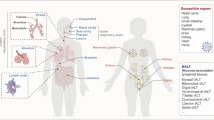
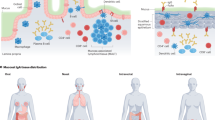
Abstract
Mucosal vaccines offer the potential to trigger robust protective immune responses at the predominant sites of pathogen infection. In principle, the induction of adaptive immunity at mucosal sites, involving secretory antibody responses and tissue-resident T cells, has the capacity to prevent an infection from becoming established in the first place, rather than only curtailing infection and protecting against the development of disease symptoms. Although numerous effective mucosal vaccines are in use, the major advances seen with injectable vaccines (including adjuvanted subunit antigens, RNA and DNA vaccines) have not yet been translated into licensed mucosal vaccines, which currently comprise solely live attenuated and inactivated whole-cell preparations. The identification of safe and effective mucosal adjuvants allied to innovative antigen discovery and delivery strategies is key to advancing mucosal vaccines. Significant progress has been made in resolving the mechanisms that regulate innate and adaptive mucosal immunity and in understanding the crosstalk between mucosal sites, and this provides valuable pointers to inform mucosal adjuvant design. In particular, increased knowledge on mucosal antigen-presenting cells, innate lymphoid cell populations and resident memory cells at mucosal sites highlights attractive targets for vaccine design. Exploiting these insights will allow new vaccine technologies to be leveraged to facilitate rational mucosal vaccine design for pathogens including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and for cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
03 August 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41577-021-00599-8
References
WHO. The top 10 causes of death. World Health Organization https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (2020).
Troeger, C. et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18, 1191–1210 (2018).
Shi, T. et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 390, 946–958 (2017).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396, 1204–1222 (2020).
Hoft, D. F. et al. Comparisons of the humoral and cellular immune responses induced by live attenuated influenza vaccine and inactivated influenza vaccine in adults. Clin. Vaccine Immunol. 24, 1–9 (2017).
Lartey, S. et al. Live-attenuated influenza vaccine induces tonsillar follicular T helper cell responses that correlate with antibody induction. J. Infect. Dis. 221, 21–32 (2020).
Jahnmatz, M. et al. Safety and immunogenicity of the live attenuated intranasal pertussis vaccine BPZE1: a phase 1b, double-blind, randomised, placebo-controlled dose-escalation study. Lancet Infect. Dis. 20, 1290–1301 (2020).
Lin, A. et al. Live attenuated pertussis vaccine BPZE1 induces a broad antibody response in humans. J. Clin. Invest. 130, 2332–2346 (2020). This study provides evidence supporting the safety and immunogenicity of a novel live attenuated nasal B. pertussis vaccine in humans.
Bull, N. C. et al. Enhanced protection conferred by mucosal BCG vaccination associates with presence of antigen-specific lung tissue-resident PD-1+KLRG1−CD4+ T cells. Mucosal Immunol. 12, 555–564 (2019).
WHO. WHO coronavirus disease (COVID-19) dashboard. World Health Organization https://covid19.who.int/ (2021)
International Monetary Fund. World Economic Outlook Update June 2020 — A Crisis Like No Other, An Uncertain Recovery (International Monetary Fund, 2020).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Giurgea, L. T., Han, A. & Memoli, M. J. Universal coronavirus vaccines: the time to start is now. NPJ Vaccines 5, 43 (2020).
Troeger, C. et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18, 1211–1228 (2018).
WHO. Global task force on cholera control: overview of ending cholera — a global roadmap to 2030 (WHO, 2018).
Sharma, T. et al. Development of Hillchol®, a low-cost inactivated single strain Hikojima oral cholera vaccine. Vaccine 38, 7998–8009 (2020).
WHO. Cervical cancer. World Health Organization https://www.who.int/health-topics/cervical-cancer#tab=tab_1 (2021).
Cavarelli, M. & Scarlatti, G. HIV-1 infection: the role of the gastrointestinal tract. Am. J. Reprod. Immunol. 71, 537–542 (2014).
Jones, A. T. et al. HIV-1 vaccination by needle-free oral injection induces strong mucosal immunity and protects against SHIV challenge. Nat. Commun. 10, 798 (2019).
Russell, M. W., Moldoveanu, Z., Ogra, P. L. & Mestecky, J. Mucosal immunity in COVID-19: a neglected but critical aspect of SARS-CoV-2 infection. Front. Immunol. 11, 1–5 (2020).
Lycke, N. Recent progress in mucosal vaccine development: potential and limitations. Nat. Rev. Immunol. 12, 592–605 (2012).
