Abstract
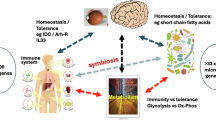
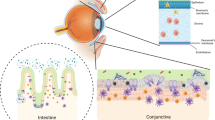
Balanced immune responses in the eyes are crucial to preserve vision. The ocular immune system has long been considered distinct, owing to the so-called ‘immune privilege’ of its component tissues. More recently, intravital imaging and transcriptomic techniques have reshaped scientific understanding of the ocular immune landscape, such as revealing the specialization of immune cell populations in the various tissues of the eye. As knowledge of the phenotypes of corneal and retinal immune cells has evolved, links to both the systemic immune system, and the central and peripheral nervous systems, have been identified. Using intravital imaging, T cells have recently been found to reside in, and actively patrol, the healthy human cornea. Disease-associated retinal microglia with links to retinal degeneration have also been identified. This Review provides an updated guide to the ocular immune system, highlighting current knowledge of the immune cells that are present in steady-state and specific diseased ocular tissues, as well as evidence for their relationship to systemic disease. In addition, we discuss emerging intravital imaging techniques that can be used to visualize immune cell morphology and dynamics in living human eyes and how these could be applied to advance understanding of the human immune system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wieghofer, P. et al. Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling. EMBO J. 40, e105123 (2021).
Downie, L. E. et al. Redefining the human corneal immune compartment using dynamic intravital imaging. Proc. Natl Acad. Sci. USA 120, e2217795120 (2023). This study is the first to show that intraepithelial lymphocytes make up a substantial portion of cells in human corneal epithelium.
Hammer, D. X., Agrawal, A., Villanueva, R., Saeedi, O. & Liu, Z. Label-free adaptive optics imaging of human retinal macrophage distribution and dynamics. Proc. Natl Acad. Sci. USA 117, 30661–30669 (2020). This study characterizes the distribution and dynamics of retinal macrophages in living humans.
Colorado, L. H., Edwards, K., Chinnery, H. R. & Bazan, H. E. In vivo immune cell dynamics in the human cornea. Exp. Eye Res. 199, 108168 (2020).
Loi, J. K. et al. Corneal tissue-resident memory T cells form a unique immune compartment at the ocular surface. Cell Rep. 39, 110852 (2022). This study shows the formation of tissue-resident memory T cells in the mouse cornea following viral infection.
Sim, R., Yong, K., Liu, Y. C. & Tong, L. In vivo confocal microscopy in different types of dry eye and meibomian gland dysfunction. J. Clin. Med. 11, 2349 (2022).
Cavalcanti, B. M. et al. In vivo confocal microscopy detects bilateral changes of corneal immune cells and nerves in unilateral herpes zoster ophthalmicus. Ocul. Surf. 16, 101–111 (2018).
Chirapapaisan, C. et al. In vivo confocal microscopy demonstrates increased immune cell densities in corneal graft rejection correlating with signs and symptoms. Am. J. Ophthalmol. 203, 26–36 (2019).
Dehghani, C. et al. Morphometric changes to corneal dendritic cells in individuals with mild cognitive impairment. Front. Neurosci. 14, 556137 (2020).
Dick, A. D. Influence of microglia on retinal progenitor cell turnover and cell replacement. Eye 23, 1939–1945 (2009).
Vendomele, J., Khebizi, Q. & Fisson, S. Cellular and molecular mechanisms of anterior chamber-associated immune deviation (ACAID): what we have learned from knockout mice. Front. Immunol. 8, 1686 (2017).
Jiang, L. Q., Jorquera, M. & Streilein, J. W. Subretinal space and vitreous cavity as immunologically privileged sites for retinal allografts. Invest. Ophthalmol. Vis. Sci. 34, 3347–3354 (1993).
Rosenbaum, J. T., Woods, A., Kezic, J., Planck, S. R. & Rosenzweig, H. L. Contrasting ocular effects of local versus systemic endotoxin. Invest. Ophthalmol. Vis. Sci. 52, 6472–6477 (2011).
