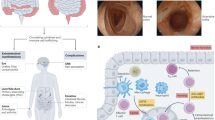
Abstract
Cytokines are involved in intestinal homeostasis and pathological processes associated with inflammatory bowel disease (IBD). The biological effects of cytokines, including several involved in the pathology of Crohn’s disease and ulcerative colitis, occur as a result of receptor-mediated signalling through the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) DNA-binding families of proteins. Although therapies targeting cytokines have revolutionized IBD therapy, they have historically targeted individual cytokines, and an unmet medical need exists for patients who do not respond to or lose response to these treatments. Several small-molecule inhibitors of JAKs that have the potential to affect multiple pro-inflammatory cytokine-dependent pathways are in clinical development for the treatment of IBD, with one agent, tofacitinib, already approved for ulcerative colitis and several other agents with demonstrated efficacy in early phase trials. This Review describes the current understanding of JAK–STAT signalling in intestinal homeostasis and disease and the rationale for targeting this pathway as a treatment for IBD. The available evidence for the efficacy, safety and pharmacokinetics of JAK inhibitors in IBD as well as the potential approaches to optimize treatment with these agents, such as localized delivery or combination therapy, are also discussed.
Key points
-
Cytokines contribute to both normal intestinal homeostasis and pathological processes associated with the chronic and relapsing (acute) nature of inflammatory bowel disease (IBD).
-
Therapies targeting individual pro-inflammatory cytokines have revolutionized the treatment of IBD, although many patients are unresponsive or lose response to therapy.
-
The Janus kinase (JAK) tyrosine kinases and signal transducer and activator of transcription (STAT) DNA-binding proteins mediate the signalling and downstream biological effects in response to cytokine receptor binding, including several effects involved in IBD pathology.
-
Small-molecule JAK inhibitors, which have the potential to affect multiple cytokine-dependent pathways, have been shown to be efficacious in treating IBD.
-
However, the degeneracy of JAK-mediated signalling and the targeting of multiple cytokine pathways could increase the potential for unpredictable effects (including adverse effects).
-
New approaches such as selective or gut-specific JAK inhibitors, and a greater understanding of disease-specific mechanisms of action to facilitate personalized approaches to treatment, could improve the risk–benefit profiles of this class of therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342 (2014).
Yu, H. et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment. Pharmacol. Ther. 47, 364–370 (2018).
Lamb, C. A. et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68 (Suppl. 3), 1–106 (2019).
Shuai, K. & Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3, 900–911 (2003).
Schwartz, D. M. et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 17, 78 (2017). This review describes the cellular and molecular rationale for JAK inhibition for various chronic inflammatory diseases as well as the efficacy and safety data available from clinical trials, and provides an overview of potential next-generation and future approaches to JAK targeting.
Darnell, J. E. Jr., Kerr, I. M. & Stark, G. R. JAK-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264, 1415–1421 (1994).
Villarino, A. V., Kanno, Y. & O’Shea, J. J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 18, 374–384 (2017). This review provides an overview of current knowledge regarding JAK–STAT biology, including immune cell function, disease aetiology and therapeutic intervention; broader principles of gene regulation and signal-dependent transcription factors are also discussed.
Perez-Jeldres, T. et al. Targeting cytokine signaling and lymphocyte traffic via small molecules in inflammatory bowel disease: JAK inhibitors and S1PR agonists. Front. Pharmacol. 10, 212 (2019).
Miklossy, G., Hilliard, T. S. & Turkson, J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 12, 611–629 (2013).
Johnson, D. E., O’Keefe, R. A. & Grandis, J. R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 (2018).
Yan, R., Small, S., Desplan, C., Dearolf, C. R. & Darnell, J. E. Jr. Identification of a Stat gene that functions in Drosophila development. Cell 84, 421–430 (1996).
Liongue, C., Taznin, T. & Ward, A. C. Signaling via the CytoR/JAK/STAT/SOCS pathway: emergence during evolution. Mol. Immunol. 71, 166–175 (2016).
Rane, S. G. & Reddy, E. P. JAK3: a novel JAK kinase associated with terminal differentiation of hematopoietic cells. Oncogene 9, 2415–2423 (1994).
Yamaoka, K. et al. The Janus kinases (Jaks). Genome Biol. 5, 253 (2004).
Levy, D. E. & Darnell, J. E. Jr. STATs: transcriptional control and biological impact. Nat. Rev. Mol. Cell Biol. 3, 651–662 (2002).
McBride, K. M., Banninger, G., McDonald, C. & Reich, N. C. Regulated nuclear import of the STAT1 transcription factor by direct binding of importin-alpha. EMBO J. 21, 1754–1763 (2002).