Strugnell, R. A. & Wijburg, O. L. C. The role of secretory antibodies in infection immunity. Nat. Rev. Microbiol. 8, 656–667 (2010).
Perez-Lopez, A., Behnsen, J., Nuccio, S.-P. & Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 16, 135–148 (2016).
Ahmad, R., Sorrell, M. F., Batra, S. K., Dhawan, P. & Singh, A. B. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol. 10, 307–317 (2017).
LaMere, M. W. et al. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J. Virol. 85, 5027–5035 (2011).
Wang, N., Shang, J., Jiang, S. & Du, L. Subunit vaccines against emerging pathogenic human coronaviruses. Front. Microbiol. 11, 298 (2020).
Pardi, N., Hogan, M. J., Porter, F. W. & Weissman, D. mRNA vaccines — a new era in vaccinology. Nat. Rev. Drug Discov. 17, 261–279 (2018).
Heyningen, S. V. Cholera toxin: interaction of subunits with ganglioside GM1. Science 183, 656–657 (1974).
Antonio-Herrera, L. et al. The nontoxic cholera B subunit is a potent adjuvant for intradermal DC-targeted vaccination. Front. Immunol. 9, 2212 (2018).
Eriksson, K., Fredriksson, M., Nordström, I. & Holmgren, J. Cholera toxin and its B subunit promote dendritic cell vaccination with different influences on TH1 and TH2 development. Infect. Immun. 71, 1740–1747 (2003).
Qadri, F. et al. Safety and immunogenicity of the oral, inactivated, enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi children and infants: a double-blind, randomised, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 20, 208–219 (2020). This study shows that the inclusion of dmLT adjuvant in an oral ETEC vaccine enhances intestinal IgA responses in infants.
Akhtar, M. et al. Evaluation of the safety and immunogenicity of the oral inactivated multivalent enterotoxigenic Escherichia coli vaccine ETVAX in Bangladeshi adults in a double-blind, randomized, placebo-controlled phase I trial using electrochemiluminescence and ELISA assays for immunogenicity analyses. Vaccine 37, 5645–5656 (2019).
Engeroff, P. & Bachmann, M. F. The 5th Virus-Like Particle and Nano-Particle Vaccines (VLPNPV) conference. Expert Rev. Vaccines 18, 1–3 (2019).
Mohsen, M. O., Zha, L., Cabral-Miranda, G. & Bachmann, M. F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 34, 123–132 (2017).
Mörbe, U. M. et al. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 14, 793–802 (2021).
Macpherson, A. J., McCoy, K. D., Johansen, F.-E. & Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 1, 11–22 (2008).
Agace, W. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Lett. 128, 21–23 (2010).
van Splunter, M. et al. Oral cholera vaccination promotes homing of IgA+ memory B cells to the large intestine and the respiratory tract. Mucosal Immunol. 11, 1254–1264 (2018). This study shows that oral cholera vaccination (Dukoral) in humans leads to the induction of IgA+ memory B cells expressing homing markers for the airways and colon.
Helander, H. F. & Fändriks, L. Surface area of the digestive tract — revisited. Scand. J. Gastroenterol. 49, 681–689 (2014).
Caruso, R., Lo, B. C. & Núñez, G. Host–microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 20, 411–426 (2020).
Bain, C. C. et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 6, 498–510 (2013).
Esterházy, D. et al. Compartmentalized gut lymph node drainage dictates adaptive immune responses. Nature 569, 126–130 (2019).
Zhu, Q. et al. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 18, 1291–1296 (2012).
Mowat, A. M. I., Scott, C. L. & Bain, C. C. Barrier-tissue macrophages: functional adaptation to environmental challenges. Nat. Med. 23, 1258–1270 (2017).
Jakubzick, C. V., Randolph, G. J. & Henson, P. M. Monocyte differentiation and antigen-presenting functions. Nat. Rev. Immunol. 17, 349–362 (2017).
Mazzini, E., Massimiliano, L., Penna, G. & Rescigno, M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1+ macrophages to CD103+ dendritic cells. Immunity 40, 248–261 (2014).
Leonardi, I. et al. CX3CR1+, mononuclear phagocytes control immunity to intestinal fungi. Science 359, 232–236 (2018).
Esterházy, D. et al. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral Treg cells and tolerance. Nat. Immunol. 17, 545–555 (2016).
Koscsó, B. et al. Gut-resident CX3CR1hi macrophages induce tertiary lymphoid structures and IgA response in situ. Sci. Immunol. 5, eaax0062 (2020).
Granot, T. et al. Dendritic cells display subset and tissue-specific maturation dynamics over human life. Immunity 46, 504–515 (2017).
