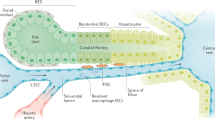
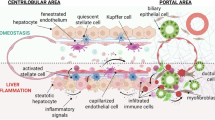
Abstract
Regulated cell death is a hallmark of inflammatory liver disease, and its intensity influences disease progression and severity. However, it is now clear that the form of cell death could also have an important role. In addition to apoptosis, various forms of regulated necrosis are increasingly reported to contribute to inflammatory liver disease due to their lytic nature. In this Review, we discuss the key regulatory molecules that govern regulated necrosis pathways and summarize our current understanding of the involvement of necroptosis, pyroptosis and ferroptosis in liver injury in preclinical murine models of acute and chronic liver disease and liver cancer development. Furthermore, we highlight the existing controversies and knowledge gaps regarding the relevance of these cell death modalities in hepatocytes and non-hepatocytic liver cells as well as the emerging mechanisms controlling these pathways. Finally, we discuss efforts to specifically modulate these regulated cell death pathways in liver disease and hepatocarcinogenesis in the attempt to prevent liver disease progression or to elicit more potent antitumour immune responses. Outstanding issues and methodological advances that will help to translate preclinical findings into therapeutic applications are also presented.
Key points
-
Regulated cell death (RCD) is a hallmark of liver disease with stage-dependent, protumorigenic and tumour-suppressive effects.
-
Different forms of regulated necrosis (necroptosis, pyroptosis and ferroptosis) have been associated with liver disease progression, but the ability of hepatocytes to execute them remains controversial.
-
Activation of RCD might not always entail full cell death execution, as conditions of sublethal activation are starting to emerge.
-
Ninjurin 1 (NINJ1) has been identified as a driver of plasma membrane rupture in the final step of multiple lytic RCD modalities.
-
RCD inhibition seems to be a promising treatment strategy in the early stages of liver disease.
-
RCD induction, particularly regulated necrosis, could be a promising therapeutic approach in liver cancer treatment by reshaping the tumour microenvironment and acting synergistically with immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Devarbhavi, H. et al. Global burden of liver disease: 2023 update. J. Hepatol. 79, 516–537 (2023).
Schwabe, R. F. & Luedde, T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat. Rev. Gastroenterol. Hepatol. 15, 738–752 (2018).
Mihm, S. Danger-associated molecular patterns (DAMPs): molecular triggers for sterile inflammation in the liver. Int J. Mol. Sci. 19, 3104 (2018).
Kondylis, V. & Pasparakis, M. RIP kinases in liver cell death, inflammation and cancer. Trends Mol. Med. 25, 47–63 (2019).
Loosen, S. H. et al. An elevated FIB-4 score is associated with an increased incidence of liver cancer: a longitudinal analysis among 248,224 outpatients in Germany. Eur. J. Cancer 168, 41–50 (2022).
Sterling, R. K. et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325 (2006).
Wu, X., Cao, J., Wan, X. & Du, S. Programmed cell death in hepatocellular carcinoma: mechanisms and therapeutic prospects. Cell Death Discov. 10, 356 (2024).
Galluzzi, L. et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018).
Shojaie, L., Iorga, A. & Dara, L. Cell death in liver diseases: a review. Int J. Mol. Sci. 21, 9682 (2020).
Vucur, M. et al. RIP3 inhibits inflammatory hepatocarcinogenesis but promotes cholestasis by controlling caspase-8- and JNK-dependent compensatory cell proliferation. Cell Rep. 4, 776–790 (2013).
Luedde, T. et al. Deletion of NEMO/IKKγ in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell 11, 119–132 (2007).
Kondylis, V. et al. NEMO prevents steatohepatitis and hepatocellular carcinoma by inhibiting RIPK1 kinase activity-mediated hepatocyte apoptosis. Cancer Cell 28, 582–598 (2015).
Krishna-Subramanian, S. et al. RIPK1 and death receptor signaling drive biliary damage and early liver tumorigenesis in mice with chronic hepatobiliary injury. Cell Death Differ. 26, 2710–2726 (2019).
Boege, Y. et al. A dual role of caspase-8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development. Cancer Cell 32, 342–359.e10 (2017).
Weber, A. et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology 51, 1226–1236 (2010).
Hikita, H. et al. Bak deficiency inhibits liver carcinogenesis: a causal link between apoptosis and carcinogenesis. J. Hepatol. 57, 92–100 (2012).
Liedtke, C. et al. Loss of caspase-8 protects mice against inflammation-related hepatocarcinogenesis but induces non-apoptotic liver injury. Gastroenterology 141, 2176–2187 (2011).
Guicciardi, M. E., Malhi, H., Mott, J. L. & Gores, G. J. Apoptosis and necrosis in the liver. Compr. Physiol. 3, 977–1010 (2013).
Luedde, T., Kaplowitz, N. & Schwabe, R. F. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology 147, 765–783.e4 (2014).
Green, D. R. Cell Death: Apoptosis and other Means to an End 2nd edn (Cold Spring Harbor Laboratory Press, 2018).
Kalkavan, H. & Green, D. R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 25, 46–55 (2018).
Bao, Q. & Shi, Y. Apoptosome: a platform for the activation of initiator caspases. Cell Death Differ. 14, 56–65 (2007).
Jost, P. J. et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460, 1035–1039 (2009).
Sachet, M., Liang, Y. Y. & Oehler, R. The immune response to secondary necrotic cells. Apoptosis 22, 1189–1204 (2017).
Giampazolias, E. et al. Mitochondrial permeabilization engages NF-κB-dependent anti-tumour activity under caspase deficiency. Nat. Cell Biol. 19, 1116–1129 (2017).
Marchi, S., Guilbaud, E., Tait, S. W. G., Yamazaki, T. & Galluzzi, L. Mitochondrial control of inflammation. Nat. Rev. Immunol. 23, 159–173 (2023).
Rongvaux, A. et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell 159, 1563–1577 (2014).
White, M. J. et al. Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production. Cell 159, 1549–1562 (2014).
Wang, Y. et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017).
Weinlich, R., Oberst, A., Beere, H. M. & Green, D. R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18, 127–136 (2017).
Lawlor, K. E., Murphy, J. M. & Vince, J. E. Gasdermin and MLKL necrotic cell death effectors: signaling and diseases. Immunity 57, 429–445 (2024).
Lin, J. et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 540, 124–128 (2016).
Newton, K. et al. RIPK1 inhibits ZBP1-driven necroptosis during development. Nature 540, 129–133 (2016).
Davies, K. A., Czabotar, P. E. & Murphy, J. M. Death at a funeral: activation of the dead enzyme, MLKL, to kill cells by necroptosis. Curr. Opin. Struct. Biol. 88, 102891 (2024).
Hildebrand, J. M. et al. Activation of the pseudokinase MLKL unleashes the four-helix bundle domain to induce membrane localization and necroptotic cell death. Proc. Natl Acad. Sci. USA 111, 15072–15077 (2014).
Broz, P., Pelegrin, P. & Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 20, 143–157 (2020).
Barnett, K. C., Li, S., Liang, K. & Ting, J. P. A 360° view of the inflammasome: mechanisms of activation, cell death, and disease. Cell 186, 2288–2312 (2023).
Van Opdenbosch, N. & Lamkanfi, M. Caspases in cell death, inflammation, and disease. Immunity 50, 1352–1364 (2019).
Liu, X., Xia, S., Zhang, Z., Wu, H. & Lieberman, J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 20, 384–405 (2021).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Soula, M. et al. Metabolic determinants of cancer cell sensitivity to canonical ferroptosis inducers. Nat. Chem. Biol. 16, 1351–1360 (2020).
Zhang, Y., Chen, X., Gueydan, C. & Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 28, 9–21 (2018).
Kayagaki, N. et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature 591, 131–136 (2021).