Dando, S. J., Kazanis, R. & McMenamin, P. G. Myeloid cells in the mouse retina and uveal tract respond differently to systemic inflammatory stimuli. Invest. Ophthalmol. Vis. Sci. 62, 10 (2021).
McDermott, A. M. Antimicrobial compounds in tears. Exp. Eye Res. 117, 53–61 (2013).
Taylor, A. A review of the influence of aqueous humor on immunity. Ocul. Immunol. Inflamm. 11, 231–241 (2003).
Shin, H. et al. Changes in the eye microbiota associated with contact lens wearing. mBio 7, e00198 (2016).
Graham, J. E. et al. Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest. Ophthalmol. Vis. Sci. 48, 5616–5623 (2007).
Song, H., Xiao, K., Min, H., Chen, Z. & Long, Q. Characterization of conjunctival sac microbiome from patients with allergic conjunctivitis. J. Clin. Med. 11, 1130 (2022).
Wang, X. et al. An ocular glymphatic clearance system removes beta-amyloid from the rodent eye. Sci. Transl. Med. 12, eaaw3210 (2020).
Yin, X. et al. Compartmentalized ocular lymphatic system mediates eye–brain immunity. Nature 628, 204–211 (2024). This study shows that antigens from the posterior compartment of the eye drain to the deep cervical lymph nodes via lymphatic vasculature in the optic nerve sheath.
Liu, J. & Li, Z. Resident innate immune cells in the cornea. Front. Immunol. 12, 620284 (2021).
Britten-Jones, A. C., Rajan, R., Craig, J. P. & Downie, L. E. Quantifying corneal immune cells from human in vivo confocal microscopy images: can manual quantification be improved with observer training? Exp. Eye Res. 216, 108950 (2022).
Tajbakhsh, Z. et al. Dendritiform immune cells with reduced antigen-capture capacity persist in the cornea during the asymptomatic phase of allergic conjunctivitis. Eye 37, 2768–2775 (2023).
Dou, S. et al. Single-cell atlas of keratoconus corneas revealed aberrant transcriptional signatures and implicated mechanical stretch as a trigger for keratoconus pathogenesis. Cell Discov. 8, 66 (2022).
Dijkgraaf, F. E. et al. Tissue patrol by resident memory CD8(+) T cells in human skin. Nat. Immunol. 20, 756–764 (2019).
Gebhardt, T. et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature 477, 216–219 (2011).
Beura, L. K. et al. Intravital mucosal imaging of CD8(+) resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat. Immunol. 19, 173–182 (2018).
Yamagami, S. et al. Distinct populations of dendritic cells in the normal human donor corneal epithelium. Invest. Ophthalmol. Vis. Sci. 46, 4489–4494 (2005).
Wu, M. et al. Intravital imaging of the human cornea reveals the differential effects of season on innate and adaptive immune cell morphodynamics. Ophthalmology https://doi.org/10.1016/j.ophtha.2024.04.020 (2024).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Strobl, J. & Haniffa, M. Functional heterogeneity of human skin-resident memory T cells in health and disease. Immunol. Rev. 316, 104–119 (2023).
Arnous, R. et al. Tissue resident memory T cells inhabit the deep human conjunctiva. Sci. Rep. 12, 6077 (2022).
Schenkel, J. M., Fraser, K. A., Vezys, V. & Masopust, D. Sensing and alarm function of resident memory CD8(+) T cells. Nat. Immunol. 14, 509–513 (2013).
Park, S. L. et al. Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol. 19, 183–191 (2018).
Bitirgen, G. et al. Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long covid. Br. J. Ophthalmol. 106, 1635–1641 (2022).
Li, W. et al. The miR-183/96/182 cluster is a checkpoint for resident immune cells and shapes the cellular landscape of the cornea. Ocul. Surf. 30, 17–41 (2023).
DeMaio, A., Mehrotra, S., Sambamurti, K. & Husain, S. The role of the adaptive immune system and T cell dysfunction in neurodegenerative diseases. J. Neuroinflamm. 19, 251 (2022).
Deliyanti, D. et al. CD8(+) T cells promote pathological angiogenesis in ocular neovascular disease. Arterioscler. Thromb. Vasc. Biol. 43, 522–536 (2023).
