Abstract
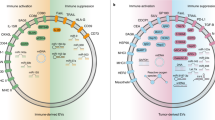
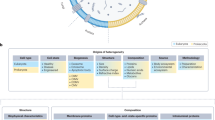
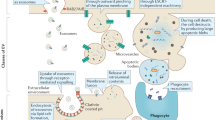
The twenty-first century has witnessed major developments in the field of extracellular vesicle (EV) research, including significant steps towards defining standard criteria for the separation and detection of EVs. The recent recognition that EVs have the potential to function as biomarkers or as therapeutic tools has attracted even greater attention to their study. With this progress in mind, an updated comprehensive overview of the roles of EVs in the immune system is timely. This Review summarizes the roles of EVs in basic processes of innate and adaptive immunity, including inflammation, antigen presentation, and the development and activation of B cells and T cells. It also highlights key progress related to deciphering the roles of EVs in antimicrobial defence and in allergic, autoimmune and antitumour immune responses. It ends with a focus on the relevance of EVs to immunotherapy and vaccination, drawing attention to ongoing or recently completed clinical trials that aim to harness the therapeutic potential of EVs.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Couch, Y. et al. A brief history of nearly EV-erything – the rise and rise of extracellular vesicles. J. Extracell. Vesicles 10, e12144 (2021).
Théry, C. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 (2018).
György, B. et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68, 2667–2688 (2011).
Toyofuku, M., Nomura, N. & Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 17, 13–24 (2019).
Tulkens, J., De Wever, O. & Hendrix, A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nat. Protoc. 15, 40–67 (2020).
Verweij, F. J. et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat. Methods 18, 1013–1026 (2021).
van Niel, G. et al. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 23, 369–382 (2022).
Mathieu, M., Martin-Jaular, L., Lavieu, G. & Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 (2019).
Jeppesen, D. K. et al. Reassessment of exosome composition. Cell 177, 428–445.e18 (2019).
Barman, B. et al. VAP-A and its binding partner CERT drive biogenesis of RNA-containing extracellular vesicles at ER membrane contact sites. Dev. Cell 57, 974–994.e8 (2022).
Arya, S. B., Chen, S., Jordan-Javed, F. & Parent, C. A. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat. Cell Biol. https://doi.org/10.1038/s41556-022-00934-8 (2022).
Valcz, G. et al. En bloc release of MVB-like small extracellular vesicle clusters by colorectal carcinoma cells. J. Extracell. Vesicles 8, 1596668 (2019).
Kerviel, A., Zhang, M. & Altan-Bonnet, N. A new infectious unit: extracellular vesicles carrying virus populations. Annu. Rev. Cell Dev. Biol. 37, 171–197 (2021).
Wu, Q., Zhang, H., Sun, S., Wang, L. & Sun, S. Extracellular vesicles and immunogenic stress in cancer. Cell Death. Dis. 12, 894 (2021).
Zhang, H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 20, 332–343 (2018).
Zhang, Q. et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 23, 1240–1254 (2021).
Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4, 27066 (2015).
Boilard, E. Extracellular vesicles and their content in bioactive lipid mediators: more than a sack of microRNA. J. Lipid Res. 59, 2037–2046 (2018).
Esser, J. U. et al. Exosomes from human macrophages and dendritic cells contain enzymes for leukotriene biosynthesis and promote granulocyte migration. J. Allergy Clin. Immunol. 126, 1032–1040 (2010).
Rossaint, J. et al. Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response. Nat. Commun. 7, 13464 (2016).
Duchez, A. C. et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc. Natl Acad. Sci. USA 112, E3564–E3573 (2015).
Buzás, E. I., Tóth, E. Á., Sódar, B. W. & Szabó-Taylor, K. É. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 40, 453–464 (2018).
Sung, B. H., Parent, C. A. & Weaver, A. M. Extracellular vesicles: critical players during cell migration. Dev. Cell 56, 1861–1874 (2021).
