Abstract
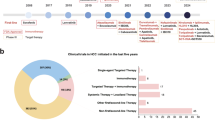
Hepatocellular carcinoma (HCC) is a prevalent disease with a progression that is modulated by the immune system. Systemic therapy is used in the advanced stage and until 2017 consisted only of antiangiogenic tyrosine kinase inhibitors (TKIs). Immunotherapy with checkpoint inhibitors has shown strong anti-tumour activity in a subset of patients and the combination of the anti-PDL1 antibody atezolizumab and the VEGF-neutralizing antibody bevacizumab has or will soon become the standard of care as a first-line therapy for HCC, whereas the anti-PD1 agents nivolumab and pembrolizumab are used after TKIs in several regions. Other immune strategies such as adoptive T-cell transfer, vaccination or virotherapy have not yet demonstrated consistent clinical activity. Major unmet challenges in HCC checkpoint immunotherapy are the discovery and validation of predictive biomarkers, advancing treatment to earlier stages of the disease, applying the treatment to patients with liver dysfunction and the discovery of more effective combinatorial or sequential approaches. Combinations with other systemic or local treatments are perceived as the most promising opportunities in HCC and some are already under evaluation in large-scale clinical trials. This Review provides up-to-date information on the best use of currently available immunotherapies in HCC and the therapeutic strategies under development.
Key points
-
Multiple immune mechanisms are important in the development and progression of hepatocellular carcinoma (HCC) and correlate with prognosis.
-
Checkpoint inhibitors targeting PD1 and PDL1 and CTLA4 are active, tolerable and clinically beneficial against advanced HCC.
-
At present, the best available first-line treatment for advanced HCC is a combination of PDL1 blockade with atezolizumab and VEGF blockade with bevacizumab.
-
There is as yet an almost complete lack of suitable biomarkers to guide the development of checkpoint inhibitors and their combinations in HCC.
-
Immunotherapy is likely to synergize with local and locoregional interventions in earlier stages of HCC.
-
Other promising forms of immunotherapy for HCC such as additional checkpoint inhibitors, adoptive cell transfer, vaccination and virotherapy are being actively pursued.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ferlay, J. et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 144, 1941–1953 (2019).
WHO. Liver. http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf (2018).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2, 16018 (2016).
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 69, 182–236 (2018). A thorough and updated guidance on the management of HCC.
Heimbach, J. K. et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67, 358–380 (2018).
Vogel, A. et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 29, iv238–iv255 (2018).
Omata, M. et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol. Int. 11, 317–370 (2017).
Llovet, J. M. et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 359, 378–390 (2008).
Lencioni, R. et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J. Hepatol. 64, 1090–1098 (2016).
Kudo, M. et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology 60, 1697–1707 (2014).
Bruix, J. et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 16, 1344–1354 (2015).
Kudo, M. et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391, 1163–1173 (2018).
Bruix, J. et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389, 56–66 (2017).
Abou-Alfa, G. K. et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 379, 54–63 (2018).
Zhu, A. X. et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 20, 282–296 (2019).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Rabinovich, G. A., Gabrilovich, D. & Sotomayor, E. M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296 (2007).
Cai, L. et al. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol. Immunother. 67, 999–1009 (2018).
Velcheti, V. & Schalper, K. Basic overview of current immunotherapy approaches in cancer. Am. Soc. Clin. Oncol. Educ. Book 35, 298–308 (2016).
Chen, L. & Flies, D. B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 13, 227–242 (2013).
He, X. & Xu, C. Immune checkpoint signaling and cancer immunotherapy. Cell Res. 30, 660–669 (2020).
Jain, N., Nguyen, H., Chambers, C. & Kang, J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl Acad. Sci. USA 107, 1524–1528 (2010).
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
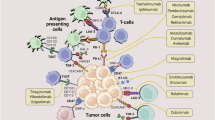
Prieto, J., Melero, I. & Sangro, B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 12, 681–700 (2015).
Flecken, T. et al. Immunodominance and functional alterations of tumor-associated antigen-specific CD8+ T-cell responses in hepatocellular carcinoma. Hepatology 59, 1415–1426 (2014).
Yarchoan, M., Johnson, B. A., Lutz, E. R., Laheru, D. A. & Jaffee, E. M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 17, 209–222 (2017).
Luksza, M. et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 551, 517–520 (2017).
Malekzadeh, P. et al. Antigen experienced T cells from peripheral blood recognize p53 neoantigens. Clin. Cancer Res. 26, 1267–1276 (2020).
