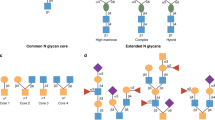
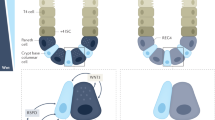
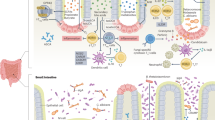
Abstract
Glycans are essential components of homeostatic networks, acting as fine tuners of immunological responses, and are therefore promising targets for manipulating immune tolerance. Glycans shield the entire gut mucosa surface, contributing to epithelial barrier integrity. Moreover, most microorganisms expose glycoconjugates on their surfaces, making glycans essential molecules in the crosstalk between host immune response and the gut microbiota. The vast amount of biological information encoded by mucosal glycans is deciphered by a variety of glycan-binding proteins that translate glycan recognition into either pro-inflammatory or anti-inflammatory responses. Current evidence from inflammatory bowel disease (IBD) has highlighted the prominent role of glycans in establishing and regulating key cellular and molecular pathways underlying the transition from health to intestinal inflammation, with implications for understanding IBD immunopathogenesis and for IBD prediction and prevention. In this Review, we discuss current advances, emerging challenges and future prospects in exploiting the power of the mucosal glycocalyx and the glycome as master coordinators of the immunoregulatory networks in IBD from the preclinical phase to established diagnosis. We discuss the clinical utility of the glycome as a serological biomarker with diagnostic, prognostic and predictive value, and as a potential new target for preventive intervention strategies in IBD.
Key points
-
The mucosal glycocalyx is a fundamental physical and biological barrier that shields the surface of the gut mucosa, guaranteeing epithelial barrier integrity.
-
Glycan-encoded information is recognized by a variety of glycan-binding proteins on immune cells, establishing powerful glycan-mediated immunoregulatory circuits for preserving gut homeostasis.
-
Changes in mucosal glycocalyx affect the gut permeability and the microbiota composition associated with gut dysbiosis.
-
Changes in mucosa glycosylation activate pro-inflammatory pathways culminating in intestinal inflammation.
-
Changes in glycome are detected in blood, in plasma glycoproteins and circulating antibodies (IgG), acting as biomarkers that track with disease onset and progression.
-
Targeting mucosa glycosylation through metabolic supplementation with glycans can preserve gut homeostasis with an effect in preventing the transition from health to intestinal inflammation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rudd, P. M., Elliott, T., Cresswell, P., Wilson, I. A. & Dwek, R. A. Glycosylation and the immune system. Science 291, 2370–2376 (2001).
Flynn, R. A. et al. Small RNAs are modified with N-glycans and displayed on the surface of living cells. Cell 184, 3109–3124.e22 (2021).
Luis, A. S. & Hansson, G. C. Intestinal mucus and their glycans: a habitat for thriving microbiota. Cell Host Microbe 31, 1087–1100 (2023).
Inaba, R., Vujakovic, S. & Bergstrom, K. The gut mucus network: a dynamic liaison between microbes and the immune system. Semin. Immunol. 69, 101807 (2023).
Ohtsubo, K. & Marth, J. D. Glycosylation in cellular mechanisms of health and disease. Cell 126, 855–867 (2006).
Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015).
Verhelst, X. et al. Protein glycosylation as a diagnostic and prognostic marker of chronic inflammatory gastrointestinal and liver diseases. Gastroenterology 158, 95–110 (2020).
Pinho, S. S. & Rabinovich, G. A. The glycoimmune landscape in health and disease. Semin. Immunol. 78, 101965 (2025).
Pinho, S. S., Alves, I., Gaifem, J. & Rabinovich, G. A. Immune regulatory networks coordinated by glycans and glycan-binding proteins in autoimmunity and infection. Cell Mol. Immunol. 20, 1101–1113 (2023).
Vicente, M. M., Leite-Gomes, E. & Pinho, S. S. Glycome dynamics in T and B cell development: basic immunological mechanisms and clinical applications. Trends immunol. 44, 585–597 (2023).
Crouch, L. I. et al. The role of glycans in health and disease: regulators of the interaction between gut microbiota and host immune system. Semin. Immunol. 73, 101891 (2024).