Burgelman, M., Vandendriessche, C. & Vandenbroucke, R. E. Extracellular vesicles: a double-edged sword in sepsis. Pharmaceuticals 14, 829 (2021).
Tu, F. et al. Novel role of endothelial derived exosomal HSPA12B in regulating macrophage inflammatory responses in polymicrobial sepsis. Front. Immunol. 11, 825 (2020).
Shlomovitz, I. et al. Proteomic analysis of necroptotic extracellular vesicles. Cell Death Dis. 12, 1059 (2021).
Wozniak, A. L. et al. The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J. Cell Biol. 219, e201912074 (2020).
Budden, C. F. et al. Inflammasome-induced extracellular vesicles harbour distinct RNA signatures and alter bystander macrophage responses. J. Extracell. Vesicles 10, e12127 (2021).
Fendl, B. et al. Extracellular vesicles are associated with C-reactive protein in sepsis. Sci. Rep. 11, 6996 (2021).
Capello, M. et al. Exosomes harbor B cell targets in pancreatic adenocarcinoma and exert decoy function against complement-mediated cytotoxicity. Nat. Commun. 10, 254 (2019).
Skogberg, G. et al. Human thymic epithelial primary cells produce exosomes carrying tissue-restricted antigens. Immunol. Cell Biol. 93, 727–734 (2015).
Lundberg, V. et al. Thymic exosomes promote the final maturation of thymocytes. Sci. Rep. 6, 36479 (2016).
Ayre, D. C. et al. Dynamic regulation of CD24 expression and release of CD24-containing microvesicles in immature B cells in response to CD24 engagement. Immunology 146, 217–233 (2015).
Phan, H. D. et al. CD24 and IgM stimulation of B cells triggers transfer of functional B cell receptor to B cell recipients via extracellular vesicles. J. Immunol. 12, ji2100025 (2021).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996). This hallmark paper reported the release of B cell-derived EVs with functional pMHC complexes on their surface.
Skokos, D. et al. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. Immunology 170, 3037–3045 (2003).
Utsugi-Kobukai, S., Fujimaki, H., Hotta, C., Nakazawa, M. & Minami, M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol. Lett. 89, 125–131 (2003).
Qazi, K. R., Gehrmann, U., Jordö, E. D., Karlsson, M. C. I. & Gabrielsson, S. Antigen-loaded exosomes alone induce Th1-type memory through a B-cell-dependent mechanism. Blood 113, 2673–2683 (2009).
Vincent-Schneider, H. et al. Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int. Immunol. 14, 713–722 (2002).
Théry, C. et al. Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat. Immunol. 3, 1156–1162 (2002).
André, F. et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 172, 2126–2136 (2004).
Balan, S. & Bhardwaj, N. Cross-presentation of tumor antigens is ruled by synaptic transfer of vesicles among dendritic cell subsets. Cancer Cell 37, 751–753 (2020).
Fu, C. et al. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl Acad. Sci. USA 117, 23730–23741 (2020).
Marcoux, G. et al. Platelet EVs contain an active proteasome involved in protein processing for antigen presentation via MHC-I molecules. Blood 138, 2607–2620 (2021). This study shows the cross-presentation capacity of platelet-derived EVs, which contain a functional proteasome.
Bálint, Š. et al. Supramolecular attack particles are autonomous killing entities released from cytotoxic T cells. Science 368, 897–901 (2020).
Mittelbrunn, M. et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2, 282 (2011).
Saliba, D. G. et al. Composition and structure of synaptic ectosomes exporting antigen receptor linked to functional CD40 ligand from helper T. Elife 8, e47528 (2019).
Choudhuri, K. et al. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature 507, 118–123 (2014).
Céspedes, P. F. et al. T-cell trans-synaptic vesicles are distinct and carry greater effector content than constitutive extracellular vesicles. Nat. Commun. 13, 3460 (2022).
Kim, H. R. et al. T cell microvilli constitute immunological synaptosomes that carry messages to antigen-presenting cells. Nat. Commun. 9, 3630 (2018).