Sekimoto, T. & Yoneda, Y. Nuclear import and export of proteins: the molecular basis for intracellular signaling. Cytokine Growth Factor Rev. 9, 205–211 (1998).
O’Shea, J. J. & Plenge, R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36, 542–550 (2012).
Johnston, J. A. et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature 370, 151–153 (1994).
Rochman, Y., Spolski, R. & Leonard, W. J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 9, 480–490 (2009).
Arai, K. I. et al. Cytokines: coordinators of immune and inflammatory responses. Annu. Rev. Biochem. 59, 783–836 (1990).
Lutticken, C. et al. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science 263, 89–92 (1994).
Guschin, D. et al. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 14, 1421–1429 (1995).
Stahl, N. et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6β receptor components. Science 263, 92–95 (1994).
Zou, J., Presky, D. H., Wu, C. Y. & Gubler, U. Differential associations between the cytoplasmic regions of the interleukin-12 receptor subunits β1 and β2 and JAK kinases. J. Biol. Chem. 272, 6073–6077 (1997).
Banerjee, S., Biehl, A., Gadina, M., Hasni, S. & Schwartz, D. M. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 77, 521–546 (2017).
Chua, A. O. et al. Expression cloning of a human IL-12 receptor component. A new member of the cytokine receptor superfamily with strong homology to gp130. J. Immunol. 153, 128–136 (1994).
Bacon, C. M. et al. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J. Exp. Med.181, 399–404 (1995).
Ghoreschi, K., Laurence, A. & O’Shea, J. J. Janus kinases in immune cell signaling. Immunol. Rev. 228, 273–287 (2009).
Hunter, C. A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat. Rev. Immunol. 5, 521–531 (2005).
Stark, G. R. & Darnell, J. E. Jr. The JAK-STAT pathway at twenty. Immunity 36, 503–514 (2012). This review, co-authored by the scientists most credited with unravelling the signal transduction pathways mediating cellular responses to type I interferons, provides a personal and captivating historical perspective on the basic research findings and collaboration that led to the discovery of the JAK–STAT pathway.
Schindler, C. & Plumlee, C. Interferons pen the JAK-STAT pathway. Semin. Cell Dev. Biol. 19, 311–318 (2008).
Meraz, M. A. et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell 84, 431–442 (1996).
Shuai, K., Schindler, C., Prezioso, V. R. & Darnell, J. E. Jr. Activation of transcription by IFN-γ: tyrosine phosphorylation of a 91-kD DNA binding protein. Science 258, 1808–1812 (1992).
Park, C., Li, S., Cha, E. & Schindler, C. Immune response in Stat2 knockout mice. Immunity 13, 795–804 (2000).
Li, X., Leung, S., Qureshi, S., Darnell, J. E. Jr. & Stark, G. R. Formation of STAT1-STAT2 heterodimers and their role in the activation of IRF-1 gene transcription by interferon-α. J. Biol. Chem. 271, 5790–5794 (1996).
Lin, L. et al. STAT3 is necessary for proliferation and survival in colon cancer-initiating cells. Cancer Res.71, 7226–7237 (2011).
Kasembeli, M. M., Bharadwaj, U., Robinson, P. & Tweardy, D. J. Contribution of STAT3 to inflammatory and fibrotic diseases and prospects for its targeting for treatment. Int. J. Mol. Sci. 19, 2299 (2018).
Hillmer, E. J., Zhang, H., Li, H. S. & Watowich, S. S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 31, 1–15 (2016).
Lin, J. X. & Leonard, W. J. The common cytokine receptor γ chain family of cytokines. Cold Spring Harb. Perspect. Biol. 10, a028449 (2018).
Kohn, L. A. et al. Human lymphoid development in the absence of common gamma-chain receptor signaling. J. Immunol. 192, 5050–5058 (2014).
Villa, A. et al. Monocyte function in a severe combined immunodeficient patient with a donor splice site mutation in the Jak3 gene. Blood 88, 817–823 (1996).
Mishra, J., Waters, C. M. & Kumar, N. Molecular mechanism of interleukin-2-induced mucosal homeostasis. Am. J. Physiol. Cell Physiol. 302, C735–C747 (2012).
Mishra, J., Verma, R. K., Alpini, G., Meng, F. & Kumar, N. Role of Janus kinase 3 in mucosal differentiation and predisposition to colitis. J. Biol. Chem. 288, 31795–31806 (2013).
Hedl, M., Proctor, D. D. & Abraham, C. JAK2 disease-risk variants are gain of function and JAK signaling threshold determines innate receptor-induced proinflammatory cytokine secretion in macrophages. J. Immunol. 197, 3695–3704 (2016).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Planell, N. et al. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut 62, 967–976 (2013).
