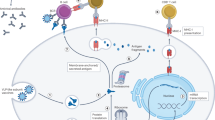
Abstract
The textbook view of vaccination is that it functions to induce immune memory of the specific pathogen components of the vaccine, leading to a quantitatively and qualitatively better response if the host is exposed to infection with the same pathogen. However, evidence accumulated over the past few decades increasingly suggests that vaccines can also have non-specific effects on unrelated infections and diseases, with important implications for childhood mortality particularly in low-income settings. Furthermore, many of these non-specific effects, as well as the pathogen-specific effects, of vaccines show differences between the sexes. Here, members of the Optimmunize consortium discuss the evidence for and potential mechanisms of non-specific and sex-differential effects of vaccines, as well as their potential policy implications. Given that the non-specific effects of some vaccines are now being tested for their ability to protect against COVID-19, the authors also comment on the broader implications of these trials.
The contributors
Peter Aaby was trained as an anthropologist but has built a large health surveillance system in Guinea-Bissau since 1978, focusing on the high levels of child mortality there. Crowding and intensive exposure to measles were key determinants of child mortality. This led to vaccine research and the discovery of the non-specific effects of measles vaccine.
Christine Stabell Benn is a professor in global health at the University of Southern Denmark. She conducts epidemiological and immunological studies of vaccines and vitamin A, with a focus on their real-life effects on overall health in Africa and Denmark. She formulated the hypothesis that these health interventions with immunomodulatory effects interact, often in a sex-differential manner.
Katie L. Flanagan is Director of Infectious Diseases for north/north-west Tasmania, an adjunct professor at the University of Tasmania and RMIT University and an adjunct associate professor at Monash University. She is Honorary Secretary of the Australasian Society for Infectious Diseases (ASID), Chair of the ASID Vaccination Special Interest Group and a member of the Australian Technical Advisory Group on Immunisation. Her current research focuses on using systems vaccinology to study the sex-differential and non-targeted effects of vaccines.
Sabra L. Klein is a professor of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health, Baltimore, USA. She is an expert on sex and gender differences in immune responses and susceptibility to infection. She is the immediate past president of the Organization for the Study of Sex Differences, a principal investigator of the Johns Hopkins Specialized Center for Research Excellence in sex and age differences in immunity to influenza and a co-director of the Johns Hopkins Center for Women’s Health, Sex, and Gender Research.
Tobias R. Kollmann is a paediatric infectious disease clinician and systems vaccinologist at Telethon Kids Institute and Perth Children’s Hospital in Perth, Australia. His expertise centres on newborn infectious diseases, immune ontogeny and early-life vaccine responses, using cutting-edge technology and analytics to extract the most information out of the typically small biological samples obtainable in early life.
David J. Lynn is Director of the Computational and Systems Biology Program and an EMBL Australia group leader at the South Australian Health and Medical Research Institute. He is also a professor at the Flinders University College of Medicine and Public Health. He leads a research programme in systems immunology, investigating how pathogenic and commensal microorganisms modulate the immune system in different contexts, including vaccination.
Frank Shann worked as a paediatrician in Papua New Guinea and then for 20 years was Director of Intensive Care at the Royal Children’s Hospital in Melbourne, Australia. He is a professorial fellow in the Department of Paediatrics, University of Melbourne, engaged in research on the non-specific effects of vaccines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Higgins, J. P. et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ 355, i5170 (2016).
Shann, F. Nonspecific effects of vaccines and the reduction of mortality in children. Clin. Ther. 35, 109–114 (2013).
Aaby, P., Kollmann, T. R. & Benn, C. S. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nat. Immunol. 15, 895–899 (2014).
Benn, C. S., Fisker, A. B., Rieckmann, A., Sørup, S. & Aaby, P. Vaccinology: time to change paradigm? Lancet Infect. Dis. (in the press).
Aaby, P., Ravn, H. & Benn, C. S. The WHO review of the possible non-specific effects of diphtheria-tetanus-pertussis vaccine. Pediatr. Infect. Dis. J. 35, 1247–1257 (2016).
Klein, S. L., Shann, F., Moss, W. J., Benn, C. S. & Aaby, P. RTS,S malaria vaccine and increased mortality in girls. mBio 7, e00514-16 (2016).
Aaby, P. et al. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open 2, e000707 (2012).
Shann, F. A live-vaccine-last schedule: saving an extra million lives a year? Clin. Infect. Dis. https://doi.org/10.1093/cid/ciaa292 (2020).
Sørup, S. et al. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA 311, 826–835 (2014).