Efremova, M., Finotello, F., Rieder, D. & Trajanoski, Z. Neoantigens generated by individual mutations and their role in cancer immunity and immunotherapy. Front. Immunol. 8, 1679 (2017).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Chan, T. A. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 30, 44–56 (2019).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Fujimoto, A. et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat. Genet. 48, 500–509 (2016).
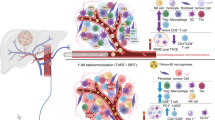
Crispe, I. N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Ebrahimkhani, M. R., Mohar, I. & Crispe, I. N. Cross-presentation of antigen by diverse subsets of murine liver cells. Hepatology 54, 1379–1387 (2011).
Krenkel, O. & Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 17, 306–321 (2017).
Ormandy, L. et al. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 65, 2457–2464 (2005).
Shetty, S., Lalor, P. F. & Adams, D. H. Liver sinusoidal endothelial cells – gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 15, 555–567 (2018).
Höchst, B. et al. Activated human hepatic stellate cells induce myeloid derived suppressor cells from peripheral blood monocytes in a CD44-dependent fashion. J. Hepatol. 59, 528–535 (2013).
Dunham, R. M. et al. Hepatic stellate cells preferentially induce Foxp3+ regulatory T cells by production of retinoic acid. J. Immunol. 190, 2009–2016 (2013).
Yu, M. C. et al. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology 40, 1312–1321 (2004).
Cariani, E. et al. HLA and killer immunoglobulin-like receptor genes as outcome predictors of hepatitis C virus-related hepatocellular carcinoma. Clin. Cancer Res. 19, 5465–5473 (2013).
Cariani, E. et al. Natural killer cells phenotypic characterization as an outcome predictor of HCV-linked HCC after curative treatments. Oncoimmunology 5, e1154249 (2016).
Hoechst, B. et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50, 799–807 (2009).
Zhang, Q. F. et al. Liver-infiltrating CD11b-CD27- NK subsets account for NK-cell dysfunction in patients with hepatocellular carcinoma and are associated with tumor progression. Cell. Mol. Immunol. 14, 819–829 (2017).
Fu, J. et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132, 2328–2339 (2007).
Gao, Q. et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J. Clin. Oncol. 25, 2586–2593 (2007).
Chen, K. J. et al. Selective recruitment of regulatory T cell through CCR6-CCL20 in hepatocellular carcinoma fosters tumor progression and predicts poor prognosis. PLoS ONE 6, e24671 (2011).
Hoechst, B. et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135, 234–243 (2008).
Veglia, F., Perego, M. & Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nature Immunology 19, 108–119 (2018).
Yeung, O. W. H. et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 62, 607–616 (2015).
Xiao, X. et al. PD-1hi identifies a novel regulatory B-cell population in human hepatoma that promotes disease progression. Cancer Discov. 6, 546–559 (2016).
Zhao, F. et al. Human CCR4+ CCR6+ Th17 cells suppress autologous CD8+ T cell responses. J. Immunol. 188, 6055–6062 (2012).
Han, Y. et al. Human CD14+CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 59, 567–579 (2014).
Affo, S., Yu, L.-X. & Schwabe, R. F. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu. Rev. Pathol. Mech. Dis. 12, 153–186 (2017).
Zhou, S. L. et al. Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology 150, 1646–1658.e17 (2016).
Wu, K., Kryczek, I., Chen, L., Zou, W. & Welling, T. H. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 69, 8067–8075 (2009).
Ji, J. et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology 62, 481–495 (2015).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Luke, J. J., Bao, R., Sweis, R. F., Spranger, S. & Gajewski, T. F. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 25, 3074–3083 (2019).
Spranger, S. et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc. Natl Acad. Sci. USA 113, E7759–E7768 (2016).
Barry, K. C. et al. A natural killer–dendritic cell axis defines checkpoint therapy–responsive tumor microenvironments. Nat. Med. 24, 1178–1191 (2018).
Bray, S. M., Vujanovic, L. & Butterfield, L. H. Dendritic cell-based vaccines positively impact natural killer and regulatory T cells in hepatocellular carcinoma patients. Clin. Dev. Immunol. 2011, 249281 (2011).
Sánchez-Paulete, A. R. et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 6, 71–79 (2016).
de Galarreta, M. R. et al. β-catenin activation promotes immune escape and resistance to anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 9, 1124–1141 (2019). A link between a frequent genetic alteration in HCC and resistance to ICIs in animal models.
Harding, J. J. et al. Prospective genotyping of hepatocellular carcinoma: clinical implications of next-generation sequencing for matching patients to targeted and immune therapies. Clin. Cancer Res. 25, 2116–2126 (2019).