Craxton, A., Draves, K. E. & Clark, E. A. Bim regulates BCR-induced entry of B cells into the cell cycle. Eur. J. Immunol. 37, 2715–2722 (2007).
Fischer, S. F. et al. Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood 110, 3978–3984 (2007).
Setz, C. S. et al. Pten controls B-cell responsiveness and germinal center reaction by regulating the expression of IgD BCR. EMBO J. 38, e100249 (2019).
Fernández-Messina, L. et al. Transfer of extracellular vesicle-microRNA controls germinal center reaction and antibody production. EMBO Rep. 21, e48925 (2020). This study provides evidence for an important role of EVs in the germinal centre reaction.
Denzer, K. et al. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J. Immunol. 165, 1259–1265 (2000).
Tew, J. G. et al. Follicular dendritic cells and presentation of antigen and costimulatory signals to B cells. Immunol. Rev. 156, 39–52 (1997).
Hofmann, L. et al. The emerging role of exosomes in diagnosis, prognosis, and therapy in head and neck cancer. Int. J. Mol. Sci. 21, 4072 (2020).
Smyth, L. A. et al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur. J. Immunol. 43, 2430–2440 (2013).
Okoye, I. S. et al. MicroRNA-containing T regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity 41, 89–103 (2014).
Tung, S. L. et al. Regulatory T cell derived extracellular vesicles modify dendritic cell function. Sci. Rep. 8, 6065 (2018).
Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018).
Xie, M. et al. Immunoregulatory effects of stem cell-derived extracellular vesicles on immune cells. Front. Immunol. 11, 13 (2020).
Hedlund, M. et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J. Immunol. 183, 340–351 (2009).
Stenqvist, A. C. et al. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol. 191, 5515–5523 (2013).
Kovács, Á. F. et al. Unravelling the role of trophoblastic-derived extracellular vesicles in regulatory T cell differentiation. Int. J. Mol. Sci. 20, 3457 (2019).
Brown, L., Wolf, J. M., Prados-Rosales, R. & Casadewall, A. Through the wall: extracellular vesicles in Gram-positive bacteria, mycobacteria and fungi. Nat. Rev. Microbiol. 13, 620–630 (2015).
Johnston, E. L., Heras, B., Kufer, T. A. & Kaparakis-Liaskos, M. Detection of bacterial membrane vesicles by NOD-like receptors. Int. J. Mol. Sci. 22, 1005 (2021).
McMillan, H. M. & Kuehn, M. J. The extracellular vesicle generation paradox: a bacterial point of view. EMBO J. 40, e108174 (2021).
Schorey, J. S., Cheng, Y., Singh, P. P. & Smith, V. L. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 16, 24–43 (2015).
Kuipers, M. E., Hokke, C. H., Smits, H. H. & Nolte’t Hoen, E. N. M. Pathogen-derived extracellular vesicle-associated molecules that affect the host immune system: an overview. Front. Microbiol. 9, 2182 (2018).
Bitto, N. J. et al. Staphylococcus aureus membrane vesicles contain immunostimulatory DNA, RNA and peptidoglycan that activate innate immune receptors and induce autophagy. J. Extracell. Vesicles 10, e12080 (2021).
Sisquella, X. et al. Malaria parasite DNA-harbouring vesicles activate cytosolic immune sensors. Nat. Commun. 8, 1985 (2017).
Erttmann, S. F. et al. The gut microbiota prime systemic antiviral immunity via the cGAS-STING-IFN-I axis. Immunity 55, 847–861.e10 (2020).
van der Grein, S. G., Defourny, K. A. Y., Slot, E. F. J. & Nolte’t Hoen, E. N. M. Intricate relationships between naked viruses and extracellular vesicles in the crosstalk between pathogen and host. Semin. Immunopathol. 40, 491–504 (2018).
Pilzer, D., Gasser, O., Moskovich, O., Schifferli, J. A. & Fishelson, Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springe. Semin. Immunopathol. 27, 375–387 (2005).