Bedford, J. G. et al. Unresponsiveness to inhaled antigen is governed by conventional dendritic cells and overridden during infection by monocytes. Sci. Immunol 5, eabb5439 (2020).
Wang, Z. et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci. Transl Med. 13, eabf1555 (2021).
Moor, K. et al. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature 544, 498–502 (2017). This study reports the mechanism by which vaccine-induced intestinal IgA can mediate protection against enteric bacterial infection.
Barría, M. I. et al. Localized mucosal response to intranasal live attenuated influenza vaccine in adults. J. Infect. Dis. 207, 115–124 (2013).
Kim, L. et al. Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI insight 3, 1–12 (2018).
Komban, R. J. et al. Activated Peyer′s patch B cells sample antigen directly from M cells in the subepithelial dome. Nat. Commun. 10, 1–15 (2019).
Parr, E. L. & Parr, M. B. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J. Virol. 72, 5137–5145 (1998).
Renegar, K. B., Small, P. A., Boykins, L. G. & Wright, P. F. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J. Immunol. 173, 1978–1986 (2004).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Schenkel, J. M. & Masopust, D. Tissue-resident memory T cells. Immunity 41, 886–897 (2014).
Bartolomé-Casado, R. et al. CD4+ T cells persist for years in the human small intestine and display a TH1 cytokine profile. Mucosal Immunol. 14, 402–410 (2021). This study shows that CD4+ TRM cells in the human intestine are long lived.
Slütter, B. et al. Dynamics of influenza-induced lung-resident memory T cells underlie waning heterosubtypic immunity. Sci. Immunol. 2, eaag2031 (2017).
Son, Y. M. et al. Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci. Immunol. 6, eabb6852 (2021).
Swarnalekha, N. et al. T resident helper cells promote humoral responses in the lung. Sci. Immunol 6, eabb6808 (2021). This study provides evidence that lung-resident T helper cells should be induced by vaccination to support local antibody responses to influenza virus.
Uddbäck, I. et al. Long-term maintenance of lung resident memory T cells is mediated by persistent antigen. Mucosal Immunol. 14, 92–99 (2021). This study shows that long-lived CD8+ TRM cell responses require airway vaccination and antigen persistence in the lungs.
Stolley, J. M. et al. Retrograde migration supplies resident memory T cells to lung-draining LN after influenza infection. J. Exp. Med. 217, e20192197 (2020).
Fonseca, R. et al. Developmental plasticity allows outside-in immune responses by resident memory T cells. Nat. Immunol. 21, 412–421 (2020).
Lindqvist, M., Persson, J., Thörn, K. & Harandi, A. M. The mucosal adjuvant effect of α-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J. Immunol. 182, 6435–6443 (2009).
Tan, H. X. et al. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal Immunol. 11, 994–1007 (2018).
Oh, J. E. et al. Migrant memory B cells secrete luminal antibody in the vagina. Nature 571, 122–126 (2019).
Logerot, S. et al. IL-7-adjuvanted vaginal vaccine elicits strong mucosal immune responses in non-human primates. Front. Immunol. 12, 1–17 (2021).
Panda, S. K. & Colonna, M. Innate lymphoid cells in mucosal immunity. Front. Immunol. 10, 1–13 (2019).
Sonnenberg, G. F. & Hepworth, M. R. Functional interactions between innate lymphoid cells and adaptive immunity. Nat. Rev. Immunol. 19, 599–613 (2019).
Klose, C. S. N. & Artis, D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 30, 475–491 (2020).
Kästele, V. et al. Intestinal-derived ILCs migrating in lymph increase IFNγ production in response to Salmonella typhimurium infection. Mucosal Immunol. 14, 717–727 (2021).
Meierovics, A., Yankelevich, W. J. C. & Cowley, S. C. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl Acad. Sci. USA 110, 3119–3128 (2013).
Toubal, A., Nel, I., Lotersztajn, S. & Lehuen, A. Mucosal-associated invariant T cells and disease. Nat. Rev. Immunol. 19, 643–657 (2019).
Trottein, F. & Paget, C. Natural killer T cells and mucosal-associated invariant T cells in lung infections. Front. Immunol. 9, 1–17 (2018).
McCarthy, N. E. & Eberl, M. Human γδ T-cell control of mucosal immunity and inflammation. Front. Immunol. 9, 985 (2018).
Clements, J. D. & Norton, E. B. The mucosal vaccine adjuvant LT(R192G/L211A) or dmLT. mSphere 3, 1–17 (2018).
Hammerschmidt, S. I. et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J. Clin. Invest. 121, 3051–3061 (2011).