Estevinho, M. M. et al. Emerging role of environmental pollutants in inflammatory bowel disease risk, outcomes and underlying mechanisms. Gut https://doi.org/10.1136/gutjnl-2024-332523 (2024).
Gaifem, J. et al. A unique serum IgG glycosylation signature predicts development of Crohn’s disease and is associated with pathogenic antibodies to mannose glycan. Nat. Immunol. https://doi.org/10.1038/s41590-024-01916-8 (2024).
Garber, K. No added sugar: antibody makers find an upside to ‘no fucose’. Nat. Biotechnol. 36, 1025–1027 (2018).
Kaneko, Y., Nimmerjahn, F. & Ravetch, J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006).
Huffman, J. E. et al. Comparative performance of four methods for high-throughput glycosylation analysis of immunoglobulin G in genetic and epidemiological research. Mol. Cell. Proteomics 13, 1598–1610 (2014).
Dressman, J. W. et al. Development of an antibody-based platform for the analysis of immune cell-specific N-linked glycosylation. Anal. Chem. 95, 10289–10297 (2023).
Marie, A. L., Gao, Y. & Ivanov, A. R. Native N-glycome profiling of single cells and ng-level blood isolates using label-free capillary electrophoresis-mass spectrometry. Nat. Commun. 15, 3847 (2024).
Hracs, L. et al. Global evolution of inflammatory bowel disease across epidemiologic stages. Nature https://doi.org/10.1038/s41586-025-08940-0 (2025).
Kaplan, G. G. The global burden of inflammatory bowel disease: from 2025 to 2045. Nat. Rev. Gastroenterol. Hepatol. 22, 708–720 (2025).
Torres, J., Ungaro, R. C. & Colombel, J. F. Is prevention the best way to modify inflammatory bowel disease? How close are we? Gastroenterology https://doi.org/10.1053/j.gastro.2021.07.051 (2021).
Torres, J., Burisch, J., Riddle, M., Dubinsky, M. & Colombel, J. F. Preclinical disease and preventive strategies in IBD: perspectives, challenges and opportunities. Gut 65, 1061–1069 (2016).
Torres, J. et al. Serum biomarkers identify patients who will develop inflammatory bowel diseases up to 5 years before diagnosis. Gastroenterology 159, 96–104 (2020).
Linden, S. K., Sutton, P., Karlsson, N. G., Korolik, V. & McGuckin, M. A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197 (2008).
Schneider, H., Pelaseyed, T., Svensson, F. & Johansson, M. E. V. Study of mucin turnover in the small intestine by in vivo labeling. Sci. Rep. 8, 5760 (2018).
Hansson, G. C. Mucins and the microbiome. Annu. Rev. Biochem. 89, 769–793 (2020).
Marth, J. D. & Grewal, P. K. Mammalian glycosylation in immunity. Nat. Rev. Immunol. 8, 874–887 (2008).
Green, R. S. et al. Mammalian N-glycan branching protects against innate immune self-recognition and inflammation in autoimmune disease pathogenesis. Immunity 27, 308–320 (2007).
Granovsky, M. et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat. Med. 6, 306–312 (2000).
Demetriou, M., Granovsky, M., Quaggin, S. & Dennis, J. W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 409, 733–739 (2001).
Dias, A. M. et al. Dysregulation of T cell receptor N-glycosylation: a molecular mechanism involved in ulcerative colitis. Hum. Mol. Genet. 23, 2416–2427 (2014).
Mortales, C. L., Lee, S. U. & Demetriou, M. N-glycan branching is required for development of mature B cells. J. Immunol. 205, 630–636 (2020).
Zhou, R. W. et al. N-glycosylation bidirectionally extends the boundaries of thymocyte positive selection by decoupling Lck from Ca2+ signaling. Nat. Immunol. 15, 1038–1045 (2014).
van Kooyk, Y. & Rabinovich, G. A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 9, 593–601 (2008).
Rodrigues, C. S. et al. Alterations in mucosa branched N-glycans lead to dysbiosis and downregulation of ILC3: a key driver of intestinal inflammation. Gut Microbes 17, 2461210 (2025).
Lau, K. S. et al. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 129, 123–134 (2007).
Chen, H. L., Li, C. F., Grigorian, A., Tian, W. & Demetriou, M. T cell receptor signaling co-regulates multiple Golgi genes to enhance N-glycan branching. J. Biol. Chem. 284, 32454–32461 (2009).