Deliyanti, D. et al. Foxp3(+) Tregs are recruited to the retina to repair pathological angiogenesis. Nat. Commun. 8, 748 (2017). This study shows that regulatory T cells are recruited to the retina and take an active role in protecting the eye from neovascularization in oxygen-induced retinopathy.
Luger, D. et al. Either a TH17 or a TH1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 205, 799–810 (2008).
Chen, Y. H. et al. Functionally distinct IFN-gamma(+) IL-17a(+) TH cells in experimental autoimmune uveitis: T-cell heterogeneity, migration, and steroid response. Eur. J. Immunol. 50, 1941–1951 (2020).
Fan, N. W. et al. Long-lived autoreactive memory CD4(+) T cells mediate the sustained retinopathy in chronic autoimmune uveitis. FASEB J. 37, e22855 (2023).
Boldison, J. et al. Tissue-resident exhausted effector memory CD8+ T cells accumulate in the retina during chronic experimental autoimmune uveoretinitis. J. Immunol. 192, 4541–4550 (2014).
Paskeviciute, E. et al. Systemic virus infection results in CD8 T cell recruitment to the retina in the absence of local virus infection. Front. Immunol. 14, 1221511 (2023).
Voigt, V. et al. Cytomegalovirus establishes a latent reservoir and triggers long-lasting inflammation in the eye. PLoS Pathog. 14, e1007040 (2018).
Urban, S. L. et al. Peripherally induced brain tissue-resident memory CD8(+) T cells mediate protection against CNS infection. Nat. Immunol. 21, 938–949 (2020).
Gate, D. et al. Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer’s disease. Nature 577, 399–404 (2020).
Conedera, F. M. et al. Assessing the role of T cells in response to retinal injury to uncover new therapeutic targets for the treatment of retinal degeneration. J. Neuroinflamm. 20, 206 (2023).
Groh, J. et al. Accumulation of cytotoxic T cells in the aged CNS leads to axon degeneration and contributes to cognitive and motor decline. Nat. Aging 1, 357–367 (2021).
Zhang, X. et al. Aged microglia promote peripheral T cell infiltration by reprogramming the microenvironment of neurogenic niches. Immun. Ageing 19, 34 (2022).
Hu, P., Pollard, J. D. & Chan-Ling, T. Breakdown of the blood–retinal barrier induced by activated T cells of nonneural specificity. Am. J. Pathol. 156, 1139–1149 (2000).
Bose, T., Lee, R., Hou, A., Tong, L. & Chandy, K. G. Tissue resident memory T cells in the human conjunctiva and immune signatures in human dry eye disease. Sci. Rep. 7, 45312 (2017).
Li, L. et al. Conjunctiva resident γδ T cells expressed high level of IL-17a and promoted the severity of dry eye. Invest. Ophthalmol. Vis. Sci. 63, 13 (2022).
Zhao, Z. et al. Choroidal γδ T cells in protection against retinal pigment epithelium and retinal injury. FASEB J. 31, 4903–4916 (2017).
Liu, J. et al. Local group 2 innate lymphoid cells promote corneal regeneration after epithelial abrasion. Am. J. Pathol. 187, 1313–1326 (2017).
Alam, J. et al. Single-cell transcriptional profiling of murine conjunctival immune cells reveals distinct populations expressing homeostatic and regulatory genes. Mucosal Immunol. 15, 620–628 (2022).
Zhang, X. et al. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS ONE 7, e36822 (2012).
Zarzuela, J. C., Reinoso, R., Armentia, A., Enriquez-de-Salamanca, A. & Corell, A. Conjunctival intraepithelial lymphocytes, lacrimal cytokines and ocular commensal microbiota: analysis of the three main players in allergic conjunctivitis. Front. Immunol. 13, 911022 (2022).
Knickelbein, J. E., Watkins, S. C., McMenamin, P. G. & Hendricks, R. L. Stratification of antigen-presenting cells within the normal cornea. Ophthalmol. Eye Dis. 1, 45–54 (2009).