Harro, C. et al. Live attenuated enterotoxigenic Escherichia coli (ETEC) vaccine with dmLT adjuvant protects human volunteers against virulent experimental ETEC challenge. Vaccine 37, 1978–1986 (2019).
Lebens, M. et al. Construction and preclinical evaluation of mmCT, a novel mutant cholera toxin adjuvant that can be efficiently produced in genetically manipulated Vibrio cholerae. Vaccine 34, 2121–2128 (2016).
Holmgren, J. et al. Preclinical immunogenicity and protective efficacy of an oral Helicobacter pylori inactivated whole cell vaccine and multiple mutant cholera toxin: a novel and non-toxic mucosal adjuvant. Vaccine 36, 6223–6230 (2018).
Ågren, L., Löwenadler, B. & Lycke, N. A novel concept in mucosal adjuvanticity: the CTA1-DD adjuvant is a B cell-targeted fusion protein that incorporates the enzymatically active cholera toxin A1 subunit. Immunol. Cell Biol. 76, 280–287 (1998).
Eriksson, A. M., Schön, K. M. & Lycke, N. Y. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J. Immunol. 173, 3310–3319 (2004).
Schussek, S. et al. The CTA1-DD adjuvant strongly potentiates follicular dendritic cell function and germinal center formation, which results in improved neonatal immunization. Mucosal Immunol. 13, 545–557 (2020).
Bernasconi, V. et al. A vaccine combination of lipid nanoparticles and a cholera toxin adjuvant derivative greatly improves lung protection against influenza virus infection. Mucosal Immunol. 14, 523–536 (2021).
Mutsch, M. et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 350, 896–903 (2004).
Lewis, D. J. M. et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE 4, 1–5 (2009).
Eickhoff, C. S., Blazevic, A., Killoran, E. A., Morris, M. S. & Hoft, D. F. Induction of mycobacterial protective immunity by sublingual BCG vaccination. Vaccine 37, 5364–5370 (2019).
Ottsjö, L. S., Flach, C. F., Raghavan, S., Holmgren, J. & Clements, J. A double mutant heat-labile toxin from Escherichia coli, LT(R192G/L211A), is an effective mucosal adjuvant for vaccination against Helicobacter pylori infection. Infect. Immun. 81, 1532–1540 (2013).
Bernstein, D. I. et al. A phase 1 dose escalating study of double mutant heat-labile toxin LTR192G/L211A (dmLT) from enterotoxigenic Escherichia coli (ETEC) by sublingual or oral immunization. Vaccine 37, 602–611 (2019).
Pan, S. C. et al. A double-blind, randomized controlled trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with the adjuvant LTh(αK): a phase II study. Vaccine 38, 1048–1056 (2020).
Pan, S. C. et al. A randomized, double-blind, controlled clinical trial to evaluate the safety and immunogenicity of an intranasally administered trivalent inactivated influenza vaccine with adjuvant LTh(αK): a phase I study. Vaccine 37, 1994–2003 (2019).
Longet, S. et al. An oral α-galactosylceramide adjuvanted Helicobacter pylori vaccine induces protective IL-1R- and IL-17R-dependent TH1 responses. NPJ Vaccines 4, 45 (2019).
Davitt, C. J. H. et al. A novel adjuvanted capsule based strategy for oral vaccination against infectious diarrhoeal pathogens. J. Control. Rel. 233, 162–173 (2016).
Davitt, C. J. H. et al. α-Galactosylceramide enhances mucosal immunity to oral whole-cell cholera vaccines. Mucosal Immunol. 12, 1055–1064 (2019).
Moran, H. B. T., Turley, J. L., Andersson, M. & Lavelle, E. C. Immunomodulatory properties of chitosan polymers. Biomaterials 184, 1–9 (2018).
Carroll, E. C. et al. The vaccine adjuvant chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity 44, 597–608 (2016).
Van Dis, E. et al. STING-activating adjuvants elicit a TH17 immune response and protect against Mycobacterium tuberculosis infection. Cell Rep. 23, 1435–1447 (2018).
Ebensen, T. et al. Mucosal administration of cycle-di-nucleotide-adjuvanted virosomes efficiently induces protection against influenza H5N1 in mice. Front. Immunol. 8, 1–15 (2017).
Madhun, A. S. et al. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional TH1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 29, 4973–4982 (2011).
Mansouri, S. et al. Immature lung TNFR2− conventional DC 2 subpopulation activates moDCs to promote cyclic di-GMP mucosal adjuvant responses in vivo. Mucosal Immunol. 12, 277–289 (2019).
Bielinska, A. U. et al. Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect. Immun. 75, 4020–4029 (2007).