Dondelinger, Y. et al. NINJ1 is activated by cell swelling to regulate plasma membrane permeabilization during regulated necrosis. Cell Death Dis. 14, 755 (2023).
Ramos, S., Hartenian, E., Santos, J. C., Walch, P. & Broz, P. NINJ1 induces plasma membrane rupture and release of damage-associated molecular pattern molecules during ferroptosis. EMBO J. 43, 1164–1186 (2024).
Deutsch, M. et al. Divergent effects of RIP1 or RIP3 blockade in murine models of acute liver injury. Cell Death Dis. 6, e1759 (2015).
Gunther, C. et al. The pseudokinase MLKL mediates programmed hepatocellular necrosis independently of RIPK3 during hepatitis. J. Clin. Invest. 126, 4346–4360 (2016).
Gautheron, J. et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Mol. Med. 6, 1062–1074 (2014).
Roychowdhury, S., McMullen, M. R., Pisano, S. G., Liu, X. & Nagy, L. E. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology 57, 1773–1783 (2013).
Roychowdhury, S. et al. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology 64, 1518–1533 (2016).
Afonso, M. B. et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clin. Sci. 129, 721–739 (2015).
Afonso, M. B. et al. Activation of necroptosis in human and experimental cholestasis. Cell Death Dis. 7, e2390 (2016).
Afonso, M. B. et al. Intricate interplay between cell metabolism and necroptosis regulation in metabolic dysfunction-associated steatotic liver disease: a narrative review. Metabolism 158, 155975 (2024).
Jaeschke, H. & Ramachandran, A. Acetaminophen hepatotoxicity: paradigm for understanding mechanisms of drug-induced liver injury. Annu. Rev. Pathol. 19, 453–478 (2024).
Li, J. X. et al. The B-Raf(V600E) inhibitor dabrafenib selectively inhibits RIP3 and alleviates acetaminophen-induced liver injury. Cell Death Dis. 5, e1278 (2014).
Wu, X., Nagy, L. E. & Gautheron, J. Mediators of necroptosis: from cell death to metabolic regulation. EMBO Mol. Med. 16, 219–237 (2024).
Dara, L. No necroptosis in hepatocytes: the final nail in the coffin? Gastroenterology 163, 1492–1495 (2022).
Schneider, A. T., Gautheron, J., Tacke, F., Vucur, M. & Luedde, T. Receptor interacting protein kinase 1 (RIPK1) in hepatocytes does not mediate murine acetaminophen toxicity. Hepatology 64, 306–308 (2016).
Ericsson, A. C. & Franklin, C. L. The gut microbiome of laboratory mice: considerations and best practices for translational research. Mamm. Genome 32, 239–250 (2021).
Fontaine, D. A. & Davis, D. B. Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse consortium. Diabetes 65, 25–33 (2016).
Gallage, S. et al. Spontaneous cholemia in C57BL/6 mice predisposes to liver cancer in NASH. Cell Mol. Gastroenterol. Hepatol. 13, 875–878 (2022).
Dara, L. et al. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology 62, 1847–1857 (2015).
Sun, X. et al. RIP3, a novel apoptosis-inducing kinase. J. Biol. Chem. 274, 16871–16875 (1999).
Samson, A. L. et al. A toolbox for imaging RIPK1, RIPK3, and MLKL in mouse and human cells. Cell Death Differ. 28, 2126–2144 (2021).
Preston, S. P. et al. Epigenetic silencing of RIPK3 in hepatocytes prevents MLKL-mediated necroptosis from contributing to liver pathologies. Gastroenterology 163, 1643–1657.e14 (2022).
Hoff, J. et al. RIPK3 promoter hypermethylation in hepatocytes protects from bile acid-induced inflammation and necroptosis. Cell Death Dis. 14, 275 (2023).
Koo, G. B. et al. Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics. Cell Res. 25, 707–725 (2015).
Yang, Z. et al. 2-HG inhibits necroptosis by stimulating DNMT1-dependent hypermethylation of the RIP3 promoter. Cell Rep. 19, 1846–1857 (2017).
Yang, C. et al. Regulation of RIP3 by the transcription factor Sp1 and the epigenetic regulator UHRF1 modulates cancer cell necroptosis. Cell Death Dis. 8, e3084 (2017).
Despoix, N. et al. Mouse CD146/MCAM is a marker of natural killer cell maturation. Eur. J. Immunol. 38, 2855–2864 (2008).
Jing, L. et al. A subpopulation of CD146+ macrophages enhances antitumor immunity by activating the NLRP3 inflammasome. Cell Mol. Immunol. 20, 908–923 (2023).
Boulter, L. et al. Macrophage-derived wnt opposes notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 18, 572–579 (2012).
Michalopoulos, G. K. & Bhushan, B. Liver regeneration: biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 18, 40–55 (2021).
Shi, S. et al. Recapitulating cholangiopathy-associated necroptotic cell death in vitro using human cholangiocyte organoids. Cell Mol. Gastroenterol. Hepatol. 13, 541–564 (2022).
Inaba, Y. et al. The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice. Nat. Commun. 14, 167 (2023).
Gallage, S. et al. A researcher’s guide to preclinical mouse NASH models. Nat. Metab. 4, 1632–1649 (2022).
Vucur, M. et al. Sublethal necroptosis signaling promotes inflammation and liver cancer. Immunity 56, 1578–1595.e8 (2023).
Geserick, P. et al. Absence of RIPK3 predicts necroptosis resistance in malignant melanoma. Cell Death Dis. 6, e1884 (2015).
Dannappel, M. et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature 513, 90–94 (2014).
Wang, W. et al. Sensing plasma membrane pore formation induces chemokine production in survivors of regulated necrosis. Dev. Cell 57, 228–245.e6 (2022).
Ting, A. T. & Bertrand, M. J. M. More to life than NF-κB in TNFR1 signaling. Trends Immunol. 37, 535–545 (2016).
Wei, S. et al. RIP3 deficiency alleviates liver fibrosis by inhibiting ROCK1-TLR4-NF-κB pathway in macrophages. FASEB J. 33, 11180–11193 (2019).
Tao, L. et al. RIP1 kinase activity promotes steatohepatitis through mediating cell death and inflammation in macrophages. Cell Death Differ. 28, 1418–1433 (2021).
Wu, X. et al. Macrophage-derived MLKL in alcohol-associated liver disease: regulation of phagocytosis. Hepatology 77, 902–919 (2023).
Yan, M. et al. Targeting endothelial necroptosis disrupts profibrotic endothelial-hepatic stellate cells crosstalk to alleviate liver fibrosis in nonalcoholic steatohepatitis. Int. J. Mol. Sci. 24, 11313 (2023).
Zelic, M. et al. RIP kinase 1-dependent endothelial necroptosis underlies systemic inflammatory response syndrome. J. Clin. Invest. 128, 2064–2075 (2018).
Strilic, B. et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 536, 215–218 (2016).
Banales, J. M. et al. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 16, 269–281 (2019).
Harrison, S. A. et al. A randomized, placebo-controlled trial of emricasan in patients with NASH and F1-F3 fibrosis. J. Hepatol. 72, 816–827 (2020).
Schuster, S., Cabrera, D., Arrese, M. & Feldstein, A. E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 15, 349–364 (2018).
Gaul, S. et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol. 74, 156–167 (2021).
Wree, A. et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. 92, 1069–1082 (2014).
Khanova, E. et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology 67, 1737–1753 (2018).
Xu, B. et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J. Hepatol. 68, 773–782 (2018).
Knorr, J., Wree, A. & Feldstein, A. E. Pyroptosis in steatohepatitis and liver diseases. J. Mol. Biol. 434, 167271 (2022).
Tilg, H., Adolph, T. E. & Trauner, M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. 34, 1700–1718 (2022).