Arijs, I. et al. Mucosal gene expression of antimicrobial peptides in inflammatory bowel disease before and after first infliximab treatment. PLoS One 4, e7984 (2009).
Kinchen, J. et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175, 372–386.e17 (2018).
Schreiber, S. et al. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut 51, 379–385 (2002).
Mudter, J. et al. Activation pattern of signal transducers and activators of transcription (STAT) factors in inflammatory bowel diseases. Am. J. Gastroenterol. 100, 64–72 (2005).
Wu, F. et al. Genome-wide gene expression differences in Crohn’s disease and ulcerative colitis from endoscopic pinch biopsies: insights into distinctive pathogenesis. Inflamm. Bowel Dis. 13, 807–821 (2007).
Bandyopadhyay, S. K. et al. Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1. Am. J. Pathol. 173, 1361–1368 (2008).
Wu, X. et al. Selective sequestration of STAT1 in the cytoplasm via phosphorylated SHP-2 ameliorates murine experimental colitis. J. Immunol. 189, 3497–3507 (2012).
Azuma, Y. T. et al. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm. Bowel Dis. 16, 1017–1028 (2010).
Diegelmann, J., Olszak, T., Goke, B., Blumberg, R. S. & Brand, S. A novel role for interleukin-27 (IL-27) as mediator of intestinal epithelial barrier protection mediated via differential signal transducer and activator of transcription (STAT) protein signaling and induction of antibacterial and anti-inflammatory proteins. J. Biol. Chem. 287, 286–298 (2012).
Musso, A. et al. Signal transducers and activators of transcription 3 signaling pathway: an essential mediator of inflammatory bowel disease and other forms of intestinal inflammation. Inflamm. Bowel Dis. 11, 91–98 (2005).
Atreya, R. et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat. Med. 6, 583–588 (2000).
Lu, D. et al. The phosphatase DUSP2 controls the activity of the transcription activator STAT3 and regulates TH17 differentiation. Nat. Immunol. 16, 1263–1273 (2015).
Durant, L. et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32, 605–615 (2010).
Takahashi, R. et al. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-γ and IL-17A production. J. Exp. Med. 208, 2055–2067 (2011).
Suzuki, A. et al. CIS3/SOCS3/SSI3 plays a negative regulatory role in STAT3 activation and intestinal inflammation. J. Exp. Med. 193, 471–481 (2001).
Bai, A. et al. Blockade of STAT3 by antisense oligonucleotide in TNBS-induced murine colitis. Int. J. Colorectal Dis. 22, 625–635 (2007).
Sugimoto, K. et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544 (2008).
Andoh, A. et al. Expression of IL-24, an activator of the JAK1/STAT3/SOCS3 cascade, is enhanced in inflammatory bowel disease. J. Immunol. 183, 687–695 (2009).
Alonzi, T. et al. Induced somatic inactivation of STAT3 in mice triggers the development of a fulminant form of enterocolitis. Cytokine 26, 45–56 (2004).
Takeda, K. et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity10, 39–49 (1999).
Sugimoto, K. Role of STAT3 in inflammatory bowel disease. World J. Gastroenterol. 14, 5110–5114 (2008).
Murphy, K. M. & Reiner, S. L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2, 933–944 (2002).
Kaplan, M. H. STAT4: a critical regulator of inflammation in vivo. Immunol. Res. 31, 231–242 (2005).
Zundler, S. & Neurath, M. F. Interleukin-12: functional activities and implications for disease. Cytokine Growth Factor Rev. 26, 559–568 (2015).
Monteleone, G. et al. Interleukin-21 enhances T-helper cell type I signaling and interferon-gamma production in Crohn’s disease. Gastroenterology 128, 687–694 (2005).
Glosson-Byers, N. L., Sehra, S. & Kaplan, M. H. STAT4 is required for IL-23 responsiveness in Th17 memory cells and NKT cells. JAKSTAT 3, e955393 (2014).
Harbour, S. N., Maynard, C. L., Zindl, C. L., Schoeb, T. R. & Weaver, C. T. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc. Natl Acad. Sci. USA 112, 7061–7066 (2015).
Simpson, S. J. et al. T cell-mediated pathology in two models of experimental colitis depends predominantly on the interleukin 12/Signal transducer and activator of transcription (Stat)-4 pathway, but is not conditional on interferon gamma expression by T cells. J. Exp. Med. 187, 1225–1234 (1998).
Wirtz, S. et al. Cutting edge: chronic intestinal inflammation in STAT-4 transgenic mice: characterization of disease and adoptive transfer by TNF- plus IFN-γ-producing CD4+ T cells that respond to bacterial antigens. J. Immunol. 162, 1884–1888 (1999).