Chinnery, H. R. et al. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest. Ophthalmol. Vis. Sci. 48, 1568–1574 (2007).
Hattori, T. et al. Characterization of langerin-expressing dendritic cell subsets in the normal cornea. Invest. Ophthalmol. Vis. Sci. 52, 4598–4604 (2011).
Mass, E., Nimmerjahn, F., Kierdorf, K. & Schlitzer, A. Tissue-specific macrophages: how they develop and choreograph tissue biology. Nat. Rev. Immunol. 23, 563–579 (2023).
Ma, B. et al. Mapping resident immune cells in the murine ocular surface and lacrimal gland by flow cytometry. Ocul. Immunol. Inflamm. 31, 748–759 (2023).
Senthil, K., Jiao, H., Downie, L. E. & Chinnery, H. R. Altered corneal epithelial dendritic cell morphology and phenotype following acute exposure to hyperosmolar saline. Invest. Ophthalmol. Vis. Sci. 62, 38 (2021).
Liu, Y., Hamrah, P., Zhang, Q., Taylor, A. W. & Dana, M. R. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J. Exp. Med. 195, 259–268 (2002).
Buela, K. A. & Hendricks, R. L. Cornea-infiltrating and lymph node dendritic cells contribute to CD4+ T cell expansion after herpes simplex virus-1 ocular infection. J. Immunol. 194, 379–387 (2015).
Knickelbein, J. E., Buela, K. A. & Hendricks, R. L. Antigen-presenting cells are stratified within normal human corneas and are rapidly mobilized during ex vivo viral infection. Invest. Ophthalmol. Vis. Sci. 55, 1118–1123 (2014).
Jamali, A. et al. Plasmacytoid dendritic cells in the eye. Prog. Retin. Eye Res. 80, 100877 (2021).
Colonna, M., Trinchieri, G. & Liu, Y. J. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5, 1219–1226 (2004).
Jamali, A. et al. Characterization of resident corneal plasmacytoid dendritic cells and their pivotal role in herpes simplex keratitis. Cell Rep. 32, 108099 (2020).
Luznik, Z. et al. Identification and characterization of dendritic cell subtypes in preserved and cultured cadaveric human corneolimbal tissue on amniotic membrane. Acta Ophthalmol. 97, e184–e193 (2019).
Chinnery, H. R., McMenamin, P. G. & Dando, S. J. Macrophage physiology in the eye. Pflug. Arch. 469, 501–515 (2017).
Gulka, S. M. D., Gowen, B., Litke, A. M., Delaney, K. R. & Chow, R. L. Laser-induced microinjury of the corneal basal epithelium and imaging of resident macrophage responses in a live, whole-eye preparation. Front. Immunol. 14, 1050594 (2023).
Zhuang, X. et al. Time- and stimulus-dependent characteristics of innate immune cells in organ-cultured human corneal tissue. J. Innate Immun. 14, 98–111 (2022).
Orecchioni, M., Ghosheh, Y., Pramod, A. B. & Ley, K. Macrophage polarization: different gene signatures in M1(LPS+) vs. classically and M2(LPS−) vs. alternatively activated macrophages. Front. Immunol. 10, 1084 (2019).
Jaggi, U. et al. Increased phagocytosis in the presence of enhanced M2-like macrophage responses correlates with increased primary and latent HSV-1 infection. PLoS Pathog. 16, e1008971 (2020).
Jaggi, U. et al. Essential role of M1 macrophages in blocking cytokine storm and pathology associated with murine HSV-1 infection. PLoS Pathog. 17, e1009999 (2021).
Tian, H., Wu, J. & Ma, M. Implications of macrophage polarization in corneal transplantation rejection. Transpl. Immunol. 64, 101353 (2021).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Benhar, I. et al. Temporal single-cell atlas of non-neuronal retinal cells reveals dynamic, coordinated multicellular responses to central nervous system injury. Nat. Immunol. 24, 700–713 (2023).
O’Koren, E. G., Mathew, R. & Saban, D. R. Fate mapping reveals that microglia and recruited monocyte-derived macrophages are definitively distinguishable by phenotype in the retina. Sci. Rep. 6, 20636 (2016).