Ioannou, G. N., Haigh, W. G., Thorning, D. & Savard, C. Hepatic cholesterol crystals and crown-like structures distinguish NASH from simple steatosis. J. Lipid Res. 54, 1326–1334 (2013).
Ioannou, G. N. et al. Cholesterol-lowering drugs cause dissolution of cholesterol crystals and disperse Kupffer cell crown-like structures during resolution of NASH. J. Lipid Res. 56, 277–285 (2015).
Pan, J. et al. Fatty acid activates NLRP3 inflammasomes in mouse Kupffer cells through mitochondrial DNA release. Cell Immunol. 332, 111–120 (2018).
Iracheta-Vellve, A. et al. Inhibition of sterile danger signals, uric acid and ATP, prevents inflammasome activation and protects from alcoholic steatohepatitis in mice. J. Hepatol. 63, 1147–1155 (2015).
Petrasek, J. et al. Metabolic danger signals, uric acid and ATP, mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J. Leukoc. Biol. 98, 249–256 (2015).
Sun, P. et al. Hepatocytes are resistant to cell death from canonical and non-canonical inflammasome-activated pyroptosis. Cell Mol. Gastroenterol. Hepatol. 13, 739–757 (2022).
Cai, S. Y. et al. Inflammasome is activated in the liver of cholestatic patients and aggravates hepatic injury in bile duct-ligated mouse. Cell Mol. Gastroenterol. Hepatol. 9, 679–688 (2020).
Guo, C. et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity 45, 944 (2016).
Frissen, M. et al. Bidirectional role of NLRP3 during acute and chronic cholestatic liver injury. Hepatology 73, 1836–1854 (2021).
Qu, J., Yuan, Z., Wang, G., Wang, X. & Li, K. The selective NLRP3 inflammasome inhibitor MCC950 alleviates cholestatic liver injury and fibrosis in mice. Int. Immunopharmacol. 70, 147–155 (2019).
Xu, W. F. et al. Gasdermin E-derived caspase-3 inhibitors effectively protect mice from acute hepatic failure. Acta Pharmacol. Sin. 42, 68–76 (2021).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Trauner, M., Fickert, P. & Wagner, M. MDR3 (ABCB4) defects: a paradigm for the genetics of adult cholestatic syndromes. Semin. Liver Dis. 27, 77–98 (2007).
Liao, L. et al. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut 68, 1477–1492 (2019).
Kaufmann, B. et al. Cell-specific deletion of NLRP3 inflammasome identifies myeloid cells as key drivers of liver inflammation and fibrosis in murine steatohepatitis. Cell Mol. Gastroenterol. Hepatol. 14, 751–767 (2022).
Kaufmann, B. et al. NLRP3 activation in neutrophils induces lethal autoinflammation, liver inflammation, and fibrosis. EMBO Rep. 23, e54446 (2022).
Wree, A. et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59, 898–910 (2014).
Vonderlin, J., Chavakis, T., Sieweke, M. & Tacke, F. The multifaceted roles of macrophages in NAFLD pathogenesis. Cell Mol. Gastroenterol. Hepatol. 15, 1311–1324 (2023).
Horn, C. L., Morales, A. L., Savard, C., Farrell, G. C. & Ioannou, G. N. Role of cholesterol-associated steatohepatitis in the development of NASH. Hepatol. Commun. 6, 12–35 (2022).
Duewell, P. et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 (2010).
Tall, A. R. & Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 (2015).
Hacker, G. & Haimovici, A. Sub-lethal signals in the mitochondrial apoptosis apparatus: pernicious by-product or physiological event? Cell Death Differ. 30, 250–257 (2023).
Riley, J. S. & Tait, S. W. Mitochondria and pathogen immunity: from killer to firestarter. EMBO J. 38, e102325 (2019).
Mridha, A. R. et al. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J. Hepatol. 66, 1037–1046 (2017).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489 (2012).
Vergis, N. et al. IL-1 signal inhibition in alcohol-related hepatitis: a randomized, double-blind, placebo-controlled trial of canakinumab. Clin. Gastroenterol. Hepatol. 23, 797–807.e5 (2025).
Gawrieh, S. et al. Randomized trial of anakinra plus zinc vs. prednisone for severe alcohol-associated hepatitis. J. Hepatol. 80, 684–693 (2024).
Li, H. et al. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur. J. Pharmacol. 928, 175091 (2022).
Nadella, V. & Kanneganti, T. D. Inflammasomes and their role in PANoptosomes. Curr. Opin. Immunol. 91, 102489 (2024).
Yang, C. et al. Gasdermin D protects against noninfectious liver injury by regulating apoptosis and necroptosis. Cell Death Dis. 10, 481 (2019).
Carlson, B. A. et al. Glutathione peroxidase 4 and vitamin E cooperatively prevent hepatocellular degeneration. Redox Biol. 9, 22–31 (2016).
Van Coillie, S. et al. Targeting ferroptosis protects against experimental (multi)organ dysfunction and death. Nat. Commun. 13, 1046 (2022).
MacDonald, G. A. et al. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominately in acinar zone 3. J. Gastroenterol. Hepatol. 16, 599–606 (2001).
Seki, S. et al. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J. Hepatol. 37, 56–62 (2002).
Chalasani, N. et al. Relationship of steatosis grade and zonal location to histological features of steatohepatitis in adult patients with non-alcoholic fatty liver disease. J. Hepatol. 48, 829–834 (2008).
Nikopoulou, C. et al. Spatial and single-cell profiling of the metabolome, transcriptome and epigenome of the aging mouse liver. Nat. Aging 3, 1430–1445 (2023).
Straub, B. K., Stoeffel, P., Heid, H., Zimbelmann, R. & Schirmacher, P. Differential pattern of lipid droplet-associated proteins and de novo perilipin expression in hepatocyte steatogenesis. Hepatology 47, 1936–1946 (2008).
Yeh, M. M. & Brunt, E. M. Pathological features of fatty liver disease. Gastroenterology 147, 754–764 (2014).
Wang, H. et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology 66, 449–465 (2017).
Mishima, E. et al. A non-canonical vitamin K cycle is a potent ferroptosis suppressor. Nature 608, 778–783 (2022).
Carlson, B. A. et al. Thioredoxin reductase 1 protects against chemically induced hepatocarcinogenesis via control of cellular redox homeostasis. Carcinogenesis 33, 1806–1813 (2012).
Mishima, E. et al. Recommendations for robust and reproducible research on ferroptosis. Nat. Rev. Mol. Cell Biol. https://doi.org/10.1038/s41580-025-00843-2 (2025).
Lorincz, T., Jemnitz, K., Kardon, T., Mandl, J. & Szarka, A. Ferroptosis is involved in acetaminophen induced cell death. Pathol. Oncol. Res. 21, 1115–1121 (2015).
Yamada, N. et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 11, 144 (2020).
Adelusi, O. B., Etemadi, Y., Akakpo, J. Y., Ramachandran, A. & Jaeschke, H. Effect of ferroptosis inhibitors in a murine model of acetaminophen-induced liver injury. J. Biochem. Mol. Toxicol. 38, e23791 (2024).
Adelusi, O. B., Ramachandran, A., Lemasters, J. J. & Jaeschke, H. The role of iron in lipid peroxidation and protein nitration during acetaminophen-induced liver injury in mice. Toxicol. Appl. Pharmacol. 445, 116043 (2022).
Peleman, C., Francque, S. & Berghe, T. V. Emerging role of ferroptosis in metabolic dysfunction-associated steatotic liver disease: revisiting hepatic lipid peroxidation. EBioMedicine 102, 105088 (2024).
Spickett, C. M. & Pitt, A. R. Oxidative lipidomics coming of age: advances in analysis of oxidized phospholipids in physiology and pathology. Antioxid. Redox Signal. 22, 1646–1666 (2015).
Ni, Z. et al. Guiding the choice of informatics software and tools for lipidomics research applications. Nat. Methods 20, 193–204 (2023).
