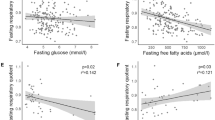
Abstract
Upon fasting, mammals undergo a fasting response in which the liver’s main role is producing fuel (glucose and ketone bodies) to supply extra-hepatic tissues. Glucose is produced by glycogenolysis and gluconeogenesis, and ketone bodies are produced by ketogenesis, which is preceded by lipolysis and fatty acid oxidation. Hepatic fuel production during fasting is controlled by hormonal and metabolic cues, collectively termed here ‘fasting cues’. In this Review, we discuss fasting cues that directly signal hepatocytes and whose plasma levels increase upon fasting, namely, glucagon, glucocorticoids, growth hormone, adrenaline, free fatty acids, asprosin and GP73. We outline the fasting-dependent increases in blood levels of these cues, how they regulate transcription and the metabolic consequences of these cues in hepatocytes. We put particular emphasis on their role in directing fuel production. The perception of endocrine control of fuel production is shifting from the classic ‘counter-regulatory’ notion that fasting cues are simply opposing insulin action, to the realization that fasting cues cooperate with each other to elicit a synergistic response and also complement each other’s actions indirectly. We discuss these modes of crosstalk and cooperation between fasting cues and describe the effects of signal integration on the transcriptional and metabolic response to fasting.
Key points
-
Circulating fasting cues originating from several organs signal hepatocytes during fasting and govern hepatic production of glucose and ketone bodies.
-
Hepatic glucose production is mainly controlled by glucagon and glucocorticoids, with contributions from other fasting cues such as adrenaline.
-
Gluconeogenesis is dependent on amino acid catabolism, and both processes are markedly transcriptionally regulated.
-
Lipolysis in white adipose tissue is controlled by adrenaline, growth hormone and glucocorticoids, leading to a flow of free fatty acids into hepatocytes.
-
Free fatty acids serve as both precursors for ketogenesis and signalling cues activating a ketogenic gene programme.
-
Fasting cues cooperate with each other to optimize fuel production by synergistic gene induction achieved by several transcriptional mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Soeters, M. R., Soeters, P. B., Schooneman, M. G., Houten, S. M. & Romijn, J. A. Adaptive reciprocity of lipid and glucose metabolism in human short-term starvation. Am. J. Physiol. Endocrinol. Metab. 303, E1397–E1407 (2012).
Secor, S. M. & Carey, H. V. Integrative physiology of fasting. Compr. Physiol. 6, 773–825 (2016).
Petersen, M. C., Vatner, D. F. & Shulman, G. I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 (2017).
Legouis, D., Faivre, A., Cippà, P. E. & de Seigneux, S. Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrol. Dial. Transpl. 37, 1417–1425 (2022).
Pietzner, M. et al. Systemic proteome adaptions to 7-day complete caloric restriction in humans. Nat. Metab. 6, 764–777 (2024).
Fukao, T., Lopaschuk, G. D. & Mitchell, G. A. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 70, 243–251 (2004).
Ruppert, P. M. M. & Kersten, S. Mechanisms of hepatic fatty acid oxidation and ketogenesis during fasting. Trends Endocrinol. Metab. 35, 107–124 (2024).
Cahill, G. F. Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 26, 1–22 (2006).
Grabner, G. F., Xie, H., Schweiger, M. & Zechner, R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 3, 1445–1465 (2021).
Wang, Y., Kwon, H., Su, X. & Wondisford, F. E. Glycerol not lactate is the major net carbon source for gluconeogenesis in mice during both short and prolonged fasting. Mol. Metab. 31, 36–44 (2020).
Goldstein, I. & Hager, G. L. Transcriptional and chromatin regulation during fasting — the genomic era. Trends Endocrinol. Metab. 26, 699–710 (2015).
Bideyan, L., Nagari, R. & Tontonoz, P. Hepatic transcriptional responses to fasting and feeding. Genes Dev. 35, 635–657 (2021).
Capozzi, M. E., D’Alessio, D. A. & Campbell, J. E. The past, present, and future physiology and pharmacology of glucagon. Cell Metab. 34, 1654–1674 (2022).
White, P. J., Wewer Albrechtsen, N. J. & Campbell, J. E. Islet hormones at the intersection of glucose and amino acid metabolism. Nat. Rev. Endocrinol. 21, 397–412 (2025).
MacDonald, P. E. & Rorsman, P. Metabolic messengers: glucagon. Nat. Metab. 5, 186–192 (2023).