Cristescu, R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018).
Fridman, W. H., Zitvogel, L., Sautès-Fridman, C. & Kroemer, G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 14, 717–734 (2017).
Peng, W. et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Skoulidis, F. et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 8, 822–835 (2018).
Spranger, S. & Gajewski, T. F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 18, 139–147 (2018).
Duruisseaux, M. et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir. Med. 6, 771–781 (2018).
Zhou, J. et al. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int. J. Cancer 125, 1640–1648 (2009).
Horwitz, E. et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 4, 730–743 (2014).
Glodde, N. et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 47, 789–802.e9 (2017).
Sanmamed, M. F. et al. Serum interleukin-8 reflects tumor burden and treatment response across malignancies of multiple tissue origins. Clin. Cancer Res. 20, 5697–5707 (2014).
Tsai, J. H. F. et al. Elevated urinary transforming growth factor-β1 level as a tumour marker and predictor of poor survival in cirrhotic hepatocellular carcinoma. Br. J. Cancer 76, 244–250 (1997).
Ritter, M. et al. Immunoregulation of dendritic and T cells by alpha-fetoprotein in patients with hepatocellular carcinoma. J. Hepatol. 41, 999–1007 (2004).
Pardee, A. D., Shi, J. & Butterfield, L. H. Tumor-derived α-fetoprotein impairs the differentiation and T cell stimulatory activity of human dendritic cells. J. Immunol. 193, 5723–5732 (2014).
Dong, L. Q. et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J. Hepatol. 72, 896–908 (2020).
Kamimura, H. et al. Reduced NKG2D ligand expression in hepatocellular carcinoma correlates with early recurrence. J. Hepatol. 56, 381–388 (2012).
Makarova-Rusher, O. V., Medina-Echeverz, J., Duffy, A. G. & Greten, T. F. The yin and yang of evasion and immune activation in HCC. J. Hepatol. 62, 1420–1429 (2015).
Berraondo, P., Ochoa, M. C., Olivera, I. & Melero, I. Immune desertic landscapes in hepatocellular carcinoma shaped by β-catenin activation. Cancer Discov. 9, 1003–1005 (2019).
Montfort, A. et al. The TNF paradox in cancer progression and immunotherapy. Front. Immunol. 10, 1818 (2019).
Zhong, M. et al. Induction of tolerogenic dendritic cells by activated TGF-β/Akt/Smad2 signaling in RIG-I-deficient stemness-high human liver cancer cells. BMC Cancer 19, 439 (2019).
Zhang, F. et al. TGF-β induces M2-like macrophage polarization via SNAIL-mediated suppression of a pro-inflammatory phenotype. Oncotarget 7, 52294–52306 (2016).
Ahmadzadeh, M. & Rosenberg, S. A. TGF-β1 attenuates the acquisition and expression of effector function by tumor antigen-specific human memory CD8 T cells. J. Immunol. 174, 5215–5223 (2005).
Okumoto, K. et al. Possible contribution of circulating transforming growth factor-beta1 to immunity and prognosis in unresectable hepatocellular carcinoma. Liver Int. 24, 21–28 (2004).
Yamagiwa, S., Gray, J. D., Hashimoto, S. & Horwitz, D. A. A role for TGF-β in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J. Immunol. 166, 7282–7289 (2001).
Peng, L. et al. High TGF-β1 expression predicts poor disease prognosis in hepatocellular carcinoma patients. Oncotarget 8, 34387–34397 (2017).
Lin, T. H. et al. High serum transforming growth factor-β1 levels predict outcome in hepatocellular carcinoma patients treated with sorafenib. Clin. Cancer Res. 21, 3678–3684 (2015).
Feun, L. G. et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 125, 3603–3614 (2019).
Stewart, C. A. et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J. Clin. Invest. 123, 4859–4874 (2013).
Kuang, D. M. et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 206, 1327–1337 (2009).
Yi, Y. et al. The functional impairment of HCC-infiltrating γδ T cells, partially mediated by regulatory T cells in a TGFβ- and IL-10-dependent manner. J. Hepatol. 58, 977–983 (2013).
Arihara, F. et al. Increase in CD14+HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol. Immunother. 62, 1421–1430 (2013).
Shi, Y., Song, Q., Hu, D., Zhuang, X. & Yu, S. Tumor-infiltrating lymphocyte activity is enhanced in tumors with low IL-10 production in HBV-induced hepatocellular carcinoma. Biochem. Biophys. Res. Commun. 461, 109–114 (2015).