Stein, J. M. & Luzio, J. P. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem. J. 274, 381–386 (1991).
Roszkowiak, J. et al. Interspecies outer membrane vesicles (OMVs) modulate the sensitivity of pathogenic bacteria and pathogenic yeasts to cationic peptides and serum complement. Int. J. Mol. Sci. 20, 5577 (2019).
Manning, A. J. & Kuehn, M. J. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol. 11, 258 (2011).
Marki, A. & Ley, K. The expanding family of neutrophil-derived extracellular vesicles. Immunol. Rev. https://doi.org/10.1111/imr.13103 (2022).
Kolonics, F., Szeifert, V., Tímár, C. I., Ligeti, E. & Lőrincz, Á. M. The functional heterogeneity of neutrophil-derived extracellular vesicles reflects the status of the parent cell. Cells 9, 2718 (2020).
Szeifert, V. et al. Mac-1 receptor clustering initiates production of pro-inflammatory, antibacterial extracellular vesicles from neutrophils. Front. Immunol. 12, 671995 (2021).
Timár, C. I. et al. Antibacterial effect of microvesicles released from human neutrophilic granulocytes. Blood 121, 510–518 (2013).
Amjadi, M. F., Avner, B. S., Greenlee-Wacker, M. C., Horswill, A. R. & Nauseef, W. M. Neutrophil-derived extracellular vesicles modulate the phenotype of naive human neutrophils. J. Leukoc. Biol. 110, 917–925 (2021).
Yipp, B. G. et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 (2012).
Marki, A. et al. Elongated neutrophil-derived structures are blood-borne microparticles formed by rolling neutrophils during sepsis. J. Exp. Med. 218, e20200551 (2021).
Prado, N. et al. Pollensomes as natural vehicles for pollen allergens. J. Immunol. 195, 445–449 (2015).
Vallhov, H. et al. Dendritic cell-derived exosomes carry the major cat allergen Fel d 1 and induce an allergic immune response. Allergy 70, 1651–1655 (2015).
Fang, S. B. et al. Plasma EVs display antigen-presenting characteristics in patients with allergic rhinitis and promote differentiation of Th2 cells. Front. Immunol. 12, 710372 (2021).
Admyre, C. et al. B cell-derived exosomes can present allergen peptides and activate allergen-specific T cells to proliferate and produce TH2-like cytokines. J. Allergy Clin. Immunol. 120, 1418–1424 (2007).
Katz-Kiriakos, E. et al. Epithelial IL-33 appropriates exosome trafficking for secretion in chronic airway disease. JCI Insight 6, e136166 (2021).
Zhang, M. et al. Epithelial exosomal contactin-1 promotes monocyte-derived dendritic cell-dominant T-cell responses in asthma. J. Allergy Clin. Immunol. 148, 1545–1558 (2021).
Molfetta, R. et al. Immune complexes exposed on mast cell-derived nanovesicles amplify allergic inflammation. Allergy 75, 1260–1263 (2020).
Li, F. et al. Mast cell-derived exosomes promote Th2 cell differentiation via OX40L-OX40 ligation. J. Immunol. Res. 2016, 3623898 (2016).
Buzas, E. I., György, B., Nagy, G., Falus, A. & Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 10, 356–364 (2014).
Lu, M. et al. The role of extracellular vesicles in the pathogenesis and treatment of autoimmune disorders. Front. Immunol. 12, 566299 (2021).
Li, Y. et al. Plasma-derived DNA containing-extracellular vesicles induce STING-mediated proinflammatory responses in dermatomyositis. Theranostics 115, 7144–7158 (2021).
Hartl, J. et al. Autoantibody-mediated impairment of DNASE1L3 activity in sporadic systemic lupus erythematosus. J. Exp. Med. 218, e20201138 (2021).