Holst, J. J. & Wewer Albrechtsen, N. J. Methods and guidelines for measurement of glucagon in plasma. Int. J. Mol. Sci. 20, 5416 (2019).
Bomholt, A. B. et al. Evaluation of commercially available glucagon receptor antibodies and glucagon receptor expression. Commun. Biol. 5, 1278 (2022).
Kajani, S., Laker, R. C., Ratkova, E., Will, S. & Rhodes, C. J. Hepatic glucagon action: beyond glucose mobilization. Physiol. Rev. 104, 1021–1060 (2024).
Wewer Albrechtsen, N. J. The glucose-mobilizing effect of glucagon at fasting is mediated by cyclic AMP. Am. J. Physiol. Endocrinol. Metab. 321, E571–E574 (2021).
Habegger, K. M. Cross talk between insulin and glucagon receptor signaling in the hepatocyte. Diabetes 71, 1842–1851 (2022).
Rodgers, R. L. Glucagon, cyclic AMP, and hepatic glucose mobilization: a half-century of uncertainty. Physiol. Rep. 10, e15263 (2022).
Cao, W. et al. p38 mitogen-activated protein kinase plays a stimulatory role in hepatic gluconeogenesis. J. Biol. Chem. 280, 42731–42737 (2005).
Jiang, Y. et al. Glucagon receptor activates extracellular signal-regulated protein kinase 1/2 via cAMP-dependent protein kinase. Proc. Natl Acad. Sci. USA 98, 10102–10107 (2001).
Ahrén, B. Glucagon — early breakthroughs and recent discoveries. Peptides 67, 74–81 (2015).
Longuet, C. et al. The glucagon receptor is required for the adaptive metabolic response to fasting. Cell Metab. 8, 359–371 (2008).
Kim, T. et al. Hepatic glucagon receptor signaling enhances insulin-stimulated glucose disposal in rodents. Diabetes 67, 2157–2166 (2018).
Winther-Sørensen, M. et al. Glucagon acutely regulates hepatic amino acid catabolism and the effect may be disturbed by steatosis. Mol. Metab. 42, 101080 (2020).
Adeva-Andany, M. M., Funcasta-Calderón, R., Fernández-Fernández, C., Castro-Quintela, E. & Carneiro-Freire, N. Metabolic effects of glucagon in humans. J. Clin. Transl. Endocrinol. 15, 45–53 (2019).
Bankir, L., Bouby, N., Speth, R. C., Velho, G. & Crambert, G. Glucagon revisited: coordinated actions on the liver and kidney. Diabetes Res. Clin. Pract. 146, 119–129 (2018).
Elmelund, E. et al. Opposing effects of chronic glucagon receptor agonism and antagonism on amino acids, hepatic gene expression, and alpha cells. iScience 25, 105296 (2022).
Vega, R. B. et al. A metabolomic signature of glucagon action in healthy individuals with overweight/obesity. J. Endocr. Soc. 5, bvab118 (2021).
Dean, E. D. et al. Interrupted glucagon signaling reveals hepatic α cell axis and role for L-glutamine in α cell proliferation. Cell Metab. 25, 1362–1373 (2017).
Kim, J. et al. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic α cell hyperplasia in mice. Cell Metab. 25, 1348–1361 (2017).
Solloway, M. J. et al. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of α-cell mass. Cell Rep. 12, 495–510 (2015).
Watanabe, C. et al. Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides. Diabetes 61, 74–84 (2012).
Tellez, K. et al. In vivo studies of glucagon secretion by human islets transplanted in mice. Nat. Metab. 2, 547–557 (2020).
Perry, R. J. et al. Glucagon stimulates gluconeogenesis by INSP3R1-mediated hepatic lipolysis. Nature 579, 279–283 (2020).
Galsgaard, K. D., Pedersen, J., Knop, F. K., Holst, J. J. & Wewer Albrechtsen, N. J. Glucagon receptor signaling and lipid metabolism. Front. Physiol. 10, 413 (2019).
Swenson, T. L. & Porter, J. W. Mechanism of glucagon inhibition of liver acetyl-CoA carboxylase. Interrelationship of the effects of phosphorylation, polymer–protomer transition, and citrate on enzyme activity. J. Biol. Chem. 260, 3791–3797 (1985).