Yamada, N. et al. Iron overload as a risk factor for hepatic ischemia-reperfusion injury in liver transplantation: potential role of ferroptosis. Am. J. Transpl. 20, 1606–1618 (2020).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Wu, S. et al. Macrophage extracellular traps aggravate iron overload-related liver ischaemia/reperfusion injury. Br. J. Pharmacol. 178, 3783–3796 (2021).
Bebber, C. M., Muller, F., Prieto Clemente, L., Weber, J. & von Karstedt, S. Ferroptosis in cancer cell biology. Cancers 12, 164 (2020).
Huang, S. et al. Hepatic TGFβr1 deficiency attenuates lipopolysaccharide/D-galactosamine-induced acute liver failure through inhibiting GSK3β-Nrf2-mediated hepatocyte apoptosis and ferroptosis. Cell Mol. Gastroenterol. Hepatol. 13, 1649–1672 (2022).
Wu, J. et al. Nrf2-mediated ferroptosis inhibition exerts a protective effect on acute-on-chronic liver failure. Oxid. Med. Cell Longev. 2022, 4505513 (2022).
Zhao, C., Xiao, C., Feng, S. & Bai, J. Artemisitene alters LPS-induced oxidative stress, inflammation and ferroptosis in liver through Nrf2/HO-1 and NF-kB pathway. Front. Pharmacol. 14, 1177542 (2023).
Ran, Q. et al. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J. Biol. Chem. 279, 55137–55146 (2004).
Kang, R. et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe 24, 97–108.e4 (2018).
Ali, N., Ferrao, K. & Mehta, K. J. Liver iron loading in alcohol-associated liver disease. Am. J. Pathol. 193, 1427–1439 (2023).
Buzzetti, E. et al. Evaluating the association of serum ferritin and hepatic iron with disease severity in non-alcoholic fatty liver disease. Liver Int. 39, 1325–1334 (2019).
Nelson, J. E. et al. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology 53, 448–457 (2011).
Li, X. et al. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 40, 1378–1394 (2020).
Liu, C. Y. et al. Ferroptosis is involved in alcohol-induced cell death in vivo and in vitro. Biosci. Biotechnol. Biochem. 84, 1621–1628 (2020).
Luo, J. et al. Ferroptosis contributes to ethanol-induced hepatic cell death via labile iron accumulation and GPx4 inactivation. Cell Death Discov. 9, 311 (2023).
Qi, J., Kim, J. W., Zhou, Z., Lim, C. W. & Kim, B. Ferroptosis affects the progression of nonalcoholic steatohepatitis via the modulation of lipid peroxidation-mediated cell death in mice. Am. J. Pathol. 190, 68–81 (2020).
Tong, J. et al. Ferroptosis inhibitor liproxstatin-1 alleviates metabolic dysfunction-associated fatty liver disease in mice: potential involvement of PANoptosis. Acta Pharmacol. Sin. 44, 1014–1028 (2023).
Tsurusaki, S. et al. Hepatic ferroptosis plays an important role as the trigger for initiating inflammation in nonalcoholic steatohepatitis. Cell Death Dis. 10, 449 (2019).
Dixon, S. J. et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 10, 1604–1609 (2015).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Duan, J. et al. Therapeutic targeting of hepatic ACSL4 ameliorates NASH in mice. Hepatology 75, 140–153 (2022).
Askari, B. et al. Rosiglitazone inhibits acyl-CoA synthetase activity and fatty acid partitioning to diacylglycerol and triacylglycerol via a peroxisome proliferator-activated receptor-γ-independent mechanism in human arterial smooth muscle cells and macrophages. Diabetes 56, 1143–1152 (2007).
Kim, J. H., Lewin, T. M. & Coleman, R. A. Expression and characterization of recombinant rat Acyl-CoA synthetases 1, 4, and 5. Selective inhibition by triacsin C and thiazolidinediones. J. Biol. Chem. 276, 24667–24673 (2001).
Singh, A. B., Kan, C. F. K., Kraemer, F. B., Sobel, R. A. & Liu, J. Liver-specific knockdown of long-chain acyl-CoA synthetase 4 reveals its key role in VLDL-TG metabolism and phospholipid synthesis in mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 316, E880–E894 (2019).
Angendohr, C. et al. The ferroptosis mediator ACSL4 fails to prevent disease progression in mouse models of MASLD. Hepatol. Commun. 9, e0684 (2025).
Magtanong, L. et al. Context-dependent regulation of ferroptosis sensitivity. Cell Chem. Biol. 29, 1409–1418.e6 (2022).
Tang, D., Chen, X., Kang, R. & Kroemer, G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 31, 107–125 (2021).
Kayagaki, N. et al. Inhibiting membrane rupture with NINJ1 antibodies limits tissue injury. Nature 618, 1072–1077 (2023).
Kim, M. W. et al. Deficiency of ninjurin1 attenuates LPS/D-galactosamine-induced acute liver failure by reducing TNF-α-induced apoptosis in hepatocytes. J. Cell Mol. Med. 26, 5122–5134 (2022).
Park, S. Y. et al. Loss of ninjurin1 alleviates acetaminophen-induced liver injury via enhancing AMPKα-NRF2 pathway. Life Sci. 350, 122782 (2024).
Hu, Y. et al. The Ninj1/Dusp1 axis contributes to liver ischemia reperfusion injury by regulating macrophage activation and neutrophil infiltration. Cell Mol. Gastroenterol. Hepatol. 15, 1071–1084 (2023).
Llovet, J. M. et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 7, 6 (2021).
Yahoo, N., Dudek, M., Knolle, P. & Heikenwalder, M. Role of immune responses in the development of NAFLD-associated liver cancer and prospects for therapeutic modulation. J. Hepatol. 79, 538–551 (2023).
Finn, R. S. et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 382, 1894–1905 (2020).
Abou-Alfa, G. K. et al. Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 1, EVIDoa2100070 (2022).
Mohammed, S. et al. Absence of either Ripk3 or mlkl reduces incidence of hepatocellular carcinoma independent of liver fibrosis. Mol. Cancer Res. 21, 933–946 (2023).
Afonso, M. B. et al. RIPK3 acts as a lipid metabolism regulator contributing to inflammation and carcinogenesis in non-alcoholic fatty liver disease. Gut 70, 2359–2372 (2021).
Seifert, L. et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and Mincle-induced immune suppression. Nature 532, 245–249 (2016).
Schneider, A. T. et al. A decision point between transdifferentiation and programmed cell death priming controls KRAS-dependent pancreatic cancer development. Nat. Commun. 16, 1765 (2025).
Hockendorf, U. et al. RIPK3 restricts myeloid leukemogenesis by promoting cell death and differentiation of leukemia initiating cells. Cancer Cell 30, 75–91 (2016).
Jiang, X. et al. A RIPK3-independent role of MLKL in suppressing parthanatos promotes immune evasion in hepatocellular carcinoma. Cell Discov. 9, 7 (2023).
Seehawer, M. et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature 562, 69–75 (2018).
Yamagishi, R. et al. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci. Immunol. 7, eabl7209 (2022).
Lv, T. et al. Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression. J. Immunother. Cancer 10, e004763 (2022).
Humphries, F. et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020).
Bresciani, G. et al. Novel potential pharmacological applications of dimethyl fumarate — an overview and update. Front. Pharmacol. 14, 1264842 (2023).
Hassannia, B., Vandenabeele, P. & Vanden Berghe, T. Targeting ferroptosis to iron out cancer. Cancer Cell 35, 830–849 (2019).
Hino, K., Yanatori, I., Hara, Y. & Nishina, S. Iron and liver cancer: an inseparable connection. FEBS J. 289, 7810–7829 (2022).
Sun, X. et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology 64, 488–500 (2016).
