015). This article showed that breast tissue inflammation increases at menopause.
Morris, P. G. et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 4, 1021–1029 (2011).
Bader, J. E. et al. Obesity induces PD-1 on macrophages to suppress anti-tumour immunity. Nature 630, 968–975 (2024).
Manzo, T. et al. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J. Exp. Med. 217, e20191920 (2020).
Michelet, X. et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 19, 1330–1340 (2018).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25, 141–151 (2019).
Ringel, A. E. et al. Obesity shapes metabolism in the tumor microenvironment to suppress anti-tumor immunity. Cell 183, 1848–1866 (2020). This report provides evidence that obesity shapes the tumour immune microenvironment to oppose antitumour immunity.
Vick, L. V., Canter, R. J., Monjazeb, A. M. & Murphy, W. J. Multifaceted effects of obesity on cancer immunotherapies: bridging preclinical models and clinical data. Semin. Cancer Biol. 95, 88–102 (2023).
Wu, B. et al. Adipose PD-L1 modulates PD-1/PD-L1 checkpoint blockade immunotherapy efficacy in breast cancer. Oncoimmunology 7, e1500107 (2018).
Lynch, L. A. et al. Are natural killer cells protecting the metabolically healthy obese patient? Obesity 17, 601–605 (2009).
Bahr, I. et al. Diet-induced obesity is associated with an impaired NK cell function and an increased colon cancer incidence. J. Nutr. Metab. 2017, 4297025 (2017).
Naujoks, W. et al. Characterization of surface receptor expression and cytotoxicity of human NK cells and NK cell subsets in overweight and obese humans. Front. Immunol. 11, 573200 (2020).
De Barra, C., O’Shea, D. & Hogan, A. E. NK cells vs. obesity: a tale of dysfunction and redemption. Clin. Immunol. 255, 109744 (2023).
Dyck, L. & Lynch, L. Diverse effects of obesity on antitumor immunity and immunotherapy. Trends Mol. Med. 29, 112–123 (2023).
Franco, H. L., Nagari, A. & Kraus, W. L. TNFα signaling exposes latent estrogen receptor binding sites to alter the breast cancer cell transcriptome. Mol. Cell 58, 21–34 (2015). This article showed how the oestradiol-bound ER cistrome shifts from oestrogen response elements to κBRE DNA-binding sites in the presence of NF-κB activation by TNF, to modulate NF-κB target genes.
Nettles, K. W. et al. CBP Is a dosage-dependent regulator of nuclear factor-kB suppression by the estrogen receptor. Mol. Endocrinol. 22, 263–272 (2008).
Giraud, S. N., Caron, C. M., Pham-Dinh, D., Kitabgi, P. & Nicot, A. B. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkB-dependent CCL2 expression in reactive astrocytes. Proc. Natl Acad. Sci. USA 107, 8416–8421 (2010).
Stender, J. D. et al. Structural and molecular mechanisms of cytokine-mediated endocrine resistance in human breast cancer cells. Mol. Cell 65, 1122–1135 (2017).
Stein, B. & Yang, M. X. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kB and C/EBPβ. Mol. Cell Biol. 15, 4971–4979 (1995).
Kalaitzidis, D. & Gilmore, T. D. Transcription factor cross-talk: the estrogen receptor and NF-kB. Trends Endocrinol. Metab. 16, 46–52 (2005).
Wen, Y. et al. Estrogen attenuates nuclear factor-kB activation induced by transient cerebral ischemia. Brain Res. 1008, 147–154 (2004).
Dodel, R. C., Du, Y., Bales, K. R., Gao, F. & Paul, S. M. Sodium salicylate and 17β-estradiol attenuate nuclear transcription factor NF-kB translocation in cultured rat astroglial cultures following exposure to amyloid A β(1–40) and lipopolysaccharides. J. Neurochem. 73, 1453–1460 (1999).
Galien, R. & Garcia, T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kB site. Nucleic Acids Res. 25, 2424–2429 (1997).
Ghisletti, S., Meda, C., Maggi, A. & Vegeto, E. 17β-estradiol inhibits inflammatory gene expression by controlling NF-kB intracellular localization. Mol. Cell Biol. 25, 2957–2968 (2005).