Ahmed, M. et al. A novel nanoemulsion vaccine induces mucosal interleukin-17 responses and confers protection upon Mycobacterium tuberculosis challenge in mice. Vaccine 35, 4983–4989 (2017).
Kimoto, T., Kim, H., Sakai, S., Takahashi, E. & Kido, H. Oral vaccination with influenza hemagglutinin combined with human pulmonary surfactant-mimicking synthetic adjuvant SF-10 induces efficient local and systemic immunity compared with nasal and subcutaneous vaccination and provides protective immunity in mice. Vaccine 37, 612–622 (2019).
Dolgin, E. How COVID unlocked the power of RNA vaccines. Nature 589, 189–191 (2021).
Ferber, S., Gonzalez, R. J., Cryer, A. M., von Andrian, U. H. & Artzi, N. Immunology-guided biomaterial design for mucosal cancer vaccines. Adv. Mater. 32, e1903847 (2020).
Lim, M. et al. Engineered nanodelivery systems to improve DNA vaccine technologies. Pharmaceutics 12, 1–29 (2020).
O’Driscoll, C. M., Bernkop-Schnürch, A., Friedl, J. D., Préat, V. & Jannin, V. Oral delivery of non-viral nucleic acid-based therapeutics—do we have the guts for this? Eur. J. Pharm. Sci. 133, 190–204 (2019).
Ball, R. L., Bajaj, P. & Whitehead, K. A. Oral delivery of siRNA lipid nanoparticles: fate in the GI tract. Sci. Rep. 8, 1–12 (2018).
Hajam, I. A., Senevirathne, A., Hewawaduge, C., Kim, J. & Lee, J. H. Intranasally administered protein coated chitosan nanoparticles encapsulating influenza H9N2 HA2 and M2e mRNA molecules elicit protective immunity against avian influenza viruses in chickens. Vet. Res. 51, 37 (2020).
Liebowitz, D., Lindbloom, J. D., Brandl, J. R., Garg, S. J. & Tucker, S. N. High titre neutralising antibodies to influenza after oral tablet immunisation: a phase 1, randomised, placebo-controlled trial. Lancet Infect. Dis. 15, 1041–1048 (2015).
Liebowitz, D. et al. Efficacy, immunogenicity, and safety of an oral influenza vaccine: a placebo-controlled and active-controlled phase 2 human challenge study. Lancet Infect. Dis. 20, 435–444 (2020). This paper reports that an oral tablet-based influenza vaccine induces protective immunity against viral shedding in a human influenza virus challenge model.
Joyce, C. et al. Orally administered adenoviral-based vaccine induces respiratory mucosal memory and protection against RSV infection in cotton rats. Vaccine 36, 4265–4277 (2018).
Magnusson, S. E. et al. Matrix-MTM adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol. Res. 66, 224–233 (2018).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 1–16 (2020).
Frey, S. E. et al. A phase I, dose-escalation trial in adults of three recombinant attenuated Salmonella typhi vaccine vectors producing Streptococcus pneumoniae surface protein antigen PspA. Vaccine 31, 4874–4880 (2013).
Xiang, Z. Q. et al. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J. Virol. 79, 3888–3888 (2005).
Cole, M. E. et al. Pre-existing influenza-specific nasal IgA or nasal viral infection does not affect live attenuated influenza vaccine immunogenicity in children. Clin. Exp. Immunol. 204, 125–133 (2021). This report shows that pre-existing antigen-specific nasal IgA does not compromise live attenuated influenza vaccine immunogenicity in children.
JanssenMD. Janssen COVID-19 vaccine immunity to the Ad26 vector. JanssenMD https://www.janssenmd.com/janssen-covid19-vaccine/clinical-use/janssen-covid19-vaccine-preexisting-immunity-to-the-ad26-vector (2021).
Church, J. A., Parker, E. P., Kirkpatrick, B. D., Grassly, N. C. & Prendergast, A. J. Interventions to improve oral vaccine performance: a systematic review and meta-analysis. Lancet Infect. Dis. 19, 203–214 (2019).
Zimmermann, P. & Curtis, N. The influence of probiotics on vaccine responses — a systematic review. Vaccine 36, 207–213 (2018).
Frederick, D. R. et al. Adjuvant selection regulates gut migration and phenotypic diversity of antigen-specific CD4+ T cells following parenteral immunization. Mucosal Immunol. 11, 549–561 (2018). This study shows that intradermal injection of the adjuvant dmLT promoted intestinal homing of antigen-specific CD4+ T cells.