Apte, R. S., Chen, D. S. & Ferrara, N. VEGF in signaling and disease: beyond discovery and development. Cell 176, 1248–1264 (2019).
Courau, T. et al. TGF-β and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight 1, e85974 (2016).
Brown, Z. J. et al. Indoleamine 2,3-dioxygenase provides adaptive resistance to immune checkpoint inhibitors in hepatocellular carcinoma. Cancer Immunol. Immunother. 67, 1305–1315 (2018).
Cai, X. Y. et al. Overexpression of CD39 in hepatocellular carcinoma is an independent indicator of poor outcome after radical resection. Medicine 95, e4989 (2016).
Shali, S. et al. Ecto-5′-nucleotidase (CD73) is a potential target of hepatocellular carcinoma. J. Cell. Physiol. 234, 10248–10259 (2019).
Lee, I. C. et al. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int. J. Cancer 133, 2895–2902 (2013).
Nagao, M. et al. The impact of interferon gamma receptor expression on the mechanism of escape from host immune surveillance in hepatocellular carcinoma. Hepatology 32, 491–500 (2000).
Shi, F. et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int. J. Cancer 128, 887–896 (2011).
Zabala, M. et al. Induction of immunosuppressive molecules and regulatory T cells counteracts the antitumor effect of interleukin-12-based gene therapy in a transgenic mouse model of liver cancer. J. Hepatol. 47, 807–815 (2007).
Sangro, B. et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 59, 81–88 (2013). The first clinical trial of immunotherapy in HCC, showing activity of CTLA4 blockade.
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017). The first clinical trial to show consistent antitumor activity of PD1 blockade in HCC.
Zhu, A. X. et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 19, 940–952 (2018).
Finn, R. S. et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J. Clin. Oncol. 38, 193–202 (2020).
Yau, T. et al. CheckMate 459: a randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC) [abstract LBA38_PR]. Ann. Oncol. 30 (Suppl. 5), v874–v875 (2019).
Sangro, B. et al. CheckMate 459: long-term (minimum follow-up 33.6 months) survival outcomes with nivolumab versus sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma [abstract LBA-3]. Ann. Oncol. 31 (Suppl. 3), S241–S242 (2020).
Gao, Q. et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin. Cancer Res. 15, 971–979 (2009).
Li, H. et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 56, 1342–1351 (2012).
Yan, W. et al. Tim-3 fosters HCC development by enhancing TGF-β-mediated alternative activation of macrophages. Gut 64, 1593–1604 (2015).
Du, W. et al. Tim-3 as a target for cancer immunotherapy and mechanisms of action. Int. J. Mol. Sci. 18, 645 (2017).
Gautron, A. S., Dominguez-Villar, M., de Marcken, M. & Hafler, D. A. Enhanced suppressor function of TIM-3+FoxP3+ regulatory T cells. Eur. J. Immunol. 44, 2703–2711 (2014).
Li, Z. et al. Immune checkpoint proteins PD-1 and TIM-3 are both highly expressed in liver tissues and correlate with their gene polymorphisms in patients with HBV-related hepatocellular carcinoma. Medicine 95, e5749 (2016).
Andrews, L. P., Marciscano, A. E., Drake, C. G. & Vignali, D. A. A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 276, 80–96 (2017).
Zhou, G. et al. Antibodies against immune checkpoint molecules restore functions of tumor-infiltrating T cells in hepatocellular carcinomas. Gastroenterology 153, 1107–1119.e10 (2017).
Wang, J. et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 176, 334–347.e12 (2019).
Duffy, A. G. et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J. Hepatol. 66, 545–551 (2017).
Yau, T. et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040 [abstract]. J. Clin. Oncol. 37 (Suppl. 15), 4012 (2019).
Kelley, R. K. et al. Efficacy, tolerability, and biologic activity of a novel regimen of tremelimumab (T) in combination with durvalumab (D) for patients (pts) with advanced hepatocellular carcinoma (aHCC) [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 4508 (2020).
Ascierto, P. A. et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 18, 611–622 (2017).
Callahan, M. K. et al. Peripheral and tumor immune correlates in patients with advanced melanoma treated with combination nivolumab (anti-PD-1, BMS-936558, ONO-4538) and ipilimumab [abstract]. J. Clin. Oncol. 31 (Suppl. 15), 3003 (2013).
Zhu, A. X. et al. A phase Ib study of lenvatinib (LEN) plus pembrolizumab (PEMBRO) in unresectable hepatocellular carcinoma (uHCC) [abstract]. J. Clin. Oncol. 38 (Suppl. 15), 4519 (2020).