Nwachukwu, J. C. et al. Resveratrol modulates the inflammatory response via an estrogen receptor-signal integration network. eLife 3, e02057 (2014).
Hilborn, E., Stal, O. & Jansson, A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: with a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 8, 30552–30562 (2017).
Rochefort, H. et al. Estrogen receptor mediated inhibition of cancer cell invasion and motility: an overview. J. Steroid Biochem. Mol. Biol. 65, 163–168 (1998).
Al Saleh, S., Al Mulla, F. & Luqmani, Y. A. Estrogen receptor silencing induces epithelial to mesenchymal transition in human breast cancer cells. PLoS ONE 6, e20610 (2011).
Jordan, V. C. Tamoxifen: a most unlikely pioneering medicine. Nat. Rev. Drug Discov. 2, 205–213 (2003).
Eliassen, A. H. et al. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J. Natl Cancer Inst. 98, 1406–1415 (2006).
Stanczyk, F. Z., Mathews, B. W. & Sherman, M. E. Relationships of sex steroid hormone levels in benign and cancerous breast tissue and blood: a critical appraisal of current science. Steroids 99, 91–102 (2015). One of the more comprehensive reviews of earlier available data on sex steroid hormones in benign and maligant breast tissues and blood.
van Landeghem, A. A., Poortman, J., Nabuurs, M. & Thijssen, J. H. Endogenous concentration and subcellular distribution of estrogens in normal and malignant human breast tissue. Cancer Res. 45, 2900–2906 (1985).
Kaaks, R. et al. Premenopausal serum sex hormone levels in relation to breast cancer risk, overall and by hormone receptor status — results from the EPIC cohort. Int. J. Cancer 134, 1947–1957 (2014).
Key, T. J. et al. Sex hormones and risk of breast cancer in premenopausal women: a collaborative reanalysis of individual participant data from seven prospective studies. Lancet Oncol. 14, 1009–1019 (2013).
Rohan, T. E. et al. Body fat and breast cancer risk in postmenopausal women: a longitudinal study. J. Cancer Epidemiol. 2013, 754815 (2013).
Phipps, A. I. et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol. Biomark. Prev. 20, 454–463 (2011).
Kaaks, R. et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr. Relat. Cancer 12, 1071–1082 (2005).
Pasqualini, J. R. et al. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. J. Clin. Endocrinol. Metab. 81, 1460–1464 (1996).
Bernstein, L. et al. Serum hormone levels in pre-menopausal Chinese women in Shanghai and white women in Los Angeles: results from two breast cancer case–control studies. Cancer Causes Control 1, 51–58 (1990).
Savolainen-Peltonen, H. et al. Breast adipose tissue estrogen metabolism in postmenopausal women with or without breast cancer. J. Clin. Endocrinol. Metab. 99, E2661–E2667 (2014).
Key, T., Appleby, P., Barnes, I. & Reeves, G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J. Natl Cancer Inst. 94, 606–616 (2002).
Bonney, R. C., Reed, M. J., Davidson, K., Beranek, P. A. & James, V. H. The relationship between 17 β-hydroxysteroid dehydrogenase activity and oestrogen concentrations in human breast tumours and in normal breast tissue. Clin. Endocrinol. 19, 727–739 (1983).
de Jong, P. C. et al. Inhibition of breast cancer tissue aromatase activity and estrogen concentrations by the third-generation aromatase inhibitor vorozole. Cancer Res. 57, 2109–2111 (1997).
Chetrite, G. S., Cortes-Prieto, J., Philippe, J. C., Wright, F. & Pasqualini, J. R. Comparison of estrogen concentrations, estrone sulfatase and aromatase activities in normal, and in cancerous, human breast tissues. J. Steroid Biochem. Mol. Biol. 72, 23–27 (2000).
Lonning, P. E. et al. Exploring breast cancer estrogen disposition: the basis for endocrine manipulation. Clin. Cancer Res. 17, 4948–4958 (2011).