Busatto, S., Morad, G., Guo, P. & Moses, M. A. The role of extracellular vesicles in the physiological and pathological regulation of the blood-brain barrier. FASEB Bioadv. 3, 665–675 (2021).
Ma, Y., Zhou, Y., Zou, S. S., Sun, Y. & Chen, X. F. Exosomes released from Sertoli cells contribute to the survival of Leydig cells through CCL20 in rats. Mol. Hum. Reprod. 28, gaac002 (2022).
Marino, J. et al. Donor exosomes rather than passenger leukocytes initiate alloreactive T cell responses after transplantation. Sci. Immunol. 1, aaf8759 (2016).
Liu, Q. et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response. J. Clin. Invest. 126, 2805–2820 (2016).
Morelli, A. E., Bracamonte-Baran, W. & Burlingham, W. J. Donor-derived exosomes: the trick behind the semidirect pathway of allorecognition. Curr. Opin. Organ Transplant. 22, 46–54 (2017).
Dieudé, M. et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci. Transl. Med. 7, 318ra200 (2015).
Gołębiewska, J. E., Wardowska, A., Pietrowska, M., Wojakowska, A. & Dębska-Ślizień, A. Small extracellular vesicles in transplant rejection. Cells 10, 2989 (2021).
Habertheuer, A. et al. Donor tissue-specific exosome profiling enables noninvasive monitoring of acute rejection in mouse allogeneic heart transplantation. Thorac. Cardiovasc. Surg. 155, 2479–2489 (2018).
Hao, Q., Wu, Y., Wu, Y., Wang, P. & Vadgama, J. V. Tumor-derived exosomes in tumor-induced immune suppression. Int. J. Mol. Sci. 23, 1461 (2022).
Beltraminelli, T., Perez, C. R. & De Palma, M. Disentangling the complexity of tumor-derived extracellular vesicles. Cell Rep. 35, 108960 (2021).
Szajnik, M., Czystowska, M., Szczepanski, M. J., Mandapathil, M. & Whiteside, T. L. Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One 5, e11469 (2010).
Xiang, X. et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 124, 2621–2633 (2009).
Yu, S. et al. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J. Immunol. 178, 6867–6875 (2007).
Clayton, A. et al. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 180, 7249–7258 (2008).
Wieckowski, E. U. et al. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 183, 3720–3730 (2009).
Huber, V. et al. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology 128, 1796–1804 (2005).
Shinohara, H. et al. Regulated polarization of tumor-associated macrophages by miR-145 via colorectal cancer-derived extracellular vesicles. J. Immunol. 199, 1505–1515 (2017).
Marar, C., Starich, B. & Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 22, 560–570 (2021).
Whiteside, T. L. The role of tumor-derived exosomes (TEX) in shaping anti-tumor immune competence. Cells 10, 3054 (2021).
Daassi, D., Mahoney, K. M. & Freeman, G. J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 20, 209–215 (2020).
Gao, L. et al. Tumor-derived exosomes antagonize innate antiviral immunity. Nat. Immunol. 19, 233–245 (2018).
Nakazawa, Y. et al. Tumor-derived extracellular vesicles regulate tumor-infiltrating regulatory T cells via the inhibitory immunoreceptor CD300a. Elife 10, e61999 (2021).
Zitvogel, L. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat. Med. 4, 594–600 (1998).
McAndrews, K. M., Che, S. P. Y., LeBleu, V. S. & Kalluri, R. Effective delivery of STING agonist using exosomes suppresses tumor growth and enhances antitumor immunity. J. Biol. Chem. 296, 100523 (2021).
Shen, Z. et al. Effects of mesenchymal stem cell-derived exosomes on autoimmune diseases. Front. Immunol. 12, 749192 (2021).
Tong, L. et al. Milk-derived extracellular vesicles alleviate ulcerative colitis by regulating the gut immunity and reshaping the gut microbiota. Theranostics 11, 8570–8586 (2021).
Bai, X., Findlow, J. & Borrow, R. Recombinant protein meningococcal serogroup B vaccine combined with outer membrane vesicles. Expert. Opin. Biol. Ther. 11, 969–985 (2011).