McEntee, C. P. et al. Type I IFN signalling is required for cationic adjuvant formulation (CAF)01-induced cellular immunity and mucosal priming. Vaccine 38, 635–643 (2020).
Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 27, 125–135 (2021). This paper shows human tonsil organoids can be used to assess responses to vaccines and adjuvants.
Krämer, B. et al. Compartment-specific distribution of human intestinal innate lymphoid cells is altered in HIV patients under effective therapy. PLoS Pathog. 13, 1–24 (2017).
Marquardt, N. et al. Human lung natural killer cells are predominantly comprised of highly differentiated hypofunctional CD69−CD56dim cells. J. Allergy Clin. Immunol. 139, 1321–1330.e4 (2017).
De Grove, K. C. et al. Characterization and quantification of innate lymphoid cell subsets in human lung. PLoS ONE 11, 1–12 (2016).
Golebski, K. et al. IL-1β, IL-23, and TGF-β drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat. Commun. 10, 1–15 (2019).
Deusch, K. et al. A major fraction of human intraepithelial lymphocytes simultaneously expresses the γ/δ T cell receptor, the CD8 accessory molecule and preferentially uses the Vδ1 gene segment. Eur. J. Immunol. 21, 1053–1059 (1991).
Trejdosiewicz, L. K. et al. γδ T cell receptor-positive cells of the human gastrointestinal mucosa: occurrence and V region gene expression in Heliobacter pylori-associated gastritis, coeliac disease and inflammatory bowel disease. Clin. Exp. Immunol. 84, 440–444 (1991).
Fukushima, K., Masuda, T., Ohtani, H. & Nagura, H. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor γδ in inflammatory bowel disease. Gastroenterology 101, 670–678 (1991).
Ullrich, R., Schieferdecker, H. L., Ziegler, K., Riecken, E. O. & Zeitz, M. γδ T cells in the human intestine express surface markers of activation and are preferentially located in the epithelium. Cell. Immunol. 128, 619–627 (1990).
Spencer, J., Isaacson, P. G., Diss, T. C. & Macdonald, T. T. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor γ/δ heterodimer in human intestinal intraepithelial lymphocytes. Eur. J. Immunol. 19, 1335–1338 (1989).
Jarry, A., Cerf-bensussan, N., Brousse, N., Selz, F. & Guy-grand, D. Subsets of CD3+ (T cell receptor α/β or γ/δ) and CD3− lymphocytes isolated from normal human gut epithelium display phenotypical features different from their counterparts in peripheral blood. Eur. J. Immunol. 20, 1097–1103 (1990).
Zeissig, S., Kaser, A., Dougan, S. K., Nieuwenhuis, E. E. S. & Blumberg, R. S. Role of NKT cells in the digestive system. III. Role of NKT cells in intestinal immunity. Am. J. Physiol. Gastrointest. Liver Physiol. 293, 1101–1105 (2007).
Hama, I. et al. Different distribution of mucosal-associated invariant T cells within the human cecum and colon. Cent. Eur. J. Immunol. 44, 75–83 (2019).
Dusseaux, M. et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011).
Tominaga, K. et al. Possible involvement of mucosal-associated invariant T cells in the progression of inflammatory bowel diseases. Biomed. Res. 38, 111–121 (2017).
Gold, M. C. et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 8, 1–14 (2010).
Holmgren, J., Lonnroth, I. & Svennerholm, L. Tissue receptor for cholera exotoxin: postulated structure from studies with G(M1) ganglioside and related glycolipids. Infect. Immun. 8, 208–214 (1973).
Sethi, A. et al. Cell type and receptor identity regulate cholera toxin subunit B (CTB) internalization. Interface Focus 9, 20180076 (2019).
Wands, A. M. et al. Fucosylation and protein glycosylation create functional receptors for cholera toxin. eLife 4, 1–38 (2015).
Gustafsson, T. et al. Direct interaction between cholera toxin and dendritic cells is required for oral adjuvant activity. Eur. J. Immunol. 43, 1779–1788 (2013).
Lycke, N. & Lebrero-Fernández, C. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr. Opin. Pharmacol. 41, 42–51 (2018).
Smits, H. H. et al. Cholera toxin B suppresses allergic inflammation through induction of secretory IgA. Mucosal Immunol. 2, 331–339 (2009).
Sun, J.-B., Raghavan, S., Sjöling, Å., Lundin, S. & Holmgren, J. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3−CD25−CD4+ regulatory T cells. J. Immunol. 177, 7634–7644 (2006).
Baldauf, K. J. et al. Oral administration of a recombinant cholera toxin B subunit promotes mucosal healing in the colon. Mucosal Immunol. 10, 887–900 (2017).