Jordao, M. J. C. et al. Single-cell profiling identifies myeloid cell subsets with distinct fates during neuroinflammation. Science 363, eaat7554 (2019).
Kierdorf, K., Masuda, T., Jordao, M. J. C. & Prinz, M. Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat. Rev. Neurosci. 20, 547–562 (2019).
Checchin, D., Sennlaub, F., Levavasseur, E., Leduc, M. & Chemtob, S. Potential role of microglia in retinal blood vessel formation. Invest. Ophthalmol. Vis. Sci. 47, 3595–3602 (2006).
Diaz-Araya, C. M., Provis, J. M., Penfold, P. L. & Billson, F. A. Development of microglial topography in human retina. J. Comp. Neurol. 363, 53–68 (1995).
Chen, L., Yang, P. & Kijlstra, A. Distribution, markers, and functions of retinal microglia. Ocul. Immunol. Inflamm. 10, 27–39 (2002).
Bodeutsch, N. & Thanos, S. Migration of phagocytotic cells and development of the murine intraretinal microglial network: an in vivo study using fluorescent dyes. Glia 32, 91–101 (2000).
Murenu, E., Gerhardt, M. J., Biel, M. & Michalakis, S. More than meets the eye: the role of microglia in healthy and diseased retina. Front. Immunol. 13, 1006897 (2022).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
O’Koren, E. G. et al. Microglial function is distinct in different anatomical locations during retinal homeostasis and degeneration. Immunity 50, 723–737.e7 (2019).
Xu, H., Chen, M., Mayer, E. J., Forrester, J. V. & Dick, A. D. Turnover of resident retinal microglia in the normal adult mouse. Glia 55, 1189–1198 (2007).
London, A. et al. Neuroprotection and progenitor cell renewal in the injured adult murine retina requires healing monocyte-derived macrophages. J. Exp. Med. 208, 23–39 (2011).
Guillonneau, X. et al. On phagocytes and macular degeneration. Prog. Retin. Eye Res. 61, 98–128 (2017).
Calippe, B. et al. Complement factor H inhibits CD47-mediated resolution of inflammation. Immunity 46, 261–272 (2017).
Beguier, F. et al. The 10q26 risk haplotype of age-related macular degeneration aggravates subretinal inflammation by impairing monocyte elimination. Immunity 53, 429–441.e8 (2020).
Yu, C. et al. Microglia at sites of atrophy restrict the progression of retinal degeneration via galectin-3 and Trem2. J. Exp. Med. 221, e20231011 (2024). This study shows that retinal microglia migrate into the subretinal space and undergo reprogramming by upregulating genes linked to neurodegeneration, playing a protective role for photoreceptors and retinal pigment epithelium.
Deczkowska, A. et al. Disease-associated microglia: a universal immune sensor of neurodegeneration. Cell 173, 1073–1081 (2018).
Kuchroo, M. et al. Single-cell analysis reveals inflammatory interactions driving macular degeneration. Nat. Commun. 14, 2589 (2023). This study identifies a similar glial activation profile enriched in the early phase of age-related macular degeneration, Alzheimer disease and progressive multiple sclerosis.
Mun, Y., Hwang, J. S. & Shin, Y. J. Role of neutrophils on the ocular surface. Int. J. Mol. Sci. 22, 10386 (2021).
Mahajan, A. et al. Frontline science: aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. J. Leukoc. Biol. 105, 1087–1098 (2019).
Postnikoff, C. K., Held, K., Viswanath, V. & Nichols, K. K. Enhanced closed eye neutrophil degranulation in dry eye disease. Ocul. Surf. 18, 841–851 (2020).
DeDreu, J. et al. An immune response to the avascular lens following wounding of the cornea involves ciliary zonule fibrils. FASEB J. 34, 9316–9336 (2020).
McHarg, S. et al. Mast cell infiltration of the choroid and protease release are early events in age-related macular degeneration associated with genetic risk at both chromosomes 1q32 and 10q26. Proc. Natl Acad. Sci. USA 119, e2118510119 (2022).