Bardenheier, B. H., McNeil, M. M., Wodi, A. P., McNicholl, J. M. & DeStefano, F. Risk of nontargeted infectious disease hospitalizations among US children following inactivated and live vaccines, 2005-2014. Clin. Infect. Dis. 65, 729–737 (2017).
Freyne, B. & Curtis, N. Does neonatal BCG vaccination prevent allergic disease in later life? Arch. Dis. Child. 99, 182–184 (2014).
Benitez, M. L. R. et al. Mycobacterium bovis BCG in metastatic melanoma therapy. Appl. Microbiol. Biotechnol. 103, 7903–7916 (2019).
Guallar-Garrido, S. & Julian, E. Bacillus Calmette-Guérin (BCG) therapy for bladder cancer: an update. Immunotargets Ther. 9, 1–11 (2020).
Netea, M. G. et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. https://doi.org/10.1038/s41577-020-0285-6 (2020).
Kühtreiber, W. M. & Faustman, D. L. BCG therapy for type 1 diabetes: restoration of balanced immunity and metabolism. Trends Endocrinol. Metab. 30, 80–92 (2019).
Yamazaki-Nakashimada, M. A. et al. BCG: a vaccine with multiple faces. Hum. Vaccin. Immunother. https://doi.org/10.1080/21645515.2019.1706930 (2020).
Gofrit, O. N. et al. Bacillus Calmette-Guérin (BCG) therapy lowers the incidence of Alzheimer’s disease in bladder cancer patients. PLoS One 14, e0224433 (2019).
de Castro, M. J., Pardo-Seco, J. & Martinon-Torres, F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin. Infect. Dis. 60, 1611–1619 (2015).
Biering-Sorensen, S. et al. Early BCG-Denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin. Infect. Dis. 65, 1183–1190 (2017).
Stensballe, L. G. et al. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch. Dis. Child. 102, 224–231 (2017).
Arts, R. J. W. et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23, 89–100 e105 (2018).
Wardhana, Datau, E. A., Sultana, A., Mandang, V. V. & Jim, E. The efficacy of bacillus Calmette-Guerin vaccinations for the prevention of acute upper respiratory tract infection in the elderly. Acta Med. Indones. 43, 185–190 (2011).
Aaby, P. & Benn, C. S. Developing the concept of beneficial nonspecific effect of live vaccines with epidemiological studies. Clin. Microbiol. Infect. 25, 1459–1467 (2019).
Rieckmann, A. et al. Vaccinations against smallpox and tuberculosis are associated with better long-term survival: a Danish case-cohort study 1971–2010. Int. J. Epidemiol. 46, 695–705 (2017).
Klein, S. L., Jedlicka, A. & Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 10, 338–349 (2010).
Aaby, P. et al. Long-term survival after Edmonston-Zagreb measles vaccination in Guinea-Bissau: increased female mortality rate. J. Pediatr. 122, 904–908 (1993).
Aaby, P. et al. Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: reanalysis of West African studies. Lancet 361, 2183–2188 (2003).
Aaby, P. et al. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: observations from vaccination trials in Guinea-Bissau. Pediatr. Infect. Dis. J. 26, 247–252 (2007).
Flanagan, K. L. et al. Sex differences in the vaccine-specific and non-targeted effects of vaccines. Vaccine 29, 2349–2354 (2011).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Flanagan, K. L., Fink, A. L., Plebanski, M. & Klein, S. L. Sex and gender differences in the outcomes of vaccination over the life course. Annu. Rev. Cell Dev. Biol. 33, 577–599 (2017).
Aaby, P. et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ 341, c6495 (2010).
Benn, C. S., Netea, M. G., Selin, L. K. & Aaby, P. A small jab – a big effect: nonspecific immunomodulation by vaccines. Trends Immunol. 34, 431–439 (2013).
Brook, B. et al. BCG-vaccination induced emergency granulopoiesis provides rapid protection from neonatal sepsis. Sci. Transl Med. https://stm.sciencemag.org/lookup/doi/10.1126/scitranslmed.aax4517 (2020).
Aaby, P. et al. Measles vaccination in the presence or absence of maternal measles antibody: impact on child survival. Clin. Infect. Dis. 59, 484–492 (2014).
Benn, C. S., Fisker, A. B., Whittle, H. C. & Aaby, P. Revaccination with live attenuated vaccines confer additional beneficial nonspecific effects on overall survival: a review. EBioMed. 10, 312–317 (2016).
Kollmann, T. R., Marchant, A. & Way, S. S. Vaccination strategies to enhance immunity in neonates. Science 368, 612–615 (2020).