Jertborn, M., Nordström, I., Kilander, A., Czerkinsky, C. & Holmgren, J. Local and systemic immune responses to rectal administration of recombinant cholera toxin B subunit in humans. Infect. Immun. 69, 4125–4128 (2001).
Bergquist, C., Johansson, E. L., Lagergård, T., Holmgren, J. & Rudin, A. Intranasal vaccination of humans with recombinant cholera toxin B subunit induces systemic and local antibody responses in the upper respiratory tract and the vagina. Infect. Immun. 65, 2676–2684 (1997).
Harris, J. B. Cholera: immunity and prospects in vaccine development. J. Infect. Dis. 218, S141–S146 (2018).
Clemens, J. D. et al. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158, 372–377 (1988).
Scerpella, E. G. et al. Safety, immunogenicity, and protective efficacy of the whole-cell/recombinant B subunit (WC/rBS) oral cholera vaccine against travelers’ diarrhea. J. Travel. Med. 2, 22–27 (1995).
Holmgren, J. et al. Correlates of protection for enteric vaccines. Vaccine 35, 3355–3363 (2017).
Plotkin, S. A. Updates on immunologic correlates of vaccine-induced protection. Vaccine 38, 2250–2257 (2020).
Tomic, A., Tomic, I., Dekker, C. L., Maecker, H. T. & Davis, M. M. The FluPRINT dataset, a multidimensional analysis of the influenza vaccine imprint on the immune system. Sci. Data 6, 214 (2019).
Carlsson, B., Zaman, S., Mellander, L., Jalil, F. & Hanson, L. A. Secretory and serum immunoglobulin class-specific antibodies to poliovirus after vaccination. J. Infect. Dis. 152, 1238–1244 (1985).
Ainai, A. et al. Intranasal vaccination with an inactivated whole influenza virus vaccine induces strong antibody responses in serum and nasal mucus of healthy adults. Hum. Vaccines Immunother. 9, 1962–1970 (2013).
Akhtar, M. et al. Kinetics of antibody-secreting cell and fecal IgA responses after oral cholera vaccination in different age groups in a cholera endemic country. Vaccine 35, 321–328 (2017).
Aase, A. et al. Salivary IgA from the sublingual compartment as a novel noninvasive proxy for intestinal immune induction. Mucosal Immunol. 9, 884–893 (2016).
Saletti, G., Çuburu, N., Yang, J. S., Dey, A. & Czerkinsky, C. Enzyme-linked immunospot assays for direct ex vivo measurement of vaccine-induced human humoral immune responses in blood. Nat. Protoc. 8, 1073–1087 (2013).
Chang, H. S. & Sack, D. A. Development of a novel in vitro assay (ALS assay) for evaluation of vaccine-induced antibody secretion from circulating mucosal lymphocytes. Clin. Diagn. Lab. Immunol. 8, 482–488 (2001).
Chakraborty, S. et al. Characterization of mucosal immune responses to enterotoxigenic Escherichia coli vaccine antigens in a human challenge model: response profiles after primary infection and homologous rechallenge with strain H10407. Clin. Vaccine Immunol. 23, 55–64 (2016).
Uddin, T. et al. Mucosal immunologic responses in cholera patients in Bangladesh. Clin. Vaccine Immunol. 18, 506–512 (2011).
Quiding, M. et al. Intestinal immune responses in humans. Oral cholera vaccination induces strong intestinal antibody responses and interferon-γ production and evokes local immunological memory. J. Clin. Invest. 88, 143–148 (1991).
Åhrén, C., Jertborn, M. & Svennerholm, A. M. Intestinal immune responses to an inactivated oral enterotoxigenic Escherichia coli vaccine and associated immunoglobulin a responses in blood. Infect. Immun. 66, 3311–3316 (1998).
Mottram, L., Lundgren, A., Svennerholm, A. M. & Leach, S. A systems biology approach identifies B cell maturation antigen (BCMA) as a biomarker reflecting oral vaccine induced IgA antibody responses in humans. Front. Immunol. 12, 647873 (2021).
WHO. Cancer Tomorrow (International Agency for Research on Cancer, 2020).
Nizard, M. et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat. Commun. 8, 15221 (2017). This study shows that mucosal vaccine-induced TRM cells enhance antitumour immunity in mice.
Blanc, C. et al. Targeting resident memory T cells for cancer immunotherapy. Front. Immunol. 9, 1722 (2018).
Sandoval, F. et al. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci. Transl Med. 5, 172ra20 (2013).