Liu, J. et al. Mast cells participate in corneal development in mice. Sci. Rep. 5, 17569 (2015).
Xie, Y. et al. Mast cell activation protects cornea by promoting neutrophil infiltration via stimulating ICAM-1 and vascular dilation in fungal keratitis. Sci. Rep. 8, 8365 (2018).
Elieh Ali Komi, D., Rambasek, T. & Bielory, L. Clinical implications of mast cell involvement in allergic conjunctivitis. Allergy 73, 528–539 (2018).
McMenamin, P. G. The distribution of immune cells in the uveal tract of the normal eye. Eye 11, 183–193 (1997).
Bhutto, I. A. et al. Increased choroidal mast cells and their degranulation in age-related macular degeneration. Br. J. Ophthalmol. 100, 720–726 (2016).
McLeod, D. S. et al. Mast cell-derived tryptase in geographic atrophy. Invest. Ophthalmol. Vis. Sci. 58, 5887–5896 (2017).
Downie, L. E. et al. CLEAR — anatomy and physiology of the anterior eye. Cont. Lens Anterior Eye 44, 132–156 (2021).
Shetty, R. et al. Role of in vivo confocal microscopy in dry eye disease and eye pain. Indian J. Ophthalmol. 71, 1099–1104 (2023).
Lagali, N. S. et al. Dendritic cell maturation in the corneal epithelium with onset of type 2 diabetes is associated with tumor necrosis factor receptor superfamily member 9. Sci. Rep. 8, 14248 (2018).
Gao, N., Lee, P. & Yu, F. S. Intraepithelial dendritic cells and sensory nerves are structurally associated and functional interdependent in the cornea. Sci. Rep. 6, 36414 (2016).
Choi, E. Y. et al. Langerhans cells prevent subbasal nerve damage and upregulate neurotrophic factors in dry eye disease. PLoS ONE 12, e0176153 (2017).
Wu, M. et al. The neuroregenerative effects of topical decorin on the injured mouse cornea. J. Neuroinflamm. 17, 142 (2020).
Doss, A. L. & Smith, P. G. Langerhans cells regulate cutaneous innervation density and mechanical sensitivity in mouse footpad. Neurosci. Lett. 578, 55–60 (2014).
Gao, N., Yan, C., Lee, P., Sun, H. & Yu, F. S. Dendritic cell dysfunction and diabetic sensory neuropathy in the cornea. J. Clin. Invest. 126, 1998–2011 (2016).
Parlanti, P. et al. Axonal debris accumulates in corneal epithelial cells after intraepithelial corneal nerves are damaged: a focused ion beam scanning electron microscopy (FIB-SEM) study. Exp. Eye Res. 194, 107998 (2020).
Kovacs, I. et al. Substance P released from sensory nerve endings influences tear secretion and goblet cell function in the rat. Neuropeptides 39, 395–402 (2005).
Marriott, I. & Bost, K. L. Expression of authentic substance P receptors in murine and human dendritic cells. J. Neuroimmunol. 114, 131–141 (2001).
Lai, J. P., Douglas, S. D. & Ho, W. Z. Human lymphocytes express substance P and its receptor. J. Neuroimmunol. 86, 80–86 (1998).
Twardy, B. S., Channappanavar, R. & Suvas, S. Substance P in the corneal stroma regulates the severity of herpetic stromal keratitis lesions. Invest. Ophthalmol. Vis. Sci. 52, 8604–8613 (2011).
Foldenauer, M. E., McClellan, S. A., Berger, E. A. & Hazlett, L. D. Mammalian target of rapamycin regulates IL-10 and resistance to Pseudomonas aeruginosa corneal infection. J. Immunol. 190, 5649–5658 (2013).
Lasagni Vitar, R. M. et al. Modulating ocular surface pain through neurokinin-1 receptor blockade. Invest. Ophthalmol. Vis. Sci. 62, 26 (2021).
Hazlett, L. D. et al. Spantide I decreases type I cytokines, enhances IL-10, and reduces corneal perforation in susceptible mice after Pseudomonas aeruginosa infection. Invest. Ophthalmol. Vis. Sci. 48, 797–807 (2007).