Yau, T. et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): results from CheckMate 040 [abstract]. J. Clin. Oncol. 38 (Suppl. 4), 478 (2020).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020). The pivotal clinical trial that has established a combination of an anti-PD1 and an anti -VEGF as the standard of care for first-line systemic therapy in HCC.
Finn, R. S. et al. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) [abstract]. J. Clin. Oncol. 39 (Suppl. 3), 267 (2021).
Lee, M. et al. Randomised efficacy and safety results for atezolizumab (atezo) + bevacizumab (bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC) [abstract LBA39]. Ann. Oncol. 30 (Suppl. 5), v875 (2019).
Siegel, A. B. et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J. Clin. Oncol. 26, 2992–2998 (2008).
Boige, V. et al. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist 17, 1063–1072 (2012).
Ren, Z. et al. Sintilimab plus bevacizumab biosimilar vs sorafenib as first-line treatment for advanced hepatocellular carcinoma (ORIENT-32)2 [abstract LBA2]. Ann. Oncol. 31 (Suppl. 6), S1287 (2020).
Rahma, O. E. & Hodi, F. S. The intersection between tumor angiogenesis and immune suppression. Clin. Cancer Res. 25, 5449–5457 (2019).
Feng, P.-H. et al. Bevacizumab reduces S100A9-positive MDSCs linked to intracranial control in patients with EGFR-mutant lung adenocarcinoma. J. Thorac. Oncol. 13, 958–967 (2018).
Limagne, E. et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 76, 5241–5252 (2016).
Shigeta, K. et al. Dual programmed death receptor-1 and vascular endothelial growth factor receptor-2 blockade promotes vascular normalization and enhances antitumor immune responses in hepatocellular carcinoma. Hepatology 71, 1247–1261 (2020).
Fabian, M. A. et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat. Biotechnol. 23, 329–336 (2005).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Sangro, B. et al. Diagnosis and management of toxicities of immune checkpoint inhibitors in hepatocellular carcinoma. J. Hepatol. 72, 320–341 (2020). A thorough guidance on how to manage the toxicity of ICIs in HCC.
Yau, T. et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 6, e204564 (2020). The first clinical trial to show improved activity with the dual blockade of CTLA4 and PD1.
Duan, J. et al. Use of immunotherapy with programmed cell death 1 vs programmed cell death ligand 1 inhibitors in patients with cancer: a systematic review and meta-analysis. JAMA Oncol. 6, 375–384 (2020).
Spain, L., Diem, S. & Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 44, 51–60 (2016).
Vollmer, O. et al. Characterization of auto-immune hepatitis associated with the use of anti-TNFα agents: an analysis of 389 cases in VigiBase. Autoimmun. Rev. 19, 102460 (2020).
Peeraphatdit, T. B. et al. Hepatotoxicity from immune checkpoint inhibitors: a systematic review and management recommendation. Hepatology 72, 315–329 (2020).
Geiger-Gritsch, S. et al. Safety of bevacizumab in patients with advanced cancer: a meta-analysis of randomized controlled trials. Oncologist 15, 1179–1191 (2010).
Brusilovskaya, K., Königshofer, P., Schwabl, P. & Reiberger, T. Vascular targets for the treatment of portal hypertension. Semin. Liver Dis. 39, 483–501 (2019).
Reiberger, T. et al. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J. Hepatol. 51, 865–873 (2009).
Scheiner, B. et al. Short- and long-term effects of transarterial chemoembolization on portal hypertension in patients with hepatocellular carcinoma. United Eur. Gastroenterol. J. 7, 850–858 (2019).
Rimola, J. et al. Radiological response to nivolumab in patients with hepatocellular carcinoma: a multicenter analysis of real-life practice. Eur. J. Radiol. 135, 109484 (2021).
El-Khoueiry, A. B. et al. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): subanalyses of CheckMate-040 [abstract]. J. Clin. Oncol. 36 (Suppl. 4), 475 (2018).
Reig, M. et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol. 61, 318–324 (2014).
Wong, L. et al. Association between immune-related adverse events and efficacy of immune checkpoint inhibitors in patients with advanced hepatocellular carcinoma [abstract 325P]. Ann. Oncol. 30 (Suppl. 9), ix110 (2019).
Gordan, J. D. et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol. 38, 4317–4345 (2020).
Pelizzaro, F. et al. Capecitabine in advanced hepatocellular carcinoma: a multicenter experience. Dig. Liver Dis. 51, 1713–1719 (2019).
Sangro, B. et al. Association of inflammatory biomarkers with clinical outcomes in nivolumab-treated patients with advanced hepatocellular carcinoma. J. Hepatol. 73, 1460–1469 (2020).