Chen, J., Wu, Y., Hao, W., You, J. & Wu, L. Non-canonical hepatic androgen receptor mediates glucagon sensitivity in female mice through the PGC1α/ERRα/mitochondria axis. Cell Rep. 44, 115188 (2025).
Vasileva, A., Marx, T., Beaudry, J. L. & Stern, J. H. Glucagon receptor signaling at white adipose tissue does not regulate lipolysis. Am. J. Physiol. Endocrinol. Metab. 323, E389–E401 (2022).
Gravholt, C. H., Møller, N., Jensen, M. D., Christiansen, J. S. & Schmitz, O. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J. Clin. Endocrinol. Metab. 86, 2085–2089 (2001).
Wu, M. S. et al. Does glucagon increase plasma free fatty acid concentration in humans with normal glucose tolerance? J. Clin. Endocrinol. Metab. 70, 410–416 (1990).
Jensen, M. D., Heiling, V. J. & Miles, J. M. Effects of glucagon on free fatty acid metabolism in humans. J. Clin. Endocrinol. Metab. 72, 308–315 (1991).
Capozzi, M. E. et al. The limited role of glucagon for ketogenesis during fasting or in response to SGLT2 inhibition. Diabetes 69, 882–892 (2020).
Sonnenberg, G. E., Stauffacher, W. & Keller, U. Failure of glucagon to stimulate ketone body production during acute insulin deficiency or insulin replacement in man. Diabetologia 23, 94–100 (1982).
Keller, U., Gerber, P. P. & Stauffacher, W. Fatty acid-independent inhibition of hepatic ketone body production by insulin in humans. Am. J. Physiol. 254, E694–E699 (1988).
Liljenquist, J. E. et al. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. J. Clin. Investig. 53, 190–197 (1974).
Miles, J. M., Haymond, M. W., Nissen, S. L. & Gerich, J. E. Effects of free fatty acid availability, glucagon excess, and insulin deficiency on ketone body production in postabsorptive man. J. Clin. Investig. 71, 1554–1561 (1983).
Miller, R. A. & Birnbaum, M. J. Glucagon: acute actions on hepatic metabolism. Diabetologia 59, 1376–1381 (2016).
Herzig, S. et al. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413, 179–183 (2001).
Chakravarty, K., Cassuto, H., Reshef, L. & Hanson, R. W. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit. Rev. Biochem. Mol. Biol. 40, 129–154 (2005).
Korenfeld, N. et al. Fasting hormones synergistically induce amino acid catabolism genes to promote gluconeogenesis. Cell. Mol. Gastroenterol. Hepatol. 12, 1021–1036 (2021).
Snodgrass, P. J., Lin, R. C., Müller, W. A. & Aoki, T. T. Induction of urea cycle enzymes of rat liver by glucagon. J. Biol. Chem. 253, 2748–2753 (1978).
Husson, A., Buquet, C. & Vaillant, R. Induction of the five urea-cycle enzymes by glucagon in cultured foetal rat hepatocytes. Differentiation 35, 212–218 (1987).
Lee, Y., Wang, M. Y., Du, X. Q., Charron, M. J. & Unger, R. H. Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60, 391–397 (2011).
Altarejos, J. Y. & Montminy, M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat. Rev. Mol. Cell Biol. 12, 141–151 (2011).
Zhao, Y. et al. Histone phosphorylation integrates the hepatic glucagon-PKA-CREB gluconeogenesis program in response to fasting. Mol. Cell 83, 1093–1108 (2023).
Erion, D. M. et al. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metab. 10, 499–506 (2009).
Lee, D., Le Lay, J. & Kaestner, K. H. The transcription factor CREB has no non-redundant functions in hepatic glucose metabolism in mice. Diabetologia 57, 1242–1248 (2014).
Ravnskjaer, K. et al. Glucagon regulates gluconeogenesis through KAT2B- and WDR5-mediated epigenetic effects. J. Clin. Invest. 123, 4318–4328 (2013).
Tsai, W. W. et al. PRMT5 modulates the metabolic response to fasting signals. Proc. Natl Acad. Sci. USA 110, 8870–8875 (2013).
Everett, L. J. et al. Integrative genomic analysis of CREB defines a critical role for transcription factor networks in mediating the fed/fasted switch in liver. BMC Genom. 14, 337 (2013).
Goldstein, I. et al. Transcription factor assisted loading and enhancer dynamics dictate the hepatic fasting response. Genom. Res. 27, 427–439 (2017).