Han, X. et al. Tumour necrosis factor alpha blockade induces an anti-inflammatory growth hormone signalling pathway in experimental colitis. Gut 56, 73–81 (2007).
Han, X. et al. Signal transducer and activator of transcription 5b promotes mucosal tolerance in pediatric Crohn’s disease and murine colitis. Am. J. Pathol. 169, 1999–2013 (2006).
Han, X. et al. Regulation of intestinal barrier function by signal transducer and activator of transcription 5b. Gut 58, 49–58 (2009).
Gilbert, S. et al. Activated STAT5 confers resistance to intestinal injury by increasing intestinal stem cell proliferation and regeneration. Stem Cell Reports 4, 209–225 (2015).
Laurence, A. et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity 26, 371–381 (2007).
Kisseleva, T., Bhattacharya, S., Braunstein, J. & Schindler, C. W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene 285, 1–24 (2002).
Dardalhon, V. et al. IL-4 inhibits TGF-β-induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3- effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Van Kampen, C., Gauldie, J. & Collins, S. M. Proinflammatory properties of IL-4 in the intestinal microenvironment. Am. J. Physiol. Gastrointest. Liver Physiol. 288, G111–G117 (2005).
Rosen, M. J. et al. STAT6 deficiency ameliorates severity of oxazolone colitis by decreasing expression of claudin-2 and Th2-inducing cytokines. J. Immunol. 190, 1849–1858 (2013).
Zundler, S. & Neurath, M. F. Immunopathogenesis of inflammatory bowel diseases: functional role of T cells and T cell homing. Clin. Exp. Rheumatol. 33 (4 Suppl. 92), S19–S28 (2015).
Cosin-Roger, J. et al. The activation of Wnt signaling by a STAT6-dependent macrophage phenotype promotes mucosal repair in murine IBD. Mucosal Immunol. 9, 986–998 (2016).
Li, Y. et al. STAT1, STAT6 and adenosine 3’,5’-cyclic monophosphate (cAMP) signaling drive SOCS3 expression in inactive ulcerative colitis. Mol. Med. 18, 1412–1419 (2012).
Rosen, M. J. et al. STAT6 activation in ulcerative colitis: a new target for prevention of IL-13-induced colon epithelial cell dysfunction. Inflamm. Bowel Dis. 17, 2224–2234 (2011).
Reinisch, W. et al. Anrukinzumab, an anti-interleukin 13 monoclonal antibody, in active UC: efficacy and safety from a phase IIa randomised multicentre study. Gut 64, 894–900 (2015).
Danese, S. et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut 64, 243–249 (2015).
Bamidele, A. O. et al. Disruption of FOXP3-EZH2 interaction represents a pathobiological mechanism in intestinal inflammation. Cell. Mol. Gastroenterol. Hepatol. 7, 55–71 (2019).
Goldberg, R. et al. Correction of defective T-regulatory cells from patients with Crohn’s disease by ex vivo ligation of retinoic acid receptor-α. Gastroenterology 156, 1775–1787 (2019).
Himmel, M. E., Hardenberg, G., Piccirillo, C. A., Steiner, T. S. & Levings, M. K. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology 125, 145–153 (2008).
Izcue, A., Coombes, J. L. & Powrie, F. Regulatory lymphocytes and intestinal inflammation. Annu. Rev. Immunol. 27, 313–338 (2009).
Maynard, C. L. & Weaver, C. T. Intestinal effector T cells in health and disease. Immunity 31, 389–400 (2009).
Sakaguchi, S. et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212, 8–27 (2006).
Sarmento, O. F. et al. The role of the histone methyltransferase enhancer of zeste homolog 2 (EZH2) in the pathobiological mechanisms underlying inflammatory bowel disease (IBD). J. Biol. Chem. 292, 706–722 (2017).
Britton, G. J. et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORgγt+ regulatory T cells and exacerbate colitis in mice. Immunity 50, 212–224.e4 (2019).
Kleinschek, M. A. et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 206, 525–534 (2009).
Ogino, T. et al. Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn’s disease. Gastroenterology 145, 1380–1391.e1 (2013).
Tuller, T., Atar, S., Ruppin, E., Gurevich, M. & Achiron, A. Common and specific signatures of gene expression and protein-protein interactions in autoimmune diseases. Genes Immun. 14, 67–82 (2013).
Zorzi, F. et al. Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One 8, e54562 (2013).
Holmkvist, P. et al. A major population of mucosal memory CD4+ T cells, coexpressing IL-18Rα and DR3, display innate lymphocyte functionality. Mucosal Immunol. 8, 545–558 (2015).