Morosi, L. G. et al. Control of intestinal inflammation by glycosylation-dependent lectin-driven immunoregulatory circuits. Sci. Adv. 7, eabf8630 (2021).
Pickard, J. M. et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 514, 638–641 (2014).
Newburg, D. S. & Morelli, L. Human milk and infant intestinal mucosal glycans guide succession of the neonatal intestinal microbiota. Pediatr. Res. 77, 115–120 (2015).
Briliute, J. et al. Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat. Microbiol. 4, 1571–1581 (2019).
El Kaoutari, A., Armougom, F., Gordon, J. I., Raoult, D. & Henrissat, B. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 11, 497–504 (2013).
Koropatkin, N. M., Cameron, E. A. & Martens, E. C. How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10, 323–335 (2012).
Holmberg, S. M. et al. The gut commensal Blautia maintains colonic mucus function under low-fiber consumption through secretion of short-chain fatty acids. Nat. Commun. 15, 3502 (2024).
Khan, M. T. et al. Synergy and oxygen adaptation for development of next-generation probiotics. Nature 620, 381–385 (2023).
Kappler, K. & Hennet, T. Emergence and significance of carbohydrate-specific antibodies. Genes Immun. 21, 224–239 (2020).
New, J. S. et al. Neonatal exposure to commensal-bacteria-derived antigens directs polysaccharide-specific B-1 B cell repertoire development. Immunity 53, 172–186.e6 (2020).
Polonskaya, Z. et al. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Invest. 127, 1491–1504 (2017).
Polonskaya, Z., Savage, P. B., Finn, M. G. & Teyton, L. High-affinity anti-glycan antibodies: challenges and strategies. Curr. Opin. Immunol. 59, 65–71 (2019).
Harding, C. V., Kihlberg, J., Elofsson, M., Magnusson, G. & Unanue, E. R. Glycopeptides bind MHC molecules and elicit specific T cell responses. J. Immunol. 151, 2419–2425 (1993).
Kim, M. & Kim, C. H. Regulation of humoral immunity by gut microbial products. Gut Microbes 8, 392–399 (2017).
Alves, I., Fernandes, A., Santos-Pereira, B., Azevedo, C. M. & Pinho, S. S. Glycans as a key factor in self and non-self discrimination: impact on the breach of immune tolerance. FEBS Lett. https://doi.org/10.1002/1873-3468.14347 (2022).
Pereira, M. S. et al. A (glyco)biomarker that predicts failure to standard therapy in ulcerative colitis patients. J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjy139 (2018).
Brazil, J. C. & Parkos, C. A. Finding the sweet spot: glycosylation mediated regulation of intestinal inflammation. Mucosal Immunol. 15, 211–222 (2022).
Probert, C. S. et al. South Asian and European colitics show characteristic differences in colonic mucus glycoprotein type and turnover. Gut 36, 696–702 (1995).
Pereira, M. S. et al. Genetic variants of the MGAT5 gene are functionally implicated in the modulation of T cells glycosylation and plasma IgG glycome composition in ulcerative colitis. Clin. Transl. Gastroenterol. 11, e00166 (2020).
Dias, A. M. et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc. Natl Acad. Sci. 115, E4651–E4660 (2018).
Vicente, M. M. et al. Mannosylated glycans impair normal T-cell development by reprogramming commitment and repertoire diversity. Cell Mol. Immunol. 20, 955–968 (2023).
Fujii, H. et al. Core fucosylation on T cells, required for activation of T-cell receptor signaling and induction of colitis in mice, is increased in patients with inflammatory bowel disease. Gastroenterology 150, 1620–1632 (2016).
McGovern, D. P. et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum. Mol. Genet. 19, 3468–3476 (2010).
Brown, S. J. et al. Altered immune system glycosylation causes colitis in α1,2-fucosyltransferase transgenic mice. Inflamm. Bowel Dis. 10, 546–556 (2004).
Goto, Y. et al. Innate lymphoid cells regulate intestinal epithelial cell glycosylation. Science 345, 1254009 (2014).
Yao, Y. et al. Mucus sialylation determines intestinal host-commensal homeostasis. Cell 185, 1172–1188.e1128 (2022).