Wu, Y. et al. Novel mechanism of foxo1 phosphorylation in glucagon signaling in control of glucose homeostasis. Diabetes 67, 2167–2182 (2018).
Yang, W. et al. Hepatic p38α MAPK controls gluconeogenesis via FOXO1 phosphorylation at S273 during glucagon signalling in mice. Diabetologia 66, 1322–1339 (2023).
Ozcan, L. et al. Calcium signaling through CaMKII regulates hepatic glucose production in fasting and obesity. Cell Metab. 15, 739–751 (2012).
Goldstein, I. & Hager, G. L. The three Ds of transcription activation by glucagon: direct, delayed, and dynamic. Endocrinology 159, 206–216 (2018).
Goldberg, D., Buchshtab, N., Charni-Natan, M. & Goldstein, I. Transcriptional cascades during fasting amplify gluconeogenesis and instigate a secondary wave of ketogenic gene transcription. Liver Int. 44, 2964–2982 (2024).
Gray, S. et al. Regulation of gluconeogenesis by Kruppel-like factor 15. Cell Metab. 5, 305–312 (2007).
Arizmendi, C., Liu, S., Croniger, C., Poli, V. & Friedman, J. E. The transcription factor CCAAT/enhancer-binding protein beta regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J. Biol. Chem. 274, 13033–13040 (1999).
Russell, G. & Lightman, S. The human stress response. Nat. Rev. Endocrinol. 15, 525–534 (2019).
Douglass, A. M. et al. Neural basis for fasting activation of the hypothalamic–pituitary–adrenal axis. Nature 620, 154–162 (2023).
Stalder, T. et al. The cortisol awakening response: regulation and functional significance. Endocr. Rev. 46, 43–59 (2025).
Quagliarini, F., Makris, K., Friano, M. E. & Uhlenhaut, N. H. EJE Prize 2023: genes on steroids-genomic control of hepatic metabolism by the glucocorticoid receptor. Eur. J. Endocrinol. 188, R111–R130 (2023).
Lightman, S. L. & Conway-Campbell, B. L. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat. Rev. Neurosci. 11, 710–718 (2010).
Vandevyver, S., Dejager, L. & Libert, C. On the trail of the glucocorticoid receptor: into the nucleus and back. Traffic 13, 364–374 (2012).
Præstholm, S. M., Correia, C. M. & Grøntved, L. Multifaceted control of GR signaling and its impact on hepatic transcriptional networks and metabolism. Front. Endocrinol. 11, 572981 (2020).
Presman, D. M. et al. DNA binding triggers tetramerization of the glucocorticoid receptor in live cells. Proc. Natl Acad. Sci. USA 113, 8236–8241 (2016).
Jiménez-Panizo, A. et al. The multivalency of the glucocorticoid receptor ligand-binding domain explains its manifold physiological activities. Nucleic Acids Res. 50, 13063–13082 (2022).
Britton, S. W. & Silvette, H. Some observations on the cortico-adrenal hormone. Science 73, 373–374 (1931).
Li, J. X. & Cummins, C. L. Fresh insights into glucocorticoid-induced diabetes mellitus and new therapeutic directions. Nat. Rev. Endocrinol. 18, 540–557 (2022).
Kuo, T., McQueen, A., Chen, T. C. & Wang, J. C. Regulation of glucose homeostasis by glucocorticoids. Adv. Exp. Med. Biol. 872, 99–126 (2015).
Salehidoost, R. & Korbonits, M. Glucose and lipid metabolism abnormalities in Cushing’s syndrome. J. Neuroendocrinol. 34, e13143 (2022).
Yang, J., Reshef, L., Cassuto, H., Aleman, G. & Hanson, R. W. Aspects of the control of phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 284, 27031–27035 (2009).
Opherk, C. et al. Inactivation of the glucocorticoid receptor in hepatocytes leads to fasting hypoglycemia and ameliorates hyperglycemia in streptozotocin-induced diabetes mellitus. Mol. Endocrinol. 18, 1346–1353 (2004).
Præstholm, S. M. et al. Impaired glucocorticoid receptor expression in liver disrupts feeding-induced gene expression, glucose uptake, and glycogen storage. Cell Rep. 37, 109938 (2021).
Ratman, D. et al. Chromatin recruitment of activated AMPK drives fasting response genes co-controlled by GR and PPARalpha. Nucleic Acids Res. 44, 10539–10553 (2016).