Vermeulen, A. et al. Aromatase, 17β-hydroxysteroid dehydrogenase and intratissular sex hormone concentrations in cancerous and normal glandular breast tissue in postmenopausal women. Eur. J. Cancer Clin. Oncol. 22, 515–525 (1986).
Thijssen, J. H., Blankenstein, M. A., Donker, G. H. & Daroszewski, J. Endogenous steroid hormones and local aromatase activity in the breast. J. Steroid Biochem. Mol. Biol. 39, 799–804 (1991).
Lonning, P. E. et al. Tissue estradiol is selectively elevated in receptor positive breast cancers while tumour estrone is reduced independent of receptor status. J. Steroid Biochem. Mol. Biol. 117, 31–41 (2009).
Blankenstein, M. A. et al. Intratumoral levels of estrogens in breast cancer. J. Steroid Biochem. Mol. Biol. 69, 293–297 (1999).
Honma, N. et al. Sex steroid hormones in pairs of tumor and serum from breast cancer patients and pathobiological role of androstene-3β, 17β-diol. Cancer Sci. 102, 1848–1854 (2011).
Kakugawa, Y. et al. Associations of obesity and physical activity with serum and intratumoral sex steroid hormone levels among postmenopausal women with breast cancer: analysis of paired serum and tumor tissue samples. Breast Cancer Res. Treat. 162, 115–125 (2017).
Recchione, C. et al. Testosterone, dihydrotestosterone and oestradiol levels in postmenopausal breast cancer tissues. J. Steroid Biochem. Mol. Biol. 52, 541–546 (1995).
Reed, M. J. Oestradiol-17β hydroxysteroid dehydrogenase: its family and function. J. Endocrinol. 129, 163–165 (1991).
Aminian, A. et al. Association of bariatric surgery with cancer risk and mortality in adults with obesity. JAMA 327, 2423–2433 (2022).
McTiernan, A. et al. Physical activity in cancer prevention and survival: a systematic review. Med. Sci. Sports Exerc. 51, 1252–1261 (2019).
McTiernan, A. et al. Effect of exercise on serum estrogens in postmenopausal women: a 12-month randomized clinical trial. Cancer Res. 64, 2923–2928 (2004). This article showed that exercise-induced weight loss decreases both oestradiol and oestrone levels in postmenopausal women who are overweight.
Dash, C. et al. Effect of exercise on metabolic syndrome in black women by family history and predicted risk of breast cancer: the FIERCE study. Cancer 124, 3355–3363 (2018).
Byers, T. & Sedjo, R. L. Does intentional weight loss reduce cancer risk? Diabetes Obes. Metab. 13, 1063–1072 (2011).
Neilson, H. K., Friedenreich, C. M., Brockton, N. T. & Millikan, R. C. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol. Biomark. Prev. 18, 11–27 (2009).
Dethlefsen, C., Pedersen, K. S. & Hojman, P. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res. Treat. 162, 399–408 (2017).
Bruinsma, T. J., Dyer, A. M., Rogers, C. J., Schmitz, K. H. & Sturgeon, K. M. Effects of diet and exercise-induced weight loss on biomarkers of inflammation in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol. Biomark. Prev. 30, 1048–1062 (2021).
Campbell, K. L. et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: randomized controlled trial. J. Clin. Oncol. 30, 2314–2326 (2012).
Rock, C. L. et al. Favorable changes in serum estrogens and other biologic factors after weight loss in breast cancer survivors who are overweight or obese. Clin. Breast Cancer 13, 188–195 (2013).
Friedenreich, C. M. et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J. Clin. Oncol. 28, 1458–1466 (2010).
Eliassen, A. H., Colditz, G. A., Rosner, B., Willett, W. C. & Hankinson, S. E. Adult weight change and risk of postmenopausal breast cancer. JAMA 296, 193–201 (2006). This prospective cohort study showed that weight gain in adulthood, especially after menopause, increases the risk of breast cancer among postmenopausal women, whereas weight loss after menopause is associated with a decreased breast cancer risk.
Smith, L. A. et al. Weight loss reverses the effects of aging and obesity on mammary tumor immunosuppression and progression. Cancer Prev. Res. 18, 453–463 (2025).