Kelm, M. et al. Targeting epithelium-expressed sialyl Lewis glycans improves colonic mucosal wound healing and protects against colitis. JCI Insight 5, e135843 (2020).
Parikh, K. et al. Colonic epithelial cell diversity in health and inflammatory bowel disease. Nature 567, 49–55 (2019).
Nystrom, E. E. L. et al. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science 372, eabb1590 (2021).
An, G. et al. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 204, 1417–1429 (2007).
Fu, J. et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 121, 1657–1666 (2011).
Bergstrom, K. et al. Core 1- and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol. 10, 91–103 (2017).
Bergstrom, K. et al. Defective intestinal mucin-type O-glycosylation causes spontaneous colitis-associated cancer in mice. Gastroenterology 151, 152–164.e11 (2016).
Larsson, J. M. et al. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 17, 2299–2307 (2011).
Kudelka, M. R. et al. Cosmc is an X-linked inflammatory bowel disease risk gene that spatially regulates gut microbiota and contributes to sex-specific risk. Proc. Natl Acad. Sci. 113, 14787–14792 (2016).
Roy, A. et al. N-glycosylation enzyme Mpi is essential for mucin O-glycosylation, host-microbe homeostasis, Paneth cell defense, and metabolism. Preprint at Res. Sq. https://doi.org/10.21203/rs.3.rs-6222474/v1 (2025).
Dotan, I. et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn’s disease. Gastroenterology 131, 366–378 (2006).
Sendid, B. et al. Specific antibody response to oligomannosidic epitopes in Crohn’s disease. Clin. Diagn. Lab. Immunol. 3, 219–226 (1996).
Sendid, B. et al. From ASCA breakthrough in Crohn’s disease and Candida albicans research to thirty years of investigations about their meaning in human health. Autoimmun. Rev. 23, 103486 (2024).
Morishima, N. et al. Generation and validation of antibody 42B1 recognizing galactose-deficient IgG for diagnosis of chronic inflammatory diseases. Clin. Chim. Acta 566, 120052 (2025).
Trbojevic Akmacic, I. et al. Inflammatory bowel disease associates with proinflammatory potential of the immunoglobulin G glycome. Inflamm. Bowel Dis. 21, 1237–1247 (2015).
Kaul, A. et al. Serum anti-glycan antibody biomarkers for inflammatory bowel disease diagnosis and progression: a systematic review and meta-analysis. Inflamm. Bowel Dis. 18, 1872–1884 (2012).
Bourgonje, A. R. et al. Phage-display immunoprecipitation sequencing of the antibody epitope repertoire in inflammatory bowel disease reveals distinct antibody signatures. Immunity 56, 1393–1409.e6 (2023).
Alves, I. et al. Protein mannosylation as a diagnostic and prognostic biomarker of lupus nephritis: an unusual glycan-neoepitope in systemic lupus erythematosus. Arthritis Rheumatol. https://doi.org/10.1002/art.41768 (2021).
Alves, I. et al. Host-derived mannose glycans trigger a pathogenic γδ T cell/IL-17a axis in autoimmunity. Sci. Transl. Med. 15, eabo1930 (2023).
Fekete, E. & Buret, A. G. The role of mucin O-glycans in microbiota dysbiosis, intestinal homeostasis, and host-pathogen interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 324, G452–G465 (2023).
Akdis, C. A. Does the epithelial barrier hypothesis explain the increase in allergy, autoimmunity and other chronic conditions? Nat. Rev. Immunol. 21, 739–751 (2021).
Monteleone, G., Moscardelli, A., Colella, A., Marafini, I. & Salvatori, S. Immune-mediated inflammatory diseases: common and different pathogenic and clinical features. Autoimmun. Rev. 22, 103410 (2023).
van der Post, S. et al. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 68, 2142–2151 (2019).
Turpin, W. et al. Increased intestinal permeability is associated with later development of Crohn’s disease. Gastroenterology 159, 2092–2100.e5 (2020).
Oosterhoff, J. J., Larsen, M. D., van der Schoot, C. E. & Vidarsson, G. Afucosylated IgG responses in humans — structural clues to the regulation of humoral immunity. Trends Immunol. 43, 800–814 (2022).