Huang, H. et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547, 173–178 (2017).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015).
Drugs.com. FDA approves stelara (ustekinumab) for treatment of adults with moderately to severely active Crohn’s disease. Drugs.com https://www.drugs.com/newdrugs/fda-approves-stelara-ustekinumab-adults-moderately-severely-active-crohn-s-4436.html (2016).
Johnson & Johnson. European Commission approves stelara® (ustekinumab) for treatment of adults with moderately to severely active Crohn’s disease. Johnson & Johnson https://www.jnj.com/media-center/press-releases/european-commission-approves-stelara-ustekinumab-for-treatment-of-adults-withmoderately-to-severely-active-crohns-disease (2016).
Drugs.com. Pfizer announces U.S. FDA approves Xeljanz (tofacitinib) for the treatment of moderately to severely active ulcerative colitis. Drugs.com https://www.drugs.com/newdrugs/pfizer-announces-u-s-fda-approves-xeljanz-tofacitinib-moderately-severely-active-ulcerative-colitis-4756.html (2018).
Pfizer. XELJANZ® (tofacitinib citrate) receives marketing authorization in the European Union for moderately to severely active ulcerative colitis. Pfizer Inc. https://investors.pfizer.com/investor-news/press-release-details/2018/XELJANZ-tofacitinib-citrate-Receives-Marketing-Authorization-in-the-European-Union-for-Moderately-to-Severely-Active-Ulcerative-Colitis/default.aspx (2018).
Drugs.com. Janssen announces FDA approval of stelara (ustekinumab) for the treatment of adults with moderately to severely active ulcerative colitis. Drugs.com https://www.drugs.com/newdrugs/janssen-announces-fda-approval-stelara-ustekinumab-adults-moderately-severely-active-ulcerative-5084.html (2019).
Business Wire. European Commission approves expanded use of Janssen’s STELARA® (ustekinumab) for the treatment of moderately to severely active ulcerative colitis in the European Union. Business Wire https://www.businesswire.com/news/home/20190904005566/en/European-Commission-Approves-Expanded-Janssen%E2%80%99s-STELARA%C2%AE-ustekinumab (2019).
de Jong, R. J. & Ohnmacht, C. Defining dysbiosis in inflammatory bowel disease. Immunity 50, 8–10 (2019).
Curtis, J. R. et al. The incidence of gastrointestinal perforations among rheumatoid arthritis patients. Arthritis Rheum. 63, 346–351 (2011).
Xie, F., Yun, H., Bernatsky, S. & Curtis, J. R. Brief report: risk of gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologic treatments. Arthritis Rheumatol. 68, 2612–2617 (2016).
Dai, L. et al. SARI attenuates colon inflammation by promoting STAT1 degradation in intestinal epithelial cells. Mucosal Immunol. 12, 1130–1140 (2019).
Yu, H., Lee, H., Herrmann, A., Buettner, R. & Jove, R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat. Rev. Cancer. 14, 736–746 (2014).
Beebe, J. D., Liu, J. Y. & Zhang, J. T. Two decades of research in discovery of anticancer drugs targeting STAT3, how close are we? Pharmacol. Ther. 191, 74–91 (2018).
Jonker, D. J. et al. Napabucasin versus placebo in refractory advanced colorectal cancer: a randomised phase 3 trial. Lancet Gastroenterol. Hepatol. 3, 263–270 (2018).
Buettner, R., Mora, L. B. & Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 8, 945–954 (2002).
Yu, H. & Jove, R. The STATs of cancer – new molecular targets come of age. Nat. Rev. Cancer. 4, 97–105 (2004).
Jing, N. & Tweardy, D. J. Targeting Stat3 in cancer therapy. Anticancer Drugs 16, 601–607 (2005).
Darnell, J. E. Validating Stat3 in cancer therapy. Nat. Med.11, 595–596 (2005).
Bowman, T., Garcia, R., Turkson, J. & Jove, R. STATs in oncogenesis. Oncogene 19, 2474–2488 (2000).
Turkson, J. et al. Phosphotyrosyl peptides block Stat3-mediated DNA binding activity, gene regulation, and cell transformation. J. Biol. Chem. 276, 45443–45455 (2001).
Huang, Q. et al. Revisiting signal transducer and activator of transcription 3 (STAT3) as an anticancer target and its inhibitor discovery: where are we and where should we go? Eur. J. Med. Chem. 187, 111922 (2020).
Ahmad, S. F. et al. STA-21, a STAT-3 inhibitor, attenuates the development and progression of inflammation in collagen antibody-induced arthritis. Immunobiology 222, 206–217 (2017).