Acevedo, R. et al. Bacterial outer membrane vesicles and vaccine applications. Front. Immunol. 5, 121 (2014).
Morishita, M. et al. Characterizing different probiotic-derived extracellular vesicles as a novel adjuvant for immunotherapy. Mol. Pharm. 18, 1080–1092 (2021).
Fu, W. et al. CAR exosomes derived from effector CAR-T cells have potent antitumour effects and low toxicity. Nat. Commun. 10, 4355 (2019).
Johnson, L. R. et al. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell 184, 4981–4995.e14 (2021). This study shows that CAR T cells expressing the endogenous RNA RN7SL1 release this RNA in CAR-carrying EVs, which support the function of CAR T cells.
Ji, P. et al. Smart exosomes with lymph node homing and immune-amplifying capacities for enhanced immunotherapy of metastatic breast cancer. Mol. Ther. Nucleic Acids 26, 987–996 (2021).
Cheng, Q. et al. Eliciting anti-cancer immunity by genetically engineered multifunctional exosomes. Mol. Ther. https://doi.org/10.1016/j.ymthe.2022.06.013 (2022).
Mathieu, M. et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 12, 4389 (2021).
Cao, M. et al. Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding. Elife 4, e05242 (2015).
Wang, Q. & Lu, Q. Plasma membrane-derived extracellular microvesicles mediate non-canonical intercellular NOTCH signaling. Nat. Commun. 8, 709 (2017).
Rai, A. et al. Secreted midbody remnants are a class of extracellular vesicles molecularly distinct from exosomes and microparticles. Commun. Biol. 4, 400 (2021).
Di Vizio, D. et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 181, 1573–1584 (2012).
Atkin-Smith, G. K. et al. Plexin B2 is a regulator of monocyte apoptotic cell disassembly. Cell Rep. 29, 1821–1831.e3 (2019).
Ma, L. et al. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 25, 24–38 (2015).
Melentijevic, I. et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 542, 367–371 (2017).
Szabó, G. T. et al. Critical role of extracellular vesicles in modulating the cellular effects of cytokines. Cell. Mol. Life Sci. 71, 4055–4067 (2014).
Wahlgren, J. et al. Activated human T cells secrete exosomes that participate in IL-2 mediated immune response signaling. PLoS One 7, e49723 (2012).
Lima, L. G. et al. Tumor microenvironmental cytokines bound to cancer exosomes determine uptake by cytokine receptor-expressing cells and biodistribution. Nat. Commun. 12, 3543 (2021).
Fitzgerald, W. et al. A system of cytokines encapsulated in extracellular vesicles. Sci. Rep. 8, 8973 (2018).
Shelke, G. V. et al. Endosomal signalling via exosome surface TGFβ-1. J. Extracell. Vesicles 8, 1650458 (2019).
Schmidt-Arras, D. & Rose-John, S. Endosomes as signaling platforms for IL-6 family cytokine receptors. Front. Cell Dev. Biol. 9, 688314 (2021).
El-Shennawy, L. et al. Circulating ACE2-expressing extracellular vesicles block broad strains of SARS-CoV-2. Nat. Commun. 13, 405 (2022).
Kim, H. K. et al. Engineered small extracellular vesicles displaying ACE2 variants on the surface protect against SARS-CoV-2 infection. J. Extracell. Vesicles 11, e12179 (2022).
Cocozza, F. et al. Extracellular vesicles containing ACE2 efficiently prevent infection by SARS-CoV-2 Spike protein-containing virus. J. Extracell. Vesicles 10, e12050 (2020).
Xie, F. et al. Engineering extracellular vesicles enriched with palmitoylated ACE2 as COVID-19 therapy. Adv. Mater. 19, e2103471 (2021).
van der Ley, P. A., Zariri, A., van Riet, E., Oosterhoff, D. & Kruiswijk, C. P. An intranasal OMV-based vaccine induces high mucosal and systemic protecting immunity against a SARS-CoV-2 infection. Front. Immunol. 12, 781280 (2021).