Loft, A. et al. A macrophage-hepatocyte glucocorticoid receptor axis coordinates fasting ketogenesis. Cell Metab. 34, 473–486 (2022).
Xu, C. et al. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 23, 1161–1170 (2009).
Gray, N. E. et al. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. J. Biol. Chem. 287, 8444–8456 (2012).
Mueller, K. M. et al. Adipocyte glucocorticoid receptor deficiency attenuates aging- and HFD-induced obesity and impairs the feeding-fasting transition. Diabetes 66, 272–286 (2017).
Schade, D. S., Eaton, R. P. & Standefer, J. Glucocorticoid regulation of plasma ketone body concentration in insulin deficient man. J. Clin. Endocrinol. Metab. 44, 1069–1079 (1977).
Schade, D. S., Eaton, R. P. & Peake, G. T. The ketotic effects of glucocorticoid and growth hormone in man. Acta Diabetol. Lat. 17, 161–169 (1980).
Goldstein, I. in The Liver (eds Alter Arias, H. J. et al.) 1043–1049 (Wiley, 2020).
Ratman, D. et al. How glucocorticoid receptors modulate the activity of other transcription factors: a scope beyond tethering. Mol. Cell. Endocrinol. 380, 41–54 (2013).
Swinstead, E. E., Paakinaho, V., Presman, D. M. & Hager, G. L. Pioneer factors and ATP-dependent chromatin remodeling factors interact dynamically: a new perspective: multiple transcription factors can effect chromatin pioneer functions through dynamic interactions with ATP-dependent chromatin remodeling factors. BioEssays 38, 1150–1157 (2016).
Voss, T. C. et al. Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism. Cell 146, 544–554 (2011).
Rigaud, G., Roux, J., Pictet, R. & Grange, T. In vivo footprinting of rat TAT gene: dynamic interplay between the glucocorticoid receptor and a liver-specific factor. Cell 67, 977–986 (1991).
Grontved, L. et al. C/EBP maintains chromatin accessibility in liver and facilitates glucocorticoid receptor recruitment to steroid response elements. EMBO J. 32, 1568–1583 (2013).
Hunter, A. L. et al. HNF4A modulates glucocorticoid action in the liver. Cell Rep. 39, 110697 (2022).
Eigler, N., Saccà, L. & Sherwin, R. S. Synergistic interactions of physiologic increments of glucagon, epinephrine, and cortisol in the dog: a model for stress-induced hyperglycemia. J. Clin. Investig. 63, 114–123 (1979).
Shamoon, H., Hendler, R. & Sherwin, R. S. Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans. J. Clin. Endocrinol. Metab. 52, 1235–1241 (1981).
Goldberg, D., Charni-Natan, M., Buchshtab, N., Bar-Shimon, M. & Goldstein, I. Hormone-controlled cooperative binding of transcription factors drives synergistic induction of fasting-regulated genes. Nucleic Acids Res. 50, 5528–5544 (2022).
Patel, R. et al. LXRbeta is required for glucocorticoid-induced hyperglycemia and hepatosteatosis in mice. J. Clin. invest. 121, 431–441 (2011).
Hemmer, M. C. et al. E47 modulates hepatic glucocorticoid action. Nat. Commun. 10, 306 (2019).
Hauck, A. K. et al. Nuclear receptor corepressors non-canonically drive glucocorticoid receptor-dependent activation of hepatic gluconeogenesis. Nat. Metab. 6, 825–836 (2024).
Berry, R., McGinnis, G. R., Banerjee, R. R., Young, M. E. & Frank, S. J. Differential tissue response to growth hormone in mice. FEBS Open Bio 8, 1146–1154 (2018).
Ranke, M. B. & Wit, J. M. Growth hormone — past, present and future. Nat. Rev. Endocrinol. 14, 285–300 (2018).
Huang, L., Huang, Z. & Chen, C. Rhythmic growth hormone secretion in physiological and pathological conditions: lessons from rodent studies. Mol. Cell. Endocrinol. 498, 110575 (2019).
Luque, R. M., Park, S. & Kineman, R. D. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology 148, 300–309 (2007).
Perry, R. J. et al. Leptin mediates a glucose-fatty acid cycle to maintain glucose homeostasis in starvation. Cell 172, 234–248 (2018).
Steyn, F. J. et al. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology 152, 3165–3171 (2011).
Steyn, F. J. et al. GH does not modulate the early fasting-induced release of free fatty acids in mice. Endocrinology 153, 273–282 (2012).