Yun, H., Lathrop, K. L. & Hendricks, R. L. A central role for sympathetic nerves in herpes stromal keratitis in mice. Invest. Ophthalmol. Vis. Sci. 57, 1749–1756 (2016).
Yun, H., Yin, X. T., Stuart, P. M. & St Leger, A. J. Sensory nerve retraction and sympathetic nerve innervation contribute to immunopathology of murine recurrent herpes stromal keratitis. Invest. Ophthalmol. Vis. Sci. 63, 4 (2022).
Gaddipati, S. et al. Loss of neurokinin-1 receptor alters ocular surface homeostasis and promotes an early development of herpes stromal keratitis. J. Immunol. 197, 4021–4033 (2016).
Paunicka, K. J. et al. Severing corneal nerves in one eye induces sympathetic loss of immune privilege and promotes rejection of future corneal allografts placed in either eye. Am. J. Transpl. 15, 1490–1501 (2015).
Taketani, Y. et al. Restoration of regulatory T-cell function in dry eye disease by antagonizing substance P/neurokinin-1 receptor. Am. J. Pathol. 190, 1859–1866 (2020).
Datta, A. et al. TRPA1 and TPRV1 ion channels are required for contact lens-induced corneal parainflammation and can modulate levels of resident corneal immune cells. Invest. Ophthalmol. Vis. Sci. 64, 21 (2023).
Hong, S. et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science 352, 712–716 (2016).
Werneburg, S. et al. Targeted complement inhibition at synapses prevents microglial synaptic engulfment and synapse loss in demyelinating disease. Immunity 52, 167–182.e7 (2020).
Lall, D. et al. C9orf72 deficiency promotes microglial-mediated synaptic loss in aging and amyloid accumulation. Neuron 109, 2275–2291.e8 (2021).
Lehrman, E. K. et al. CD47 protects synapses from excess microglia-mediated pruning during development. Neuron 100, 120–134.e6 (2018).
Jiang, D. et al. Neuronal signal-regulatory protein alpha drives microglial phagocytosis by limiting microglial interaction with CD47 in the retina. Immunity 55, 2318–2335.e7 (2022). This study shows that neuronal SIRPα regulates microglial phagocytosis by limiting microglial SIRPα access to neuronal CD47.
Wang, H. et al. CD47 antibody blockade suppresses microglia-dependent phagocytosis and monocyte transition to macrophages, impairing recovery in EAE. JCI Insight 6, e148719 (2021).
Wolf, Y., Yona, S., Kim, K. W. & Jung, S. Microglia, seen from the CX3CR1 angle. Front. Cell Neurosci. 7, 26 (2013).
Zieger, M., Ahnelt, P. K. & Uhrin, P. CX3CL1 (fractalkine) protein expression in normal and degenerating mouse retina: in vivo studies. PLoS ONE 9, e106562 (2014).
Jobling, A. I. et al. The role of the microglial CX3CR1 pathway in the postnatal maturation of retinal photoreceptors. J. Neurosci. 38, 4708–4723 (2018).
Wang, X. et al. Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. J. Neurosci. 36, 2827–2842 (2016).
Combadiere, C. et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Invest. 117, 2920–2928 (2007).
Biesecker, K. R. et al. Glial cell calcium signaling mediates capillary regulation of blood flow in the retina. J. Neurosci. 36, 9435–9445 (2016).
Mills, S. A. et al. Fractalkine-induced microglial vasoregulation occurs within the retina and is altered early in diabetic retinopathy. Proc. Natl Acad. Sci. USA 118, e2112561118 (2021). This study shows the role of microglia in regulating retinal capillary diameter that is defective early in diabetes.
Bisht, K. et al. Capillary-associated microglia regulate vascular structure and function through PANX1–P2RY12 coupling in mice. Nat. Commun. 12, 5289 (2021).
Csaszar, E. et al. Microglia modulate blood flow, neurovascular coupling, and hypoperfusion via purinergic actions. J. Exp. Med. 219, e20211071 (2022).