Oh, D. Y. et al. Phase I study of OPB-31121, an oral STAT3 inhibitor, in patients with advanced solid tumors. Cancer Res. Treat. 47, 607–615 (2015).
Odate, S. et al. Inhibition of STAT3 with the generation 2.5 antisense oligonucleotide, AZD9150, decreases neuroblastoma tumorigenicity and increases chemosensitivity. Clin. Cancer Res. 23, 1771–1784 (2017).
Xiang, M. et al. Gene expression-based discovery of atovaquone as a STAT3 inhibitor and anticancer agent. Blood 128, 1845–1853 (2016).
Gavino, A. C., Nahmod, K., Bharadwaj, U., Makedonas, G. & Tweardy, D. J. STAT3 inhibition prevents lung inflammation, remodeling, and accumulation of Th2 and Th17 cells in a murine asthma model. Allergy 71, 1684–1692 (2016).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017). This paper describes the efficacy and safety data from the tofacitinib clinical trials that led to regulatory approval of the first JAK inhibitor for the treatment of ulcerative colitis.
Panes, J. et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut 66, 1049–1059 (2017).
Ma, C. et al. Systematic review with meta-analysis: efficacy and safety of oral Janus kinase inhibitors for inflammatory bowel disease. Aliment. Pharmacol. Ther. 50, 5–23 (2019). This systematic review provides a summary of all placebo-controlled randomized trials of JAK inhibitors in adults with IBD conducted to date.
Ma, C., Jairath, V. & Vande Casteele, N. Pharmacology, efficacy and safety of JAK inhibitors in Crohn’s disease. Best Pract. Res. Clin. Gastroenterol. 38-39, 101606 (2019). This paper provides an overview of the available clinical trial data on JAK inhibitors for the treatment of Crohn’s disease and includes an interesting perspective on trial design factors that may have influenced the results of the phase II study (FITZROY) of the selective JAK1 inhibitor filgotinib.
Vermeire, S. et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389, 266–275 (2017). This paper describes the efficacy and safety data from a clinical trial of filgotinib for the treatment of Crohn’s disease.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02914561 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02914600 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02914535 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02914522 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03077412 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT03046056 (2020).
Panaccione, R. et al. P601 Upadacitinib improves steroid-free clinical and endoscopic endpoints in patients with Crohn’s disease: data from the CELEST study. J. Crohns Colitis. 12, S412–S413 (2018).
Sandborn, W. J. et al. OP195 Efficacy and safety of upadacitinib as an induction therapy for patients with moderately-to-severely active ulcerative colitis: data from the phase 2b study U-ACHIEVE. United Eur. Gastroenterol. J. 6, A74–A75 (2018).
Sands, B. E. et al. Peficitinib, an oral Janus kinase inhibitor, in moderate-to-severe ulcerative colitis: results from a randomised, phase 2 study. J. Crohns Colitis. 12, 1158–1169 (2018).
Clark, J. D., Flanagan, M. E. & Telliez, J. B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 57, 5023–5038 (2014).
Olivera, P., Danese, S. & Peyrin-Biroulet, L. Next generation of small molecules in inflammatory bowel disease. Gut 66, 199–209 (2017).
Panes, J. et al. Long-term safety and tolerability of oral tofacitinib in patients with Crohn’s disease: results from a phase 2, open-label, 48-week extension study. Aliment. Pharmacol. Ther. 49, 265–276 (2019).
Sandborn, W. J. et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 367, 616–624 (2012).
Cohen, S. B. et al. Long-term safety of tofacitinib for the treatment of rheumatoid arthritis up to 8.5 years: integrated analysis of data from the global clinical trials. Ann. Rheum. Dis.76, 1253–1262 (2017).
Valenzuela, F. et al. Tofacitinib in patients with moderate-to-severe chronic plaque psoriasis: long-term safety and efficacy in an open-label extension study. Br. J. Dermatol.179, 853–862 (2018).
Winthrop, K. L. et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm. Bowel Dis. 24, 2258–2265 (2018).
Sandborn, W. J. et al. Safety of tofacitinib for treatment of ulcerative colitis, based on 4.4 years of data from global clinical trials. Clin. Gastroenterol. Hepatol. 17, 1541–1550 (2019).
Curtis, J. R. et al. Tofacitinib, an oral Janus kinase inhibitor: analysis of malignancies across the rheumatoid arthritis clinical development programme. Ann. Rheum. Dis. 75, 831–841 (2016).
Curtis, J. R. et al. Analysis of non-melanoma skin cancer across the tofacitinib rheumatoid arthritis clinical programme. Clin. Exp. Rheumatol. 35, 614–622 (2017).