Tannenbaum, G. S., Rorstad, O. & Brazeau, P. Effects of prolonged food deprivation on the ultradian growth hormone rhythm and immunoreactive somatostatin tissue levels in the rat. Endocrinology 104, 1733–1738 (1979).
Aubert, M. L. et al. Metabolic control of sexual function and growth: role of neuropeptide Y and leptin. Mol. Cell. Endocrinol. 140, 107–113 (1998).
Gahete, M. D., Córdoba-Chacón, J., Luque, R. M. & Kineman, R. D. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology 154, 263–269 (2013).
Fang, F., Goldstein, J. L., Shi, X., Liang, G. & Brown, M. S. Unexpected role for IGF-1 in starvation: maintenance of blood glucose. Proc. Natl Acad. Sci. USA 119, e2208855119 (2022).
Zhao, T. J. et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl Acad. Sci. USA 107, 7467–7472 (2010).
Chia, D. J. Minireview: mechanisms of growth hormone-mediated gene regulation. Mol. Endocrinol. 28, 1012–1025 (2014).
Engblom, D. et al. Direct glucocorticoid receptor-Stat5 interaction in hepatocytes controls body size and maturation-related gene expression. Genes Dev. 21, 1157–1162 (2007).
Mueller, K. M. et al. Hepatic growth hormone and glucocorticoid receptor signaling in body growth, steatosis and metabolic liver cancer development. Mol. Cell. Endocrinol. 361, 1–11 (2012).
Quagliarini, F. et al. Cistromic reprogramming of the diurnal glucocorticoid hormone response by high-fat diet. Mol. Cell 76, 531–545 (2019).
Kopchick, J. J., Berryman, D. E., Puri, V., Lee, K. Y. & Jorgensen, J. O. L. The effects of growth hormone on adipose tissue: old observations, new mechanisms. Nat. Rev. Endocrinol. 16, 135–146 (2020).
Kineman, R. D., Del Rio-Moreno, M. & Waxman, D. J. Liver-specific actions of GH and IGF1 that protect against MASLD. Nat. Rev. Endocrinol. 21, 105–117 (2025).
Vázquez-Borrego, M. C. et al. Direct and systemic actions of growth hormone receptor (GHR)-signaling on hepatic glycolysis, de novo lipogenesis and insulin sensitivity, associated with steatosis. Metab. Clin. Exp. 144, 155589 (2023).
Bak, J. F., Møller, N. & Schmitz, O. Effects of growth hormone on fuel utilization and muscle glycogen synthase activity in normal humans. Am. J. Physiol. 260, E736–E742 (1991).
Møller, N. et al. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am. J. Physiol. 258, E86–E91 (1990).
Metcalfe, P., Johnston, D. G., Nosadini, R., Orksov, H. & Alberti, K. G. Metabolic effects of acute and prolonged growth hormone excess in normal and insulin-deficient man. Diabetologia 20, 123–128 (1981).
Keller, U., Schnell, H., Girard, J. & Stauffacher, W. Effect of physiological elevation of plasma growth hormone levels on ketone body kinetics and lipolysis in normal and acutely insulin-deficient man. Diabetologia 26, 103–108 (1984).
Dichtel, L. E., Cordoba-Chacon, J. & Kineman, R. D. Growth hormone and insulin-like growth factor 1 regulation of nonalcoholic fatty liver disease. J. Clin. Endocrinol. Metab. 107, 1812–1824 (2022).
Salomon, F., Cuneo, R. C., Hesp, R. & Sönksen, P. H. The effects of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N. Engl. J. Med. 321, 1797–1803 (1989).
Nørrelund, H., Møller, N., Nair, K. S., Christiansen, J. S. & Jørgensen, J. O. Continuation of growth hormone (GH) substitution during fasting in GH-deficient patients decreases urea excretion and conserves protein synthesis. J. Clin. Endocrinol. Metab. 86, 3120–3129 (2001).
Binnerts, A. et al. The effect of growth hormone administration in growth hormone deficient adults on bone, protein, carbohydrate and lipid homeostasis, as well as on body composition. Clin. Endocrinol. 37, 79–87 (1992).
Degerblad, M., Elgindy, N., Hall, K., Sjöberg, H. E. & Thorén, M. Potent effect of recombinant growth hormone on bone mineral density and body composition in adults with panhypopituitarism. Acta Endocrinol. 126, 387–393 (1992).