Calderaro, J. et al. Programmed death ligand 1 expression in hepatocellular carcinoma: relationship with clinical and pathological features. Hepatology 64, 2038–2046 (2016). A comprehensive study of the clinical and pathological correlations of PDL1 expression in HCC.
Sia, D. et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 153, 812–826 (2017).
Liu, X. S. & Mardis, E. R. Applications of immunogenomics to cancer. Cell 168, 600–612 (2017).
Jiang, P. et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat. Med. 24, 1550–1558 (2018).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Pillai, R. N. et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer 124, 271–277 (2018).
Latchman, Y. et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2, 261–268 (2001).
Sugiura, D. et al. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science 364, 558–566 (2019).
Davda, J. et al. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. J. Immunother. Cancer 7, 105 (2019).
van Brummelen, E. M. J., Ros, W., Wolbink, G., Beijnen, J. H. & Schellens, J. H. M. Antidrug antibody formation in oncology: clinical relevance and challenges. Oncologist 21, 1260–1268 (2016).
FDA. Highlights of Prescribing Information: TECENTRIQ. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761034s025lbl.pdf (2020).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Cappuzzello, E., Sommaggio, R., Zanovello, P. & Rosato, A. Cytokines for the induction of antitumor effectors: the paradigm of cytokine-induced killer (CIK) cells. Cytokine Growth Factor Rev. 36, 99–105 (2017).
Rosenberg, S. A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Rosenberg, S. A., Spiess, P. & Lafreniere, R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233, 1318–1321 (1986).
Takayama, T. et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 356, 802–807 (2000).
Lu, P. H. & Negrin, R. S. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J. Immunol. 153, 1687–1696 (1994).
Lee, J. H. et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148, 1383–1391.e6 (2015).
Jiang, S. S. et al. A phase I clinical trial utilizing autologous tumor-infiltrating lymphocytes in patients with primary hepatocellular carcinoma. Oncotarget 6, 41339–41349 (2015).
Lee, H. J., Kim, S. K., Cho, D. & Lee, J. J. Cellular immunotherapy as a beacon of hope for hematological malignancies. Blood Res. 50, 126–128 (2015).
Gao, H. et al. Development of T cells redirected to glypican-3 for the treatment of hepatocellular carcinoma. Clin. Cancer Res. 20, 6418–6428 (2014).
Jiang, Z. et al. Anti-GPC3-CAR T cells suppress the growth of tumor cells in patient-derived xenografts of hepatocellular carcinoma. Front. Immunol. 7, 690 (2017).
Butterfield, L. H. et al. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin. Cancer Res. 12, 2817–2825 (2006).
Sun, L. et al. Engineered cytotoxic T lymphocytes with AFP-specific TCR gene for adoptive immunotherapy in hepatocellular carcinoma. Tumor Biol. 37, 799–806 (2016).
Gerry, A. B. et al. Improved affinity AFP-specific T cell receptor for hepatocellular carcinoma. J. Immunother. Cancer 1, P10 (2013).
Malekzadeh, P. et al. Neoantigen screening identifies broad TP53 mutant immunogenicity in patients with epithelial cancers. J. Clin. Invest. 129, 1109–1114 (2019).
Hu, Z., Ott, P. A. & Wu, C. J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 18, 168–182 (2018). A review on the field of tumour neoantigen-based vaccines, discussing developments and future directions for this treatment strategy.
Tagliamonte, M. et al. Potentiating cancer vaccine efficacy in liver cancer. Oncoimmunology 7, e1488564 (2018).
Hammerich, L. et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 25, 814–824 (2019).
Coulie, P. G., Van Den Eynde, B. J., Van Der Bruggen, P. & Boon, T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014).
Sawada, Y. et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin. Cancer Res. 18, 3686–3696 (2012).
Greten, T. F. et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer 10, 209 (2010).
Rammensee, H.-G. & Singh-Jasuja, H. HLA ligandome tumor antigen discovery for personalized vaccine approach. Expert Rev. Vaccin. 12, 1211–1217 (2013).
Buonaguro, L. & HEPAVAC Consortium. Developments in cancer vaccines for hepatocellular carcinoma. Cancer Immunol. Immunother. 65, 93–99 (2016).
Chen, F. et al. Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors. J. Clin. Invest. 129, 2056–2070 (2019).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Vercher, E. et al. Identification of neoantigen-reactive T cells in hepatocellular carcinoma: implication in adoptive T cell therapy. J. Hepatol. 73, S39–S40 (2020).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Vanpouille-Box, C., Hoffmann, J. A. & Galluzzi, L. Pharmacological modulation of nucleic acid sensors – therapeutic potential and persisting obstacles. Nat. Rev. Drug Discov. 18, 845–867 (2019).