Sumithran, P. et al. Long-term persistence of hormonal adaptations to weight loss. N. Engl. J. Med. 365, 1597–1604 (2011).
Frias, J. P. et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N. Engl. J. Med. 385, 503–515 (2021).
Drucker, D. J. Expanding applications of therapies based on GLP1. Nat. Rev. Endocrinol. 21, 65–66 (2025). This is a succinct review of anti-inflammatory effects of glucagon-like peptide 1 drugs from human clinical trials so far.
Trujillo, J. M., Nuffer, W. & Smith, B. A. GLP-1 receptor agonists: an updated review of head-to-head clinical studies. Ther. Adv. Endocrinol. Metab. 12, 2042018821997320 (2021).
Baggio, L. L. & Drucker, D. J. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol. Metab. 46, 101090 (2021).
Dave, B. P. et al. From diabetes to diverse domains: the multifaceted roles of GLP-1 receptor agonists. Mol. Biol. Rep. 51, 835 (2024).
Ard, J., Fitch, A., Fruh, S. & Herman, L. Weight loss and maintenance related to the mechanism of action of glucagon-like peptide 1 receptor agonists. Adv. Ther. 38, 2821–2839 (2021).
Zhang, Q. et al. The glucose-dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS–GIPR signaling. Cell Metab. 33, 833–844 (2021).
Drucker, D. J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 27, 740–756 (2018).
Wolff Sagy, Y. et al. Glucagon-like peptide-1 receptor agonists compared with bariatric metabolic surgery and the risk of obesity-related cancer: an observational, retrospective cohort study. eClinicalMedicine 83, 103213 (2025).
Wang, L., Xu, R., Kaelber, D. C. & Berger, N. A. Glucagon-like peptide 1 receptor agonists and 13 obesity-associated cancers in patients with type 2 diabetes. JAMA Netw. Open 7, e2421305 (2024).
Glenny, E. M. et al. Tirzepatide attenuates mammary tumor progression in diet-induced obese mice. Preprint at bioRxiv https://doi.org/10.1101/2024.01.20.576484 (2024).
Huang, L., Zeng, J., Wang, Y. & Pollak, M. Tirzepatide inhibits tumor growth in mice with diet-induced obesity. Preprint at bioRxiv https://doi.org/10.1101/2023.06.22.546093 (2023).
Ben Nasr, M. et al. Glucagon-like peptide 1 receptor is a T cell-negative costimulatory molecule. Cell Metab. 36, 1302–1319 (2024).
Li, X. et al. Inflammation and aging: signaling pathways and intervention therapies. Signal Transduct. Target. Ther. 8, 239 (2023).
Fu, Y. et al. Immunosenescence: signaling pathways, diseases and therapeutic targets. Signal Transduct. Target. Ther. 10, 250 (2025).
Hilborn, E., Stal, O., Alexeyenko, A. & Jansson, A. The regulation of hydroxysteroid 17β-dehydrogenase type 1 and 2 gene expression in breast cancer cell lines by estradiol, dihydrotestosterone, microRNAs, and genes related to breast cancer. Oncotarget 8, 62183–62194 (2017).
Poirier, D. Recent advances in the development of 17β-hydroxysteroid dehydrogenase inhibitors. Steroids 213, 109529 (2025).
Stanhewicz, A. E., Wenner, M. M. & Stachenfeld, N. S. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am. J. Physiol. Heart Circ. Physiol. 315, H1569–H1588 (2018).
Prabakaran, S., Schwartz, A. & Lundberg, G. Cardiovascular risk in menopausal women and our evolving understanding of menopausal hormone therapy: risks, benefits, and current guidelines for use. Ther. Adv. Endocrinol. Metab. 12, 20420188211013917 (2021).
Lisabeth, L. & Bushnell, C. Stroke risk in women: the role of menopause and hormone therapy. Lancet Neurol. 11, 82–91 (2012).
Pavon, J. M., Whitson, H. E. & Okun, M. S. Parkinson’s disease in women: a call for improved clinical studies and for comparative effectiveness research. Maturitas 65, 352–358 (2010).
GBD 2016 Parkinson’s Disease Collaborators Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953 (2018).