Sun, Y. Y. et al. Local HPV recombinant vaccinia boost following priming with an HPV DNA vaccine enhances local HPV-specific CD8+ T-cell-mediated tumor control in the genital tract. Clin. Cancer Res. 22, 657–669 (2016).
Hildner, K. et al. Batf3 deficiency reveals a critical role for CD8+ dendritic cells in cytotoxic T cell immunity. Science 322, 1097–1100 (2008).
Salmon, H. et al. Expansion and activation of CD103+ dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity 44, 924–938 (2016).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8 + T cell responses through CD8α + dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Roberts, E. W. et al. Critical role for CD103+/CD141+ dendritic cells bearing CCR7 for tumor antigen trafficking and priming of T cell immunity in melanoma. Cancer Cell 30, 324–336 (2016).
Williford, J. M. et al. Recruitment of CD103+ dendritic cells via tumor-targeted chemokine delivery enhances efficacy of checkpoint inhibitor immunotherapy. Sci. Adv. 5, 1–16 (2019).
Parkhurst, M. R. et al. Unique neoantigens arise from somatic mutations in patients with gastrointestinal cancers. Cancer Discov. 9, 1022–1035 (2019).
Jiang, T. et al. Heterogeneity of neoantigen landscape between primary lesions and their matched metastases in lung cancer. Transl Lung Cancer Res. 9, 246–256 (2020).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Sagiv-Barfi, I. et al. Eradication of spontaneous malignancy by local immunotherapy. Sci. Transl Med. 10, eaan4488 (2018).
Norton, E. B., Lawson, L. B., Freytag, L. C. & Clements, J. D. Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin. Vaccine Immunol. 18, 546–551 (2011).
Terrinoni, M., Holmgren, J., Lebens, M. & Larena, M. Requirement for cyclic AMP/protein kinase A-dependent canonical NFκB signaling in the adjusvant action of cholera toxin and its non-toxic derivative mmCT. Front. Immunol. 10, 269 (2019).
Valli, E., Baudier, R. L., Harriett, A. J. & Norton, E. B. LTA1 and dmLT enterotoxin-based proteins activate antigen-presenting cells independent of PKA and despite distinct cell entry mechanisms. PLoS ONE 15, 1–21 (2020).
Larena, M., Holmgren, J., Lebens, M., Terrinoni, M. & Lundgren, A. Cholera toxin, and the related nontoxic adjuvants mmCT and dmLT, promote human TH17 responses via cyclic AMP–protein kinase A and inflammasome-dependent IL-1 signaling. J. Immunol. 194, 3829–3839 (2015).
Acknowledgements
The authors thank N. Muñoz-Wolf for critical reading of the manuscript. The mucosal vaccine research from the Lavelle laboratory has been funded by Science Foundation Ireland (SFI) (Grant numbers 12/IA/1421, 19/FFP/6484, SFI/12/RC/2278_2, 20/SPP/3685), the Irish Research Council and the European Union FP7 programme (FP7-SME-2012-1).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Immunology thanks J. Holmgren and J. Mestecky for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Chitosan
-
A cationic polymer derived from chitin with mucosal adjuvant properties. In addition to its mucoadhesive attributes, chitosan promotes adaptive immunity through activation of the cGAS–STING and NLRP3 inflammasome pathways.
- Enchained growth
-
A process where high-affinity IgA specific for surface antigens cross-links bacteria, preventing daughter cell separation after division and contributing to clearance of mucosal pathogens.
- Microfold (M) cells
-
Specialized antigen sampling epithelial cells generally found in the follicle-associated epithelium overlying organized mucosal lymphoid tissue.
- Polymersomes
-
Hollow vesicles generally comprising block copolymers that can incorporate vaccine antigens and adjuvants in the aqueous phase.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lavelle, E.C., Ward, R.W. Mucosal vaccines — fortifying the frontiers. Nat Rev Immunol 22, 236–250 (2022). https://doi.org/10.1038/s41577-021-00583-2
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-021-00583-2
This article is cited by
-
Intranasal S-2P and lentinan formulation confers broad protection against SARS-CoV-2 VOCs via IFN-γ-dominant mechanisms
npj Vaccines (2026)
-
Transfer of breast milk IgA to infants after oral bivalent norovirus vaccination of post-partum women
npj Vaccines (2026)
-
Core genome analysis reveals novel drug and vaccine targets in multidrug-resistant Citrobacter koseri
Molecular Genetics and Genomics (2026)
-
Engineered mucus-tethering bispecific nanobodies enhance mucosal immunity against respiratory pathogens
Nature Nanotechnology (2026)
-
Development of universal influenza vaccines: strategies for broadly cross-reactive influenza vaccine responses
Animal Diseases (2025)