Tang, H. H. et al. Targeting GPX4 to overcome sorafenib resistance of human hepatocellular carcinoma by inducing ferroptosis. J. Cell Physiol. 240, e70078 (2025).
Delgado, M. E. et al. CPEB4 modulates liver cancer progression by translationally regulating hepcidin expression and sensitivity to ferroptosis. JHEP Rep. 7, 101296 (2025).
Conche, C. et al. Combining ferroptosis induction with MDSC blockade renders primary tumours and metastases in liver sensitive to immune checkpoint blockade. Gut 72, 1774–1782 (2023).
Cheu, J. W. et al. Ferroptosis suppressor protein 1 inhibition promotes tumor ferroptosis and anti-tumor immune responses in liver cancer. Cell Mol. Gastroenterol. Hepatol. 16, 133–159 (2023).
Hilmi, M., Vienot, A., Rousseau, B. & Neuzillet, C. Immune therapy for liver cancers. Cancers 12, 77 (2019).
Zhang, J., Huang, D., Saw, P. E. & Song, E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 43, 523–545 (2022).
Rosenbaum, S. R., Wilski, N. A. & Aplin, A. E. Fueling the fire: inflammatory forms of cell death and implications for cancer immunotherapy. Cancer Discov. 11, 266–281 (2021).
Gullett, J. M., Tweedell, R. E. & Kanneganti, T. D. It’s all in the PAN: crosstalk, plasticity, redundancies, switches, and interconnectedness encompassed by PANoptosis underlying the totality of cell death-associated biological effects. Cells 11, 1495 (2022).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489–495 (2000).
Kang, S. et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 6, 7515 (2015).
Conos, S. A. et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc. Natl Acad. Sci. USA 114, E961–E969 (2017).
Gutierrez, K. D. et al. MLKL activation triggers NLRP3-mediated processing and release of IL-1β independently of gasdermin-D. J. Immunol. 198, 2156–2164 (2017).
Rogers, C. et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun. 10, 1689 (2019).
Demarco, B. et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv. 6, eabc3465 (2020).
Wang, Y. et al. Cutting edge: caspase-8 is a linchpin in caspase-3 and gasdermin D activation to control cell death, cytokine release, and host defense during influenza A virus infection. J. Immunol. 207, 2411–2416 (2021).
Hotchkiss, R. S., Strasser, A., McDunn, J. E. & Swanson, P. E. Cell death. N. Engl. J. Med. 361, 1570–1583 (2009).
Sepand, M. R. et al. Targeting non-apoptotic cell death in cancer treatment by nanomaterials: recent advances and future outlook. Nanomedicine 29, 102243 (2020).
Bakrania, A., Zheng, G. & Bhat, M. Nanomedicine in hepatocellular carcinoma: a new frontier in targeted cancer treatment. Pharmaceutics 14, 41 (2021).
Bottger, R. et al. Lipid-based nanoparticle technologies for liver targeting. Adv. Drug Deliv. Rev. 154-155, 79–101 (2020).
Wu, J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J. Pers. Med 11, 771 (2021).
Mintz, K. J. & Leblanc, R. M. The use of nanotechnology to combat liver cancer: progress and perspectives. Biochim. Biophys. Acta Rev. Cancer 1876, 188621 (2021).
Li, L., Wang, X., Xu, H., Liu, X. & Xu, K. Perspectives and mechanisms for targeting ferroptosis in the treatment of hepatocellular carcinoma. Front. Mol. Biosci. 9, 947208 (2022).
Liu, J., Chen, T., Liu, X., Li, Z. & Zhang, Y. Engineering materials for pyroptosis induction in cancer treatment. Bioact. Mater. 33, 30–45 (2024).
Zhong, J., Zhao, R., Wang, Y., Su, Y. X. & Lan, X. Nano-PROTACs: state of the art and perspectives. Nanoscale 16, 4378–4391 (2024).
Zhao, L., Zhao, J., Zhong, K., Tong, A. & Jia, D. Targeted protein degradation: mechanisms, strategies and application. Signal. Transduct. Target. Ther. 7, 113 (2022).
Niu, F. et al. A GPX4 non-enzymatic domain and MDM2 targeting peptide PROTAC for acute lymphoid leukemia therapy through ferroptosis induction. Biochem. Biophys. Res. Commun. 684, 149125 (2023).
Cai, M. et al. Design and synthesis of proteolysis-targeting chimeras (PROTACs) as degraders of glutathione peroxidase 4. Bioorg. Med. Chem. 90, 117352 (2023).
Huang, D. et al. A PROTAC augmenter for photo-driven pyroptosis in breast cancer. Adv. Mater. 36, e2313460 (2024).
Dai, X. J. et al. Degraders in epigenetic therapy: PROTACs and beyond. Theranostics 14, 1464–1499 (2024).
Springer, A. D. & Dowdy, S. F. GalNAc-siRNA conjugates: leading the way for delivery of RNAi therapeutics. Nucleic Acid. Ther. 28, 109–118 (2018).
Tanowitz, M. et al. Asialoglycoprotein receptor 1 mediates productive uptake of N-acetylgalactosamine-conjugated and unconjugated phosphorothioate antisense oligonucleotides into liver hepatocytes. Nucleic Acids Res. 45, 12388–12400 (2017).
Gao, Y. et al. Long noncoding RNA URB1-antisense RNA 1 (AS1) suppresses sorafenib-induced ferroptosis in hepatocellular carcinoma by driving ferritin phase separation. ACS Nano 17, 22240–22258 (2023).
Shojaie, L. et al. Innate and adaptive immune cell interaction drives inflammasome activation and hepatocyte apoptosis in murine liver injury from immune checkpoint inhibitors. Cell Death Dis. 15, 140 (2024).
Liu, J. et al. Cancer selective target degradation by folate-caged PROTACs. J. Am. Chem. Soc. 143, 7380–7387 (2021).
Meumann, N. et al. Adeno-associated virus serotype 2 capsid variants for improved liver-directed gene therapy. Hepatology 77, 802–815 (2023).
Meumann, N. et al. Hepatocellular carcinoma is a natural target for adeno-associated virus (AAV) 2 vectors. Cancers 14, 427 (2022).
Zeng, F. et al. Ferroptosis detection: from approaches to applications. Angew. Chem. Int. Ed. Engl. 62, e202300379 (2023).
Feng, Y. & Huang, X. Methodology for comprehensive detection of pyroptosis. Methods Mol. Biol. 2255, 149–157 (2021).
Andrews, T. S. et al. Single-cell, single-nucleus, and spatial transcriptomics characterization of the immunological landscape in the healthy and PSC human liver. J. Hepatol. 80, 730–743 (2024).
Bravo Gonzalez-Blas, C. et al. Single-cell spatial multi-omics and deep learning dissect enhancer-driven gene regulatory networks in liver zonation. Nat. Cell Biol. 26, 153–167 (2024).
Guillot, A. et al. Mapping the hepatic immune landscape identifies monocytic macrophages as key drivers of steatohepatitis and cholangiopathy progression. Hepatology 78, 150–166 (2023).
Dowbaj, A. M. et al. Mouse liver assembloids model periportal architecture and biliary fibrosis. Nature https://doi.org/10.1038/s41586-025-09183-9 (2025).
Kim, H. Y. et al. Protocol to generate human liver spheroids to study liver fibrosis induced by metabolic stress. Star. Protoc. 5, 103111 (2024).
Brugger, M. et al. High precision-cut liver slice model to study cell-autonomous antiviral defense of hepatocytes within their microenvironment. JHEP Rep. 4, 100465 (2022).
Dewyse, L. et al. Improved precision-cut liver slice cultures for testing drug-induced liver fibrosis. Front. Med. 9, 862185 (2022).
Rastovic, U. et al. Human precision-cut liver slices: a potential platform to study alcohol-related liver disease. Int J. Mol. Sci. 25, 150 (2023).