Yang, D., Han, Z. & Oppenheim, J. J. Alarmins and immunity. Immunol. Rev. 280, 41–56 (2017).
Rodriguez-Ruiz, M. E., Vitale, I., Harrington, K. J., Melero, I. & Galluzzi, L. Immunological impact of cell death signaling driven by radiation on the tumor microenvironment. Nat. Immunol. 21, 120–134 (2020).
Smith, M. et al. Trial Watch: Toll-like receptor agonists in cancer immunotherapy. OncoImmunology 7, e1526250 (2018).
Flood, B. A., Higgs, E. F., Li, S., Luke, J. J. & Gajewski, T. F. STING pathway agonism as a cancer therapeutic. Immunol. Rev. 290, 24–38 (2019).
Márquez-Rodas, I. et al. Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti-PD-1 for patients with anti-PD-1-refractory tumors. Sci. Transl. Med. 12, eabb0391 (2020).
Bhatia, S. et al. Intratumoral G100, a TLR4 agonist, induces antitumor immune responses and tumor regression in patients with Merkel cell carcinoma. Clin. Cancer Res. 25, 1185–1195 (2019).
Aznar, M. A. et al. Intratumoral delivery of immunotherapy–act locally, think globally. J. Immunol. 198, 31–39 (2017).
Tenneti, P., Borad, M. J. & Babiker, H. M. Exploring the role of oncolytic viruses in hepatobiliary cancers. Immunotherapy 10, 971–986 (2018).
Melero, I. et al. Repurposing infectious disease vaccines for intratumoral immunotherapy. J. Immunother. Cancer 8, e000443 (2020).
Rodríguez-Ruiz, M. E. et al. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 29, 1312–1319 (2018).
Mizukoshi, E. et al. Enhancement of tumor-associated antigen-specific T cell responses by radiofrequency ablation of hepatocellular carcinoma. Hepatology 57, 1448–1457 (2013).
Sangro, B. et al. Nivolumab in sorafenib-naive and -experienced patients with advanced hepatocellular carcinoma (HCC): survival, hepatic safety, and biomarker assessments in CheckMate-040 [abstract 141]. Hepatology 66 (Suppl. 1), 82A (2017).
Qin, S. et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 21, 571–580 (2020).
Acknowledgements
S.H.-S. receives funding from ISCIII/FEDER, UE (PI18/00556), and Gobierno de Navarra (Departamento de Salud (045-2017)) co-financed (50%) with FEDER funds (UE, FEDER 2014-2020 “Una manera de hacer Europa”). I.M. receives funding from MINECO SAF2014-52361-R and SAF 2017-83267-C2-1R; Worldwide Cancer Research Grant (15-1146); the Asociacion Española Contra el Cancer (AECC) Foundation under grant GCB15152947MELE; and the European Union’s Horizon 2020 Program (grant agreement no. 635122 PROCROP). B.S. receives funding from ISCIII/EU TRANSCAN-2 (AC16/00065) and ISCIII FIS (PI19/00742). P.S. receives funding from Instituto de Salud Carlos III co-financed by European FEDER funds (PI17/00249 and PI20/00260), Fundación Bancaria La Caixa “Hepacare” project, and “Murchante contra el Cáncer” initiative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
I.M. reports advisory roles with Roche-Genentech, Bristol-Myers Squibb, CYTOMX, Incyte, MedImmune, Tusk, F-Star, Genmab, Molecular Partners, Alligator, Bioncotech, MSD, Merck Serono, Boehringer Ingelheim, Astra Zeneca, Numab, Catalym and Bayer, and research funding from Roche, BMS, Alligator and Bioncotech. B.S. reports consultancy fees from Adaptimmune, Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical and Terumo and speaker fees from Astra Zeneca, Bayer, BMS, BTG, Eli Lilly, Ipsen, Novartis, Merck, Roche, Sirtex Medical, Terumo BMS and Sirtex Medical. P.S. and S.H.-S. report no competing interests.
Additional information
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks A.-L. Cheng, P. Galle, A. Zhu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrial.gov: https://www.clinicaltrials.gov/
Glossary
- Biomarker
-
Biological parameter assessed in the laboratory that enables predicting or following the effects of a given treatment; identification of predictive biomarkers is important in selecting patients who would benefit from treatment.