Mancuso, P. & Bouchard, B. The impact of aging on adipose function and adipokine synthesis. Front. Endocrinol. 10, 137 (2019).
Siiteri, P. K., Simberg, N. & Murai, J. Estrogens and breast cancer. Ann. N. Y. Acad. Sci. 464, 100–105 (1986).
Woolcott, C. G. et al. Plasma sex hormone concentrations and breast cancer risk in an ethnically diverse population of postmenopausal women: the multiethnic cohort study. Endocr. Relat. Cancer 17, 125–134 (2010).
Fuhrman, B. J. et al. Estrogen metabolism and risk of breast cancer in postmenopausal women. J. Natl Cancer Inst. 104, 326–339 (2012).
Wang, J., Trentham-Dietz, A., Hemming, J. D., Hedman, C. J. & Sprague, B. L. Serum factors and clinical characteristics associated with serum E-screen activity. Cancer Epidemiol. Biomark. Prev. 22, 962–971 (2013).
Berrino, F. et al. Serum sex hormone levels after menopause and subsequent breast cancer. J. Natl Cancer Inst. 88, 291–296 (1996).
Cauley, J. A. et al. Elevated serum estradiol and testosterone concentrations are associated with a high risk for breast cancer. Study of osteoporotic fractures research group. Ann. Intern. Med. 130, 270–277 (1999).
Dorgan, J. F. et al. Relation of prediagnostic serum estrogen and androgen levels to breast cancer risk. Cancer Epidemiol. Biomark. Prev. 5, 533–539 (1996).
Garland, C. F., Friedlander, N. J., Barrett-Connor, E. & Khaw, K. T. Sex hormones and postmenopausal breast cancer: a prospective study in an adult community. Am. J. Epidemiol. 135, 1220–1230 (1992).
Hankinson, S. E. et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J. Natl Cancer Inst. 90, 1292–1299 (1998).
Missmer, S. A., Eliassen, A. H., Barbieri, R. L. & Hankinson, S. E. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J. Natl Cancer Inst. 96, 1856–1865 (2004).
Kim, J. & Oktay, K. Baseline E2 levels are higher in BRCA2 mutation carriers: a potential target for prevention? Cancer Causes Control 24, 421–426 (2013).
England, P. C., Skinner, L. G., Cottrell, K. M. & Sellwood, R. A. Serum oestradiol-17β in women with benign and malignant breast disease. Br. J. Cancer 30, 571–576 (1974).
Krishnamoorthy, G., Govindarajulu, P. & Ramalingam, V. Serum hormones in human breast cancer subjects. Neoplasma 36, 221–231 (1989).
Awio, J. P., Galukande, M., Kituuka, O. & Fualal, J. O. High serum estradiol confers no risk for breast cancer: another disparity for sub Saharan Africa women. Pan Afr. Med. J. 12, 23 (2012).
Kaaks, R. et al. Effects of dietary intervention on IGF-I and IGF-binding proteins, and related alterations in sex steroid metabolism: the Diet and Androgens (DIANA) randomised trial. Eur. J. Clin. Nutr. 57, 1079–1088 (2003).
Zhang, X., Tworoger, S. S., Eliassen, A. H. & Hankinson, S. E. Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res. Treat. 137, 883–892 (2013).
Baglietto, L. et al. Circulating steroid hormone levels and risk of breast cancer for postmenopausal women. Cancer Epidemiol. Biomark. Prev. 19, 492–502 (2010).
Sieri, S. et al. Sex hormone levels, breast cancer risk, and cancer receptor status in postmenopausal women: the ORDET cohort. Cancer Epidemiol. Biomark. Prev. 18, 169–176 (2009).
Geisler, J. et al. Letrozole is superior to anastrozole in suppressing breast cancer tissue and plasma estrogen levels. Clin. Cancer Res. 14, 6330–6335 (2008).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. J.S. and M.S. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Michael Coleman, Carol Ann Lange and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sho, M., Qureshi, R. & Slingerland, J. Oestrogen changes at menopause: insights into obesity-associated breast risk and outcomes. Nat Rev Endocrinol (2025). https://doi.org/10.1038/s41574-025-01208-7
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41574-025-01208-7