Wang, Y. et al. Precision-cut liver slices as an ex vivo model to evaluate antifibrotic therapies for liver fibrosis and cirrhosis. Hepatol. Commun. 8, e0558 (2024).
Godoy, P. et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 87, 1315–1530 (2013).
Kaur, I. et al. Primary hepatocyte isolation and cultures: technical aspects, challenges and advancements. Bioengineering 10, 131 (2023).
Atkinson, S. R. et al. In severe alcoholic hepatitis, serum keratin-18 fragments are diagnostic, prognostic, and theragnostic biomarkers. Am. J. Gastroenterol. 115, 1857–1868 (2020).
Vatsalya, V. et al. Keratin 18 is a diagnostic and prognostic factor for acute alcoholic hepatitis. Clin. Gastroenterol. Hepatol. 18, 2046–2054 (2020).
Cusi, K. et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J. Hepatol. 60, 167–174 (2014).
Feldstein, A. E. et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 50, 1072–1078 (2009).
Lee, J. et al. Accuracy of cytokeratin 18 (M30 and M65) in detecting non-alcoholic steatohepatitis and fibrosis: a systematic review and meta-analysis. PLoS One 15, e0238717 (2020).
Lauwerys, L. et al. Development of caspase-3-selective activity-based probes for PET imaging of apoptosis. EJNMMI Radiopharm. Chem. 9, 58 (2024).
Lan, Y. et al. Visualization of receptor-interacting protein kinase 1 (RIPK1) by brain imaging with positron emission tomography. J. Med. Chem. 64, 15420–15428 (2021).
Greten, T. F. et al. Biomarkers for immunotherapy of hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 20, 780–798 (2023).
Guicciardi, M. E. & Gores, G. J. Burning, but not dying: the failure of pyroptotic cell death in hepatocytes. Cell Mol. Gastroenterol. Hepatol. 13, 974–976 (2022).
Frenette, C. T. et al. Emricasan improves liver function in patients with cirrhosis and high model for end-stage liver disease scores compared with placebo. Clin. Gastroenterol. Hepatol. 17, 774–783.e4 (2019).
Lee, F. A. et al. Randomized phase II study of the X-linked inhibitor of apoptosis (XIAP) antisense AEG35156 in combination with sorafenib in patients with advanced hepatocellular carcinoma (HCC). Am. J. Clin. Oncol. 39, 609–613 (2016).
Ramachandran, A. et al. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology 58, 2099–2108 (2013).
Kang, Y. J. et al. Regulation of NKT cell-mediated immune responses to tumours and liver inflammation by mitochondrial PGAM5-Drp1 signalling. Nat. Commun. 6, 8371 (2015).
Weinlich, R. et al. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 5, 340–348 (2013).
Liu, M. et al. RIP3 blockade prevents immune-mediated hepatitis through a myeloid-derived suppressor cell dependent mechanism. Int. J. Biol. Sci. 18, 199–213 (2022).
Saeed, W. K. et al. Mismatched effects of receptor interacting protein kinase-3 on hepatic steatosis and inflammation in non-alcoholic fatty liver disease. World J. Gastroenterol. 24, 5477–5490 (2018).
Chenxu, G. et al. Loss of RIP3 initiates annihilation of high-fat diet initialized nonalcoholic hepatosteatosis: a mechanism involving toll-like receptor 4 and oxidative stress. Free Radic. Biol. Med. 134, 23–41 (2019).
Saeed, W. K. et al. Decrease in fat de novo synthesis and chemokine ligand expression in non-alcoholic fatty liver disease caused by inhibition of mixed lineage kinase domain-like pseudokinase. J. Gastroenterol. Hepatol. 34, 2206–2218 (2019).
Xu, H. et al. The pseudokinase MLKL regulates hepatic insulin sensitivity independently of inflammation. Mol. Metab. 23, 14–23 (2019).
Wu, X., Arya, R. K., Huang, E., McMullen, M. R. & Nagy, L. E. Receptor-interacting protein 1 and 3 kinase activity are required for high-fat diet induced liver injury in mice. Front. Endocrinol. 14, 1267996 (2023).
Wu, X. et al. MLKL-dependent signaling regulates autophagic flux in a murine model of non-alcohol-associated fatty liver and steatohepatitis. J. Hepatol. 73, 616–627 (2020).
Ohene-Marfo, P. et al. Non-necroptotic roles of MLKL in diet-induced obesity, liver pathology, and insulin sensitivity: insights from a high-fat, high-fructose, high-cholesterol diet mouse model. Int J. Mol. Sci. 25, 2813 (2024).
Gautheron, J. et al. The necroptosis-inducing kinase RIPK3 dampens adipose tissue inflammation and glucose intolerance. Nat. Commun. 7, 11869 (2016).
Wang, S. et al. Increased hepatic receptor interacting protein kinase 3 expression due to impaired proteasomal functions contributes to alcohol-induced steatosis and liver injury. Oncotarget 7, 17681–17698 (2016).
Miyata, T. et al. Differential role of MLKL in alcohol-associated and non-alcohol-associated fatty liver diseases in mice and humans. JCI Insight 6, e140180 (2021).
Guo, R. et al. Loss of MLKL ameliorates liver fibrosis by inhibiting hepatocyte necroptosis and hepatic stellate cell activation. Theranostics 12, 5220–5236 (2022).
Oh, J. H. et al. Hepatic stellate cells activate and avoid death under necroptosis stimuli: hepatic fibrosis during necroptosis. J. Gastroenterol. Hepatol. 38, 2206–2214 (2023).
Ortega-Ribera, M. et al. A novel multi-organ male model of alcohol-induced acute-on-chronic liver failure reveals NET-mediated hepatocellular death, which is prevented by RIPK3 inhibition. Cell Mol. Gastroenterol. Hepatol. 19, 101446 (2025).
Dara, L. The receptor interacting protein kinases in the liver. Semin. Liver Dis. 38, 73–86 (2018).
Li, Z. et al. Inhibition of TWEAK/Tnfrsf12a axis protects against acute liver failure by suppressing RIPK1-dependent apoptosis. Cell Death Discov. 8, 328 (2022).
Imaeda, A. B. et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 119, 305–314 (2009).
Williams, C. D. et al. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol. Appl. Pharmacol. 252, 289–297 (2011).
Chen, C. J. et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13, 851–856 (2007).
Williams, C. D., Farhood, A. & Jaeschke, H. Role of caspase-1 and interleukin-1β in acetaminophen-induced hepatic inflammation and liver injury. Toxicol. Appl. Pharmacol. 247, 169–178 (2010).
Luan, J. et al. NOD-Like receptor protein 3 inflammasome-dependent IL-1β accelerated ConA-induced hepatitis. Front. Immunol. 9, 758 (2018).
Li, H. et al. Gasdermin D-mediated hepatocyte pyroptosis expands inflammatory responses that aggravate acute liver failure by upregulating monocyte chemotactic protein 1/CC chemokine receptor-2 to recruit macrophages. World J. Gastroenterol. 25, 6527–6540 (2019).
Zhu, Y. et al. Caspase-11-mediated hepatocytic pyroptosis promotes the progression of nonalcoholic steatohepatitis. Cell Mol. Gastroenterol. Hepatol. 12, 653–664 (2021).
Dixon, L. J., Berk, M., Thapaliya, S., Papouchado, B. G. & Feldstein, A. E. Caspase-1-mediated regulation of fibrogenesis in diet-induced steatohepatitis. Lab. Invest. 92, 713–723 (2012).
Koh, E. H. et al. Sphingomyelin synthase 1 mediates hepatocyte pyroptosis to trigger non-alcoholic steatohepatitis. Gut 70, 1954–1964 (2021).
Ioannou, G. N. et al. Genetic deletion or pharmacologic inhibition of the Nlrp3 inflammasome did not ameliorate experimental NASH. J. Lipid Res. 64, 100330 (2023).