Noho-Konteh, F. et al. Sex-differential non-vaccine-specific immunological effects of diphtheria-tetanus-pertussis and measles vaccination. Clin. Infect. Dis. 63, 1213–1226 (2016).
Zivkovic, I. et al. Sexual diergism in antibody response to whole virus trivalent inactivated influenza vaccine in outbred mice. Vaccine 33, 5546–5552 (2015).
Zivkovic, I. et al. Sex bias in mouse humoral immune response to influenza vaccine depends on the vaccine type. Biologicals 52, 18–24 (2018).
Fink, A. L., Engle, K., Ursin, R. L., Tang, W. Y. & Klein, S. L. Biological sex affects vaccine efficacy and protection against influenza in mice. Proc. Natl Acad. Sci. USA 115, 12477–12482 (2018).
Potluri, T. et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 4, 29 (2019).
Andersen, A. et al. National immunization campaigns with oral polio vaccine reduce all-cause mortality: a natural experiment within seven randomized trials. Front. Public Health 6, 13 (2018).
Uthayakumar, D. et al. Non-specific effects of vaccines illustrated through the BCG example: from observations to demonstrations. Front. Immunol. 9, 2869 (2018).
Miller, A. et al. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. Preprint at medRxiv https://doi.org/10.1101/2020.03.24.20042937 (2020).
Shet, A. et al. Differential COVID-19-attributable mortality and BCG vaccine use in countries. Preprint at medRxiv https://doi.org/10.1101/2020.04.01.20049478 (2020).
Sala, G. & Miyakawa, T. Association of BCG vaccination policy with prevalence and mortality of COVID-19. Preprint at medRxiv https://doi.org/10.1101/2020.03.30.20048165 (2020).
Floc’h, F. & Werner, G. H. Increased resistance to virus infections of mice inoculated with BCG (bacillus Calmette-Guérin). Ann. Immunol. 127, 173–186 (1976).
Curtis, N., Sparrow, A., Ghebreyesus, T. A. & Neteta, M. G. Considering BCG vaccination to reduce the impact of COVID-19. Lancet https://doi.org/10.1016/S0140-6736(20)31025-4 (2020).
Wenham, C., Smith, J. & Morgan, R. COVID-19: the gendered impacts of the outbreak. Lancet 395, 846–848 (2020).
Zeng, F. et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J. Med. Virol. https://doi.org/10.1002/jmv.25989 (2020).
Acknowledgements
T.R.K. is supported by the NIH National Institute of Allergy and Infectious Diseases (U19AI118608-02) and Telethon Kids and Perth Children’s Hospital Foundation. D.J.L.’s work on the non-specific effects of vaccines is supported by the Flinders Foundation and the Australian National Health and Medical Research Council. The BRACE trial is supported by the Bill & Melinda Gates Foundation, Sarah and Lachlan Murdoch, the Royal Children’s Hospital Foundation, the Minderoo Foundation, the South Australian government, the NAB Foundation, the Calvert-Jones Foundation and individual donors.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding authors
Ethics declarations
Competing interests
K.L.F. has received consultation and lecture fees from Sanofi Pasteur, Seqirus and Pfizer in the past 5 years and is a member of the Australian Technical Advisory Group on Immunisation. The other authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
BRACE trial: https://www.mcri.edu.au/BRACE
Optimmunize consortium: https://www.bandim.org/optimmunize
Optimmunize meeting, February 2020: https://coursesandconferences.wellcomegenomecampus.org/our-events/optimmunize/
Rights and permissions
About this article
Cite this article
Aaby, P., Benn, C.S., Flanagan, K.L. et al. The non-specific and sex-differential effects of vaccines. Nat Rev Immunol 20, 464–470 (2020). https://doi.org/10.1038/s41577-020-0338-x
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-020-0338-x
This article is cited by
-
Taxonomy of the Full Health and Societal Value of Maternal Vaccination to Prevent Infant Respiratory Syncytial Virus Disease
Infectious Diseases and Therapy (2026)
-
Immunisation status and clinical outcomes in children admitted to a Paediatric emergency unit in Ghana: a prospective cohort study
BMC Pediatrics (2025)
-
A natural experiment on the effect of herpes zoster vaccination on dementia
Nature (2025)
-
Androgens inhibit protective CD8+ T cell responses against pre-erythrocytic malaria parasites in mice
Nature Communications (2025)
-
Fast, Present and Future of the Concept of Spondyloarthritis
Current Rheumatology Reports (2025)