Sakharova, A. A. et al. Role of growth hormone in regulating lipolysis, proteolysis, and hepatic glucose production during fasting. J. Clin. Endocrinol. Metab. 93, 2755–2759 (2008).
Höybye, C. et al. Contribution of gluconeogenesis and glycogenolysis to hepatic glucose production in acromegaly before and after pituitary microsurgery. Horm. Metab. Res. 40, 498–501 (2008).
Ghanaat, F. & Tayek, J. A. Growth hormone administration increases glucose production by preventing the expected decrease in glycogenolysis seen with fasting in healthy volunteers. Metab. Clin. Exp. 54, 604–609 (2005).
Kaplan, W., Sunehag, A. L., Dao, H. & Haymond, M. W. Short-term effects of recombinant human growth hormone and feeding on gluconeogenesis in humans. Metab. Clin. Exp. 57, 725–732 (2008).
Schwarz, J. M. et al. Effects of recombinant human growth hormone on hepatic lipid and carbohydrate metabolism in HIV-infected patients with fat accumulation. J. Clin. Endocrinol. Metab. 87, 942 (2002).
Cordoba-Chacon, J. et al. Growth hormone inhibits hepatic de novo lipogenesis in adult mice. Diabetes 64, 3093–3103 (2015).
Liu, J. et al. Growth hormone receptor disrupts glucose homeostasis via promoting and stabilizing retinol binding protein 4. Theranostics 11, 8283–8300 (2021).
Kim, Y. D. et al. Orphan nuclear receptor small heterodimer partner negatively regulates growth hormone-mediated induction of hepatic gluconeogenesis through inhibition of signal transducer and activator of transcription 5 (STAT5) transactivation. J. Biol. Chem. 287, 37098–37108 (2012).
Jo, J. R., An, S., Ghosh, S., Nedumaran, B. & Kim, Y. D. Growth hormone promotes hepatic gluconeogenesis by enhancing BTG2-YY1 signaling pathway. Sci. Rep. 11, 18999 (2021).
Sharma, R., Kopchick, J. J., Puri, V. & Sharma, V. M. Effect of growth hormone on insulin signaling. Mol. Cell. Endocrinol. 518, 111038 (2020).
Esposito, D. et al. Diabetes mellitus in patients with acromegaly: pathophysiology, clinical challenges and management. Nat. Rev. Endocrinol. 20, 541–552 (2024).
Møller, N. & Jørgensen, J. O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 30, 152–177 (2009).
Beauloye, V. et al. Impairment of liver GH receptor signaling by fasting. Endocrinology 143, 792–800 (2002).
Gevers, E. F., Hannah, M. J., Waters, M. J. & Robinson, I. C. Regulation of rapid signal transducer and activator of transcription-5 phosphorylation in the resting cells of the growth plate and in the liver by growth hormone and feeding. Endocrinology 150, 3627–3636 (2009).
Lelou, E. et al. The role of catecholamines in pathophysiological liver processes. Cells 11, 1021 (2022).
Jensen, M. D., Haymond, M. W., Gerich, J. E., Cryer, P. E. & Miles, J. M. Lipolysis during fasting. Decreased suppression by insulin and increased stimulation by epinephrine. J. Clin. invest. 79, 207–213 (1987).
Exton, J. H. & Park, C. R. Control of gluconeogenesis in liver. II. Effects of glucagon, catecholamines, and adenosine 3’,5’-monophosphate on gluconeogenesis in the perfused rat liver. J. Biol. Chem. 243, 4189–4196 (1968).
Kersten, S. Integrated physiology and systems biology of PPARalpha. Mol. Metab. 3, 354–371 (2014).
Fougerat, A. et al. Lipid sensing by PPARα: role in controlling hepatocyte gene regulatory networks and the metabolic response to fasting. Prog. Lipid Res. 96, 101303 (2024).
Fougerat, A. et al. ATGL-dependent white adipose tissue lipolysis controls hepatocyte PPARα activity. Cell Rep. 39, 110910 (2022).
Selen, E. S., Choi, J. & Wolfgang, M. J. Discordant hepatic fatty acid oxidation and triglyceride hydrolysis leads to liver disease. JCI Insight 6, e135626 (2021).
Geng, L., Lam, K. S. L. & Xu, A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat. Rev. Endocrinol. 16, 654–667 (2020).
Romere, C. et al. Asprosin, a fasting-induced glucogenic protein hormone. Cell 165, 566–579 (2016).