Lee, S. H. et al. Anti-microbial antibody response is associated with future onset of Crohn’s disease independent of biomarkers of altered gut barrier function, subclinical inflammation, and genetic risk. Gastroenterology 161, 1540–1551 (2021).
Mortha, A. et al. Neutralizing anti-granulocyte macrophage-colony stimulating factor autoantibodies recognize post-translational glycosylations on granulocyte macrophage-colony stimulating factor years before diagnosis and predict complicated Crohn’s disease. Gastroenterology 163, 659–670 (2022).
Dias, A. M. et al. Glycans as critical regulators of gut immunity in homeostasis and disease. Cell Immunol. https://doi.org/10.1016/j.cellimm.2018.07.007 (2018).
Huffman, J. E. et al. Polymorphisms in B3GAT1, SLC9A9 and MGAT5 are associated with variation within the human plasma N-glycome of 3533 European adults. Hum. Mol. Genet. 20, 5000–5011 (2011).
Klaric, L. et al. Glycosylation of immunoglobulin G is regulated by a large network of genes pleiotropic with inflammatory diseases. Sci. Adv. 6, eaax0301 (2020).
Simurina, M. et al. Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology 154, 1320–1333.e10 (2018).
Choi, E. K. et al. The manganese transporter SLC39A8 links alkaline ceramidase 1 to inflammatory bowel disease. Nat. Commun. 15, 4775 (2024).
Moller, F. T., Andersen, V., Wohlfahrt, J. & Jess, T. Familial risk of inflammatory bowel disease: a population-based cohort study 1977-2011. Am. J. Gastroenterol. 110, 564–571 (2015).
Martens, E. C., Neumann, M. & Desai, M. S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 16, 457–470 (2018).
Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011).
Ananthakrishnan, A. N., Whelan, K., Allegretti, J. R. & Sokol, H. Diet and microbiome-directed therapy 2.0 for IBD. Clin. Gastroenterol. Hepatol. 23, 406–418 (2025).
Agrawal, M. et al. Per- and poly-fluoroalkyl substances exposure is associated with later occurrence of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 22, 1728–1730.e8 (2024).
Liu, J. et al. Associations between the serum levels of PFOS/PFOA and IgG N-glycosylation in adult or children. Env. Pollut. 265, 114285 (2020).
Irving, P. M. & Gibson, P. R. Infections and IBD. Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 18–27 (2008).
Craviotto, V. et al. Viral infections in inflammatory bowel disease: tips and tricks for correct management. World J. Gastroenterol. 27, 4276–4297 (2021).
Nandy, A. et al. Epstein–Barr virus exposure precedes Crohn’s disease development. Gastroenterology https://doi.org/10.1053/j.gastro.2025.01.247 (2025).
Aspinall, G. O. et al. Lipopolysaccharides from Campylobacter jejuni associated with Guillain–Barré syndrome patients mimic human gangliosides in structure. Infect. Immun. 62, 2122–2125 (1994).
Lee, S. H., Lopes, E., Colombel, J. F. & Ungaro, R. Identifying potential targets for the interception of inflammatory bowel disease: toward precision prevention. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izaf168 (2025).
Leite-Gomes, E. et al. T-cell branched glycosylation as a mediator of colitis-associated colorectal cancer progression: a potential new risk biomarker in inflammatory bowel disease. J. Crohns Colitis 19, jjaf043 (2025).
Clerc, F. et al. Plasma N-glycan signatures are associated with features of inflammatory bowel diseases. Gastroenterology 155, 829–843 (2018).
Shubhakar, A. et al. Serum N-glycomic biomarkers predict treatment escalation in inflammatory bowel disease. J. Crohns Colitis 17, 919–932 (2023).
Bergstrom, K. et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 370, 467–472 (2020).
Fancy, N. et al. Fecal-adherent mucus is a non-invasive source of primary human MUC2 for structural and functional characterization in health and disease. J. Biol. Chem. 300, 105675 (2024).
Masselot, C. R. et al. Fecal mucin O-glycans as novel biomarkers in inflammatory bowel diseases. Inflamm. Bowel Dis. 29, e12 (2023).
van Tol, B. D. M. et al. Comprehensive immunoglobulin G, A, and M glycopeptide profiling for large-scale biomedical research. Mol. Cell. Proteomics 24, 100928 (2025).