Mariette, X., Chen, C., Biswas, P., Kwok, K. & Boy, M. G. Lymphoma in the tofacitinib rheumatoid arthritis clinical development program. Arthritis Care Res. 70, 685–694 (2018).
Verden, A., Dimbil, M., Kyle, R., Overstreet, B. & Hoffman, K. B. Analysis of spontaneous postmarket case reports submitted to the FDA regarding thromboembolic adverse events and JAK inhibitors. Drug Saf. 41, 357–361 (2018).
Desai, R. J., Pawar, A., Weinblatt, M. E. & Kim, S. C. Comparative risk of venous thromboembolism with tofacitinib versus tumor necrosis factor inhibitors: a cohort study of rheumatoid arthritis patients. Arthritis Rheumatol. 71, 892–900 (2019).
Scott, I. C., Hider, S. L. & Scott, D. L. Thromboembolism with Janus kinase (JAK) inhibitors for rheumatoid arthritis: how real is the risk? Drug Saf. 41, 645–653 (2018).
US Food and Drug Administration. FDA approves Boxed Warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). US Food and Drug Administration https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (2019).
Rigby, W. F. C., Lampl, K., Low, J. M. & Furst, D. E. Review of routine laboratory monitoring for patients with rheumatoid arthritis receiving biologic or nonbiologic DMARDs. Int. J. Rheumatol. 2017, 9614241 (2017).
Clowse, M. E. et al. Pregnancy outcomes in the tofacitinib safety databases for rheumatoid arthritis and psoriasis. Drug Saf. 39, 755–762 (2016).
Mahadevan, U. et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm. Bowel Dis. 24, 2494–2500 (2018).
Takeuchi, T. et al. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann. Rheum. Dis. 75, 1057–1064 (2016).
Panes, J. et al. P273 Efficacy and safety of upadacitinib maintenance treatment for moderate to severe Crohn’s disease: results from the CELEST study. J. Crohns Colitis. 12 (Suppl. 1), 238–239 (2018).
Sandborn, W. J. et al. P041 The gut-selective, orally administered, pan-JAK inhibitor TD-1473 demonstrates favorable safety, tolerability, pharmacokinetic, and signal for clinical activity in subjects with moderately-to-severely active ulcerative colitis. Gastroenterology 156 (Suppl.), 29–30 (2019).
Van Rompaey, L. et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J. Immunol. 191, 3568–3577 (2013).
Beattie, D. et al. P069 TD-1473, a novel, potent, and orally administered, GI-targeted, pan-Janus kinase (JAK) inhibitor. J. Crohns Colitis. 10 (Suppl. 1), 123 (2016).
Voss, J. et al. THU0127 Pharmacodynamics of a novel JAK1 selective inhibitor in rat arthritis and anemia models and in healthy human subjects. Ann. Rheum. Dis. 73, 222–222 (2014).
Mukherjee, A. et al. Exposure-response characterization of tofacitinib efficacy in moderate to severe ulcerative colitis: results from a dose-ranging phase 2 trial. Br. J. Clin. Pharmacol. 84, 1136–1145 (2018).
Dowty, M. E. et al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a Janus kinase inhibitor, in humans. Drug Metab. Dispos. 42, 759–773 (2014).
Galien, R. et al. Analysis of the JAK1 selectivity of GLPG0634 and its main metabolite in different species, healthy volunteers and rheumatoid arthritis patients. Arthritis Rheum. 65, S209–S210 (2013).
Namour, F. et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin. Pharmacokinet. 54, 859–874 (2015).
Ferslew, B., Graham, R., Sherman, C. & Nguyen, D. P469 Safety, tolerability, and pharmacokinetics of the intestine-restricted oral pan-Janus kinase inhibitor TD-1473 after single and multiple oral doses in healthy subjects. J. Crohns Colitis. 11 (Suppl. 1), 317–318 (2017).
Sandborn, W. J. et al. LB05 The intestinally restricted, orally administered, pan-Jak inhibitor TD-1473 demonstrates favorable safety, tolerability, pharmacokinetics, and signal for clinical activity in subjects with moderately-to-severely active ulcerative colitis. United Eur. Gastroenterol. J. 6, 1588–1599 (2018).
Mohamed, M. F. et al. Pharmacokinetics, safety and tolerability of ABT-494, a novel selective JAK 1 inhibitor, in healthy volunteers and subjects with rheumatoid arthritis. Clin. Pharmacokinet. 55, 1547–1558 (2016).
Bressler, B. et al. Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the Toronto consensus. Gastroenterology 148, 1035–1058.e3 (2015).
Gomollon, F. et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J. Crohns Colitis. 11, 3–25 (2017).