Ma, E. B., Javaid, H. M. A., Jung, D. H., Park, J. H. & Huh, J. Y. Gasdermin D deficiency does not protect mice from high-fat diet-induced glucose intolerance and adipose tissue inflammation. Mediators Inflamm. 2022, 7853482 (2022).
Dixon, L. J., Flask, C. A., Papouchado, B. G., Feldstein, A. E. & Nagy, L. E. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One 8, e56100 (2013).
Shkarina, K. & Broz, P. Selective induction of programmed cell death using synthetic biology tools. Semin. Cell Dev. Biol. 156, 74–92 (2024).
Bekes, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022).
Gupta, U., Ruli, T., Buttar, D., Shoreibah, M. & Gray, M. Metabolic dysfunction associated steatotic liver disease: current practice, screening guidelines and management in the primary care setting. Am. J. Med. Sci. 367, 77–88 (2024).
Wei, S., Wang, L., Evans, P. C. & Xu, S. NAFLD and NASH: etiology, targets and emerging therapies. Drug Discov. Today 29, 103910 (2024).
Chan, W. K. et al. Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-art review. J. Obes. Metab. Syndr. 32, 197–213 (2023).
Teng, M. L. et al. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 29, S32–S42 (2023).
Mackowiak, B., Fu, Y., Maccioni, L. & Gao, B. Alcohol-associated liver disease. J. Clin. Invest. 134, e176345 (2024).
Osna, N. A., Rasineni, K., Ganesan, M., Donohue, T. M. Jr. & Kharbanda, K. K. Pathogenesis of alcohol-associated liver disease. J. Clin. Exp. Hepatol. 12, 1492–1513 (2022).
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70, 151–171 (2019).
Gratacos-Gines, J., Arino, S., Sancho-Bru, P., Bataller, R. & Pose, E. MetALD: clinical aspects, pathophysiology and treatment. JHEP Rep. 7, 101250 (2025).
Lazaridis, K. N. & LaRusso, N. F. Primary sclerosing cholangitis. N. Engl. J. Med. 375, 1161–1170 (2016).
Gallage, S. et al. The therapeutic landscape of hepatocellular carcinoma. Med 2, 505–552 (2021).
Leone, V., Ali, A., Weber, A., Tschaharganeh, D. F. & Heikenwalder, M. Liver inflammation and hepatobiliary cancers. Trends Cancer 7, 606–623 (2021).
Clements, O., Eliahoo, J., Kim, J. U., Taylor-Robinson, S. D. & Khan, S. A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J. Hepatol. 72, 95–103 (2020).
Ducreux, M. et al. The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 24th ESMO/World Congress on Gastrointestinal Cancer, Barcelona, 2022. ESMO Open 8, 101567 (2023).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Ghallab, A. et al. Interruption of bile acid uptake by hepatocytes after acetaminophen overdose ameliorates hepatotoxicity. J. Hepatol. 77, 71–83 (2022).
Liu, Y., Hao, H. & Hou, T. Concanavalin A-induced autoimmune hepatitis model in mice: mechanisms and future outlook. Open Life Sci. 17, 91–101 (2022).
Heymann, F., Hamesch, K., Weiskirchen, R. & Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 49, 12–20 (2015).
Boland, M. L. et al. Towards a standard diet-induced and biopsy-confirmed mouse model of non-alcoholic steatohepatitis: impact of dietary fat source. World J. Gastroenterol. 25, 4904–4920 (2019).
Drescher, H. K. et al. The Influence of different fat sources on steatohepatitis and fibrosis development in the western diet mouse model of non-alcoholic steatohepatitis (NASH). Front. Physiol. 10, 770 (2019).
Matsumoto, M. et al. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 94, 93–103 (2013).
Rinella, M. E. & Green, R. M. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J. Hepatol. 40, 47–51 (2004).
Anstee, Q. M. & Goldin, R. D. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int. J. Exp. Pathol. 87, 1–16 (2006).
Febbraio, M. A. et al. Preclinical models for studying NASH-driven HCC: how useful are they? Cell Metab. 29, 18–26 (2019).
Nevzorova, Y. A., Boyer-Diaz, Z., Cubero, F. J. & Gracia-Sancho, J. Animal models for liver disease — a practical approach for translational research. J. Hepatol. 73, 423–440 (2020).
Gabele, E. et al. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcohol. Clin. Exp. Res. 35, 1361–1367 (2011).
Schonfeld, M. et al. A Western diet with alcohol in drinking water recapitulates features of alcohol-associated liver disease in mice. Alcohol. Clin. Exp. Res. 45, 1980–1993 (2021).
Van Campenhout, S., Van Vlierberghe, H. & Devisscher, L. Common bile duct ligation as model for secondary biliary cirrhosis. Methods Mol. Biol. 1981, 237–247 (2019).
Smit, J. J. et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell 75, 451–462 (1993).
Scholten, D., Trebicka, J., Liedtke, C. & Weiskirchen, R. The carbon tetrachloride model in mice. Lab. Anim. 49, 4–11 (2015).
Gu, C. Y. & Lee, T. K. W. Preclinical mouse models of hepatocellular carcinoma: an overview and update. Exp. Cell Res. 412, 113042 (2022).
Ma, W., Zhang, J., Chen, W., Liu, N. & Wu, T. Notch-driven cholangiocarcinogenesis involves the hippo pathway effector TAZ via METTL3-m6A-YTHDF1. Cell Mol. Gastroenterol. Hepatol. 19, 101417 (2024).
Dhani, S., Zhao, Y. & Zhivotovsky, B. A long way to go: caspase inhibitors in clinical use. Cell Death Dis. 12, 949 (2021).
Mandal, P. et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 56, 481–495 (2014).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Rathkey, J. K. et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 3, 2738 (2018).
Muller, A. J., DuHadaway, J. B., Donover, P. S., Sutanto-Ward, E. & Prendergast, G. C. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat. Med. 11, 312–319 (2005).
Teng, X. et al. Structure-activity relationship study of novel necroptosis inhibitors. Bioorg. Med. Chem. Lett. 15, 5039–5044 (2005).
Gardner, C. R. et al. From (Tool)bench to bedside: the potential of necroptosis inhibitors. J. Med. Chem. 66, 2361–2385 (2023).
Newton, K., Sun, X. & Dixit, V. M. Kinase RIP3 is dispensable for normal NF-κBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and toll-like receptors 2 and 4. Mol. Cell Biol. 24, 1464–1469 (2004).
Murphy, J. M. et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443–453 (2013).
Wu, J. et al. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994–1006 (2013).
Kaiser, W. J. et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc. Natl Acad. Sci. USA 111, 7753–7758 (2014).
Newton, K. et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357–1360 (2014).
Polykratis, A. et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 193, 1539–1543 (2014).
Garcia, L. R. et al. Ubiquitylation of MLKL at lysine 219 positively regulates necroptosis-induced tissue injury and pathogen clearance. Nat. Commun. 12, 3364 (2021).
Coll, R. C. et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 21, 248–255 (2015).
Primiano, M. J. et al. Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary inflammation. J. Immunol. 197, 2421–2433 (2016).
Hill, J. R. et al. Sulfonylureas as concomitant insulin secretagogues and NLRP3 inflammasome inhibitors. ChemMedChem 12, 1449–1457 (2017).
Lamkanfi, M. et al. Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Mariani, S. M., Matiba, B., Armandola, E. A. & Krammer, P. H. Interleukin 1β-converting enzyme related proteases/caspases are involved in TRAIL-induced apoptosis of myeloma and leukemia cells. J. Cell Biol. 137, 221–229 (1997).
Wannamaker, W. et al. (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2 R,3 S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an orally available selective interleukin (IL)-converting enzyme/caspase-1 inhibitor, exhibits potent anti-inflammatory activities by Inhibiting the release of IL-1β and IL-18. J. Pharmacol. Exp. Ther. 321, 509–516 (2007).