Levin, R. M., Krieger, N. N. & Winzler, R. J. Glucmsumine and acetylglucosamine tolerance in man. J. Lab. Clin. Med. 58, 927–932 (1961).
Salvatore, S. et al. A pilot study of N-acetyl glucosamine, a nutritional substrate for glycosaminoglycan synthesis, in paediatric chronic inflammatory bowel disease. Aliment. Pharmacol. Ther. 14, 1567–1579 (2000).
Schalich, K. M. et al. A human milk oligosaccharide prevents intestinal inflammation in adulthood via modulating gut microbial metabolism. mBio 15, e0029824 (2024).
Acknowledgements
S.S.P. acknowledges funding from the European Union (GlycanTrigger, grant agreement number 101093997; GlycanSwitch, grant agreement number 101071386). The views and opinions expressed here are, however, those of the author(s) only and do not necessarily reflect those of the European Union or the European Research Council Executive Agency. S.S.P. also acknowledges funding from the US Department of Defense, US Army Medical Research Acquisition Activity and FY18 Peer-Reviewed Medical Research Program Investigator-Initiated Research Award (award number W81XWH1920053); and funding from the European Crohn’s and Colitis Organisation (ECCO) Pioneer Award 2021, the International Organization for the study of Inflammatory Bowel Disease (IOIBD), the Portuguese Group of Study on IBD (GEDII), Mizutani Foundation for Glycoscience (Grant 250007) and the Portuguese Foundation for Science and Technology (FCT; 2023.16654.ICDT). J.-F.C. and J.T. acknowledge funding from the Innovative Health Initiative Joint Undertaking (IHI JU) under grant agreement no. 101194780 (INTERCEPT). The IHI JU receives support from the European Union’s Horizon Europe Research and Innovation Programme and COCIR, EFPIA, Europa Bío, MedTech Europe, Vaccines Europe, Ludger Ltd, Celltrion Inc. and Prometheus Laboratories Inc. J.-F.C. and J.T. also acknowledge funding from the European Union (GlycanTrigger, grant agreement number 101093997). Views and opinions expressed here are, however, those of the author(s) only and do not necessarily reflect those of the aforementioned parties. Neither of the aforementioned parties can be held responsible for them. The authors acknowledge J. Gregory, Senior Medical Illustrator at Mount Sinai, for her outstanding artwork prepared for this review.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article. All authors contributed substantially to discussion of the content. All authors wrote the article. S.S.P. reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Shinichiro Shinzaki, Jennifer Brazil and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GlycanTrigger Project: https://glycantrigger.eu/
Glossary
- Glycan mimicry
-
Similarity of glycan structures between microorganisms and host cells. Microorganisms can engage in glycan mimicry by expressing glycan structures similar to those of the host to evade immune detection and, therefore, colonize and infect the host. Glycan mimicry can also underlie the inability of the host immune system to distinguish self from non-self glycans, leading to the loss of immunological tolerance and autoimmune reactions.
- Glycan-binding proteins
-
Proteins expressed or secreted by host cells (immune cells, epithelial cells, endothelial cells) that specifically recognize and bind to carbohydrate structures (glycans), being important in immune regulation.
- Glycans
-
Complex carbohydrate structures composed of at least two monosaccharides linked together, attached to proteins or lipids. N-linked glycans are carbohydrate chains attached to the nitrogen atom of asparagine residues in proteins. O-linked glycans are carbohydrate chains attached to the oxygen atom of serine or threonine residues in proteins.
- Glycome
-
The complete repertoire of glycan structures (free or attached to proteins and lipids) expressed or secreted by a cell, tissue or organism. It contains a large amount of important biological information that adds to the information provided by the genome and proteome. It contributes to biological diversity and to speciation.
- Gut mucosal glycocalyx
-
A dense gel-like layer, composed of a diverse repertoire of glycan structures attached to glycoproteins, glycolipids and mucins that coats the apical surface of epithelial cells of gut mucosa. It functions as a protective shield, promoting epithelial barrier integrity and regulating interactions between the host immune system and the microbiota.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pinho, S.S., Torres, J. & Colombel, JF. Mucosal glycans: key drivers of the development of inflammatory bowel disease and a potential new therapeutic target. Nat Rev Gastroenterol Hepatol (2026). https://doi.org/10.1038/s41575-025-01164-7
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41575-025-01164-7