Harbord, M. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J. Crohns Colitis. 11, 769–784 (2017).
American Gastroenterological Association. IBD & bowel disorders guidelines. Gastro.org https://www.gastro.org/guidelines/ibd-and-bowel-disorders (2020).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 362, 1383–1395 (2010).
Panaccione, R. et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 146, 392–400.e3 (2014).
Hirten, R. P., Iacucci, M., Shah, S., Ghosh, S. & Colombel, J. F. Combining biologics in inflammatory bowel disease and other immune mediated inflammatory disorders. Clin. Gastroenterol. Hepatol. 16, 1374–1384 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/show/NCT02764762 (2020).
Burmester, G. R. et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381, 451–460 (2013).
Fleischmann, R. et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. Lancet 390, 457–468 (2017).
Hanauer, S. et al. Tofacitinib induction therapy reduces symptoms within 3 days for patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 17, 139–147 (2019).
Kaur, M. et al. Perianal Crohn’s disease is associated with distal colonic disease, stricturing disease behavior, IBD-associated serologies and genetic variation in the JAK-STAT pathway. Inflamm. Bowel Dis. 22, 862–869 (2016).
Ito, M. et al. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J. Pharmacol. Sci. 133, 25–33 (2017).
Parmentier, J. M. et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494). BMC Rheumatol. 2, 23 (2018).
Fensome, A. et al. Dual inhibition of TYK2 and JAK1 for the treatment of autoimmune diseases: discovery of ((S)-2,2-difluorocyclopropyl)((1R,5S)-3-(2-((1-methyl-1 H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone (PF-06700841). J. Med. Chem. 61, 8597–8612 (2018).
Telliez, J. B. et al. Discovery of a JAK3-selective inhibitor: functional differentiation of JAK3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chem. Biol. 11, 3442–3451 (2016).
Acknowledgements
The authors thank L. M. Shackelton for technical review and editing, and M. Andic for editorial assistance.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
A.S. reports research grants from Roche, Genentech, Boehringer Ingelheim and AbbVie, lecture fees from Roche, Boehringer Ingelheim and Pfizer, and consultancy fees from Genentech and GSK; M.D. reports advisory fees from Echo Pharma and Robarts Clinical Trials, speaker fees from Janssen, Merck, Pfizer, Takeda and Tillotts Pharma, and nonfinancial support from Dr. Falk Pharma. W.F. reports consulting or advisory fees from AbbVie, Boehringer Ingelheim Pharma, MediBeacon, Celegene, Janssen Research and Development, Robarts Clinical Trials, Takeda Pharma, Eli Lily, Hire, Velocity Pharma, S&B Pharms and Connecticut Children’s Medical Center. D.M. reports consulting fees from Pfizer, Gilead, Janssen, Qu Biologics, Bridge Therapeutics, Precision IBD, and grant support from Janssen, and Second Genome. S.Vermeire reports grants/research support from MSD, AbbVie, Takeda, Janssen, and Pfizer, honoraria or consultation fees from AbbVie, MSD, Takeda, Ferring, Genentech/Roche, Shire, Pfizer, Galapagos, Mundipharma, Hospira, Celgene, Second Genome, Progenity, Lilly, Arena, Gilead and Janssen, participation on speaker’s bureaus for AbbVie, MSD, Takeda, Ferring, Hospira, Pfizer, Janssen and Tillots, and employment of spouse by Biogen. N.V.C. reports research support from R-Biopharm and Takeda and consulting fees from Boehringer Ingelheim, Janssen, Pfizer, Progenity, Prometheus and Takeda, outside of the submitted work. C.H.-R. and S. Vetrano declare no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks M. Gadina, X. Roblin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Salas, A., Hernandez-Rocha, C., Duijvestein, M. et al. JAK–STAT pathway targeting for the treatment of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 17, 323–337 (2020). https://doi.org/10.1038/s41575-020-0273-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-020-0273-0
This article is cited by
-
Inflammatory bowel diseases: pathological mechanisms and therapeutic perspectives
Molecular Biomedicine (2026)
-
microRNA-34 mediates a negative feedback loop in the JAK-STAT pathway to attenuate immune overactivation in an invertebrate
Cell Communication and Signaling (2025)
-
Targeting JAK2 with a multifunctional nanoinhibitor for long-lasting anti-inflammatory effects and bone repair
Journal of Nanobiotechnology (2025)
-
Upadacitinib induction therapy in Crohn’ s disease: a retrospective real-world study in biologic-refractory patients
BMC Gastroenterology (2025)
-
Helicobacter pylori in peptic ulcer disease: pathogenesis, gastric microbiome, and innovative therapies
Bulletin of the National Research Centre (2025)