Li, E. et al. OLFR734 mediates glucose metabolism as a receptor of asprosin. Cell Metab. 30, 319–328 (2019).
Acevedo, J. et al. Asprosin as a regulator of hepatic lipogenesis and its association with hepatic steatosis. J. Lipid Res. 66, 100921 (2025).
Duerrschmid, C. et al. Asprosin is a centrally acting orexigenic hormone. Nat. Med. 23, 1444–1453 (2017).
Wan, L. et al. GP73 is a glucogenic hormone contributing to SARS-CoV-2-induced hyperglycemia. Nat. Metab. 4, 29–43 (2022).
Yang, X. et al. GP73 blockade alleviates abnormal glucose homeostasis in diabetic mice. J. Mol. Endocrinol. 70, e220103 (2023).
Madsen, M. S., Siersbæk, R., Boergesen, M., Nielsen, R. & Mandrup, S. Peroxisome proliferator-activated receptor γ and C/EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol. Cell. Biol. 34, 939–954 (2014).
Hiltunen, J. et al. Androgen receptor-mediated assisted loading of the glucocorticoid receptor modulates transcriptional responses in prostate cancer cells. Genome Res. 35, 1717–1732 (2025).
Biddie, S. C. et al. Transcription factor AP1 potentiates chromatin accessibility and glucocorticoid receptor binding. Mol. Cell 43, 145–155 (2011).
Goldstein, I., Paakinaho, V., Baek, S., Sung, M. H. & Hager, G. L. Synergistic gene expression during the acute phase response is characterized by transcription factor assisted loading. Nat. Commun. 8, 1849 (2017).
Kraus-Friedmann, N. Hormonal regulation of hepatic gluconeogenesis. Physiol. Rev. 64, 170–259 (1984).
Malbon, C. C., Rapiejko, P. J. & Watkins, D. C. Permissive hormone regulation of hormone-sensitive effector systems. Trends Pharmacol. Sci. 9, 33–36 (1988).
Takahashi, H. et al. Metabolomics reveal 1-palmitoyl lysophosphatidylcholine production by peroxisome proliferator-activated receptor α. J. Lipid Res. 56, 254–265 (2015).
Gachon, F. et al. Proline- and acidic amino acid-rich basic leucine zipper proteins modulate peroxisome proliferator-activated receptor alpha (PPARalpha) activity. Proc. Natl Acad. Sci. USA 108, 4794–4799 (2011).
Haemmerle, G. et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17, 1076–1085 (2011).
Chakravarthy, M. V. et al. ‘New’ hepatic fat activates PPARalpha to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab. 1, 309–322 (2005).
Milona, A. et al. Steroidogenic control of liver metabolism through a nuclear receptor-network. Mol. Metab. 30, 221–229 (2019).
Huang, H., Starodub, O., McIntosh, A., Kier, A. B. & Schroeder, F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. J. Biol. Chem. 277, 29139–29151 (2002).
Korenfeld, N. et al. Repeated fasting events sensitize enhancers, transcription factor activity and gene expression to support augmented ketogenesis. Nucleic Acids Res. 53, gkae1161 (2025).
Korenfeld, N. et al. LXR-dependent enhancer activation regulates the temporal organization of the liver’s response to refeeding leading to lipogenic gene overshoot. PLoS Biol. 22, e3002735 (2024).
Dulloo, A. G., Miles-Chan, J. L. & Schutz, Y. Collateral fattening in body composition autoregulation: its determinants and significance for obesity predisposition. Eur. J. Clin. Nutr. 72, 657–664 (2018).
Hillgartner, F. B., Salati, L. M. & Goodridge, A. G. Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol. Rev. 75, 47–76 (1995).
Horton, J. D., Bashmakov, Y., Shimomura, I. & Shimano, H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc. Natl Acad. Sci. USA 95, 5987–5992 (1998).
Author information
Authors and Affiliations
Contributions
D.G. and I.G. researched data for the article. D.G. and I.G. contributed substantially to discussion of the content. I.G. wrote the article. D.G. and I.G. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Sander Kersten, Thomas Scherer and Nina Henriette Uhlenhaut for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goldberg, D., Goldstein, I. Endocrine regulation of the hepatic fasting response: cues, cooperation and consequences. Nat Rev Endocrinol (2026). https://doi.org/10.1038/s41574-025-01228-3
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41574-025-01228-3