Jiang, L. et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. J. Extracell. Vesicles 11, e12192 (2022).
Tsai, S. J. et al. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J. Biol. Chem. 297, 101266 (2021).
Sengupta, V. et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cell Dev. 29, 747–754 (2020).
Fujita, Y. et al. Early prediction of COVID-19 severity using extracellular vesicle COPB2. J. Extracell. Vesicles 10, e12092 (2021).
Uto, K. et al. Identification of Plexin D1 on circulating extracellular vesicles as a potential biomarker of polymyositis and dermatomyositis. Rheumatology 61, 1669–1679 (2021).
Garcia-Contreras, M. et al. Plasma-derived exosome characterization reveals a distinct microRNA signature in long duration type 1 diabetes. Sci. Rep. 7, 5998 (2017).
Rodríguez-Muguruza, S. et al. A serum biomarker panel of exomiR-451a, exomiR-25-3p and soluble TWEAK for early diagnosis of rheumatoid arthritis. Front. Immunol. 12, 790880 (2021).
Acknowledgements
This work was supported by National Research, Development and Innovation Office NKFIH, Hungary, NVKP_16-1-2016-0017, the Hungarian Scientific Research Fund (OTKA K120237), VEKOP-2.3.2-16-2016-000002, VEKOP-2.3.3-15-2017-00016, H2020-MSCA-ITN-2017-722148 TRAIN EV and Higher Education Excellence Program (FIKP) Therapeutic Thematic Programme. This study was supported by the Hungarian Thematic Excellence Programme No. TKP2020-NKA-26. The project has received funding from the EU’s Horizon 2020 Research and Innovation Programme under grant agreement No. 739593.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.I.B. is a member of the Advisory Board of Sphere Gene Therapeutics Inc. (Boston, MA, USA).
Peer review
Peer review information
Nature Reviews Immunology thanks E. Boilard, A. Morelli and F. Sánchez-Madrid for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Multivesicular bodies
-
(MVBs). Membrane-enclosed endosomal organelles with intraluminal vesicles formed by inward budding of the limiting membrane.
- Amphisomes
-
Hybrid organelles formed by the fusion of autophagosomes and MVBs.
- Migrasomes
-
Large extracellular vesicles with numerous small inner vesicles, formed at the tips and intersections of retraction fibres of migrating cells.
- Exophers
-
Large membrane-enclosed extracellular vesicles released by physiologically normal cells to remove damaged organelles or aggregated proteins to maintain homeostasis.
- Large oncosomes
-
Large (micrometre-sized) extracellular vesicles derived from the plasma membrane of tumour cells.
- Quorum sensing
-
A process of cell–cell communication by which bacteria share information about cell density and modify their gene expression accordingly.
- Membrane attack complexes
-
Multiprotein pore-forming complexes generated on target surfaces upon activation of the complement system.
- Dermatomyositis
-
A rare chronic inflammatory disease affecting the skin and the muscles.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat Rev Immunol 23, 236–250 (2023). https://doi.org/10.1038/s41577-022-00763-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-022-00763-8
This article is cited by
-
Large extracellular vesicles and blebbisomes in cancer: emerging and translational opportunities highlights
Cell Communication and Signaling (2026)
-
Tetraspanin-based immunocapture for high-depth proteomic profiling of extracellular vesicles from cerebrospinal fluid for biomarker discovery
Clinical Proteomics (2026)
-
Unlocking the power of extracellular vesicles: multi-omics integration for cancer biomarker discovery
Biomarker Research (2026)
-
Tumor-associated macrophages display differential protein cargo sorting in extracellular vesicles associated with poor survival in ovarian cancer
Molecular Medicine (2026)
-
Iridium complex-loaded biomimetic vesicles enable enhanced photodynamic therapy and immune modulation
Microsystems & Nanoengineering (2026)