- Vascular endothelial growth factor
-
(VEGF). A family of soluble proteins that regulate angiogenesis of blood and lymphatic vessels acting on receptors encompassing intracellular tyrosine kinase domains; VEGFA is considered the main pro-angiogenic factor in human malignancies including HCC.
- Tumour microenvironment
-
Cellular and molecular components of the malignant tissue in which tumour and stromal cells exist in a mutual relationship in which each cell type shapes the functions of the other.
- Myeloid-derived suppressor cells
-
Immature haematopoietic cells of myeloid lineage resembling either macrophages or neutrophils that are abundant in patients with advanced cancer and are able to inhibit T cell responses.
- Immune checkpoint
-
Surface receptor expressed on immune system cells that downregulates the intensity of immune responses or prevents their onset; neutralization with monoclonal antibodies of checkpoint receptors or their ligands known as checkpoint inhibitors restores immune responses to cancer.
- Cytotoxic T lymphocyte-associated antigen 4
-
(CTLA4). Co-inhibitory surface glycoprotein also known as CD152 that is expressed on activated T cells and constitutively on regulatory T cells and that shows homology and shares ligands with CD28; it is known as an immune checkpoint inhibitor that upon ligation downregulates T cell-mediated immune responses to avoid autoimmunity.
- PD1
-
Checkpoint inhibitor molecule also known as CD279 expressed on the membrane of activated and exhausted T cells that, upon ligation by its cognate ligand PDL1, downregulates immune responses bringing tyrosine phosphatases, mainly SHP-2, to activating immune synapses.
- Therapeutic cancer vaccines
-
Formulation of cancer-expressed antigens in an immunogenic fashion, given to patients to elicit specific immune responses against malignant cells; adjuvant components enhance immunogenicity by promoting focal inflammation at the site of administration.
- Cancer neoantigens
-
Exclusive protein sequence expressed by cancer cells as a result of a non-synonymous mutation that is presented by self-MHC antigen-presenting molecules and is able to elicit cancer-specific immune responses.
- T cell receptor
-
(TCR). A clonally distributed antigen-specific receptor expressed on the surface of T cells that specifically recognizes peptides presented by MHC antigen-presenting molecules and initiates intracellular signalling via the CD3 complex.
- Tumour mutational burden
-
(TMB). Number of mutations per megabase of exonic DNA in tumour cells as compared with non-transformed cells of the same individual; it is usually expressed as number of mutations per megabase and correlates with the probability of T cell recognizable neoantigens in a given tumour.
- Ectonucleotidases CD39 and CD73
-
Surface enzymes expressed by tumour and non-tumour cells in the tumour microenvironment that synthesize the immunosuppressive metabolite adenosine from ATP.
- Immune-mediated adverse events
-
(IMAEs). Series of adverse effects following treatment with checkpoint inhibitors that resemble organ-specific autoimmune conditions.
- Chimeric antigen receptors
-
(CARs). Fusion proteins composed of a single-chain antibody that recognizes a surface tumour protein linked to a transmembrane domain; these constructs are usually transfected to T cells with retroviral vectors to artificially confer the ability to recognize and destroy cancer cells.
- Immunogenic cell death
-
A form of cell death that provokes immunity against the cell’s antigenic components and that usually accompanies stressful or non-programmed cell death; it is mediated by alarmins that are recognized by innate receptors on immune cells, especially dendritic cells.
- Toll-like receptor
-
A member of the family of surface and endosomal proteins that have evolved to detect pathogen-associated molecular patterns that denote the presence of moieties exclusively present in prokaryotic cells.
Rights and permissions
About this article
Cite this article
Sangro, B., Sarobe, P., Hervás-Stubbs, S. et al. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 18, 525–543 (2021). https://doi.org/10.1038/s41575-021-00438-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41575-021-00438-0
This article is cited by
-
Radiogenomics predicts immune microenvironment heterogeneity and response to combination immunotherapy in hepatocellular carcinoma
Journal of Translational Medicine (2026)
-
Serum N-glycan NA3Fb identified as a prognostic biomarker of poor outcome in HBV-related hepatocellular carcinoma
Cancer Cell International (2026)
-
Functional characterization of angiogenesis genes in hepatocellular carcinoma via integrated bulk and single cell RNA sequencing
Scientific Reports (2026)
-
Evaluation of immuno-oncologic biomarkers and β-catenin expression in response of hepatocellular carcinomas to immunotherapy
npj Precision Oncology (2026)
-
Conversion therapy using transarterial chemoembolization plus tislelizumab for unresectable hepatocellular carcinoma: effects on tumor necrosis and anti-tumor immune response
Clinical and Translational Oncology (2026)