Hu, J. J. et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 21, 736–745 (2020).
Wei, S., Feng, M. & Zhang, S. Molecular characteristics of cell pyroptosis and its inhibitors: a review of activation, regulation, and inhibitors. Int J. Mol. Sci. 23, 16115 (2022).
Man, S. M., Karki, R. & Kanneganti, T. D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 277, 61–75 (2017).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Sun, S., Shen, J., Jiang, J., Wang, F. & Min, J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal. Transduct. Target. Ther. 8, 372 (2023).
Rong, X. et al. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. eLife 4, e06557 (2015).
Peleman, C. et al. Ferroptosis is a targetable detrimental factor in metabolic dysfunction-associated steatotic liver disease. Cell Death Differ. 31, 1113–1126 (2024).
Wang, Y. et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science 354, aad6872 (2016).
Yu, S. W. et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297, 259–263 (2002).
Huang, K. et al. PARP1-mediated PPARα poly(ADP-ribosyl)ation suppresses fatty acid oxidation in non-alcoholic fatty liver disease. J. Hepatol. 66, 962–977 (2017).
Mukhopadhyay, P. et al. PARP inhibition protects against alcoholic and non-alcoholic steatohepatitis. J. Hepatol. 66, 589–600 (2017).
Bai, P. et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 13, 461–468 (2011).
Fuchs, T. A. et al. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 (2007).
Li, P. et al. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 (2010).
Papayannopoulos, V., Metzler, K. D., Hakkim, A. & Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 (2010).
Arelaki, S. et al. Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch. 481, 455–465 (2022).
van der Windt, D. J. et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 68, 1347–1360 (2018).
Tsvetkov, P. et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375, 1254–1261 (2022).
Ala, A., Walker, A. P., Ashkan, K., Dooley, J. S. & Schilsky, M. L. Wilson’s disease. Lancet 369, 397–408 (2007).
Ferenci, P. et al. Diagnosis and phenotypic classification of Wilson disease. Liver Int. 23, 139–142 (2003).
Chen, L., Min, J. & Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal. Transduct. Target. Ther. 7, 378 (2022).
Moriwaki, K. & Chan, F. K. Necroptosis-independent signaling by the RIP kinases in inflammation. Cell Mol. Life Sci. 73, 2325–2334 (2016).
Daniels, B. P. et al. RIPK3 restricts viral pathogenesis via cell death-independent neuroinflammation. Cell 169, 301–313.e11 (2017).
Moriwaki, K., Balaji, S., Bertin, J., Gough, P. J. & Chan, F. K. Distinct kinase-independent role of RIPK3 in CD11c+ mononuclear phagocytes in cytokine-induced tissue repair. Cell Rep. 18, 2441–2451 (2017).
Matter, M. S. et al. Destruction of lymphoid organ architecture and hepatitis caused by CD4+ T cells. PLoS One 6, e24772 (2011).
Zinkernagel, R. M. et al. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J. Exp. Med. 164, 1075–1092 (1986).
Teijaro, J. R. et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 340, 207–211 (2013).
Wilson, E. B. et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 340, 202–207 (2013).
Preston, S. P. et al. A necroptosis-independent function of RIPK3 promotes immune dysfunction and prevents control of chronic LCMV infection. Cell Death Dis. 14, 123 (2023).
Afonso, M. B. et al. RIPK3 dampens mitochondrial bioenergetics and lipid droplet dynamics in metabolic liver disease. Hepatology 77, 1319–1334 (2023).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Mohammed, S. et al. Impact of Mlkl or Ripk3 deletion on age-associated liver inflammation, metabolic health, and lifespan. Geroscience 47, 4465–4483 (2025).
Wang, Y. et al. MLKL as an emerging machinery for modulating organelle dynamics: regulatory mechanisms, pathophysiological significance, and targeted therapeutics. Front. Pharmacol. 16, 1512968 (2025).
Sai, K., Parsons, C., House, J. S., Kathariou, S. & Ninomiya-Tsuji, J. Necroptosis mediators RIPK3 and MLKL suppress intracellular Listeria replication independently of host cell killing. J. Cell Biol. 218, 1994–2005 (2019).
Yoon, S., Bogdanov, K. & Wallach, D. Site-specific ubiquitination of MLKL targets it to endosomes and targets Listeria and Yersinia to the lysosomes. Cell Death Differ. 29, 306–322 (2022).
Yoon, S., Kovalenko, A., Bogdanov, K. & Wallach, D. MLKL, the protein that mediates necroptosis, also regulates endosomal trafficking and extracellular vesicle generation. Immunity 47, 51–65.e7 (2017).
Zhan, C., Huang, M., Yang, X. & Hou, J. MLKL: functions beyond serving as the executioner of necroptosis. Theranostics 11, 4759–4769 (2021).
Tye, H. et al. Divergent roles of RIPK3 and MLKL in high-fat diet-induced obesity and MAFLD in mice. Life Sci. Alliance 8, e202302446 (2025).
Majdi, A. et al. Inhibition of receptor-interacting protein kinase 1 improves experimental non-alcoholic fatty liver disease. J. Hepatol. 72, 627–635 (2020).
Xuan Yuan, H. N. et al. Adenosine triphosphate-binding pocket inhibitor for mixed lineage kinase domain-like protein attenuated alcoholic liver disease via necroptosis-independent pathway. World J. Gastroenterol. 31, 96782 (2025).
Fan, W. et al. Flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci. Signal 12, eaaw3423 (2019).
Gong, Y. N. et al. ESCRT-III acts downstream of MLKL to regulate necroptotic cell death and its consequences. Cell 169, 286–300.e16 (2017).
Wright, S. S. et al. Transplantation of gasdermin pores by extracellular vesicles propagates pyroptosis to bystander cells. Cell 188, 280–291.er17 (2025).
Roeck, B. F. et al. Ferroptosis spreads to neighboring cells via plasma membrane contacts. Nat. Commun. 16, 2951 (2025).
Frank, D., Vaux, D. L., Murphy, J. M., Vince, J. E. & Lindqvist, L. M. Activated MLKL attenuates autophagy following its translocation to intracellular membranes. J. Cell Sci. 132, jcs220996 (2019).
Mauthe, M. et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 14, 1435–1455 (2018).
Yu, X. et al. MLKL promotes hepatocarcinogenesis through inhibition of AMPK-mediated autophagy. Cell Death Differ. 31, 1085–1098 (2024).
Acknowledgements
M.V. was supported by the German Cancer Aid (Deutsch Krebshilfe; 70114893) and the Ministry of Culture and Science of the State of North Rhine-Westphalia (CANTAR: NW21-062E). V.K. was supported by a grant from the research committee of the Medical Faculty of the Heinrich Heine University (grant number 2023-32). Work in the laboratory of P.B. was supported by the Swiss National Science Foundation (grant numbers 310030B_198005, 310030_219286). T.L. was supported by the European Research Council (ERC-CoG 771083: PhaseControl), the German Research Foundation (DFG: 279874820, 461704932 and 440603844), the German Cancer Aid (Deutsch Krebshilfe: 70114893) and the Ministry of Culture and Science of the State of North Rhine-Westphalia (CANTAR: NW21-062E, and MODS: PROFILNRW-2020-107-A). During the preparation of this work, the authors used an artificial intelligence-based tool (perplexity.ai) for the correction of grammar and language. After using this tool/service, the authors reviewed and edited the text as needed and take full responsibility for the content of the published article.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Deepa Sathyaseelan, who co-reviewed with Phoebe Ohene-Marfo; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vucur, M., Kondylis, V., Broz, P. et al. Regulated necrosis at the crossroads of liver inflammation and cancer development. Nat Rev Gastroenterol Hepatol (2025). https://doi.org/10.1038/s41575-025-01147-8
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41575-025-01147-8