Zabel, M. K. et al. Microglial phagocytosis and activation underlying photoreceptor degeneration is regulated by CX3CL1–CX3CR1 signaling in a mouse model of retinitis pigmentosa. Glia 64, 1479–1491 (2016).
Pfeifer, C. W. et al. Dysregulated CD200–CD200R signaling in early diabetes modulates microglia-mediated retinopathy. Proc. Natl Acad. Sci. USA 120, e2308214120 (2023).
D’Onofrio, L. et al. Small nerve fiber damage and Langerhans cells in type 1 and type 2 diabetes and LADA measured by corneal confocal microscopy. Invest. Ophthalmol. Vis. Sci. 62, 5 (2021).
O’Brien, J. A. et al. T lymphocyte and monocyte subsets are dysregulated in type 1 diabetes patients with peripheral neuropathic pain. Brain Behav. Immun. Health 15, 100283 (2021).
So, W. Z. et al. Diabetic corneal neuropathy as a surrogate marker for diabetic peripheral neuropathy. Neural Regen. Res. 17, 2172–2178 (2022).
Koronyo, Y. et al. Retinal pathological features and proteome signatures of Alzheimer’s disease. Acta Neuropathol. 145, 409–438 (2023).
Hayden, E. Y. & Teplow, D. B. Amyloid beta-protein oligomers and Alzheimer’s disease. Alzheimers Res. Ther. 5, 60 (2013).
Lambert, J. C. et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 45, 1452–1458 (2013).
van der Poel, M. et al. Transcriptional profiling of human microglia reveals grey-white matter heterogeneity and multiple sclerosis-associated changes. Nat. Commun. 10, 1139 (2019).
Biessels, G. J. & Reijmer, Y. D. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 63, 2244–2252 (2014).
Chai, Y. H. et al. Association between diabetic retinopathy, brain structural abnormalities, and cognitive impairment for accumulated evidence in observational studies. Am. J. Ophthalmol. 239, 37–53 (2022).
Xie, H. et al. Erythropoietin protects the inner blood–retinal barrier by inhibiting microglia phagocytosis via SRC/AKT/cofilin signalling in experimental diabetic retinopathy. Diabetologia 64, 211–225 (2021).
Chang, K. C., Shieh, B. & Petrash, J. M. Role of aldose reductase in diabetes-induced retinal microglia activation. Chem. Biol. Interact. 302, 46–52 (2019).
Huang, Z. et al. RIP3-mediated microglial necroptosis promotes neuroinflammation and neurodegeneration in the early stages of diabetic retinopathy. Cell Death Dis. 14, 227 (2023).
Jiang, M. et al. Enhancing fractalkine/CX3CR1 signalling pathway can reduce neuroinflammation by attenuating microglia activation in experimental diabetic retinopathy. J. Cell Mol. Med. 26, 1229–1244 (2022).
Kugadas, A., Wright, Q., Geddes-McAlister, J. & Gadjeva, M. Role of microbiota in strengthening ocular mucosal barrier function through secretory IgA. Invest. Ophthalmol. Vis. Sci. 58, 4593–4600 (2017).
Horai, R. et al. Microbiota-dependent activation of an autoreactive T cell receptor provokes autoimmunity in an immunologically privileged site. Immunity 43, 343–353 (2015).
Wu, M., Hill, L. J., Downie, L. E. & Chinnery, H. R. Neuroimmune crosstalk in the cornea: the role of immune cells in corneal nerve maintenance during homeostasis and inflammation. Prog. Retin. Eye Res. 91, 101105 (2022).
de Paiva, C. S., St Leger, A. J. & Caspi, R. R. Mucosal immunology of the ocular surface. Mucosal Immunol. 15, 1143–1157 (2022).
Reekie, I. R. et al. The cellular composition of the uveal immune environment. Front. Med. 8, 721953 (2021).
McPherson, S. W., Heuss, N. D., Pierson, M. J. & Gregerson, D. S. Retinal antigen-specific regulatory T cells protect against spontaneous and induced autoimmunity and require local dendritic cells. J. Neuroinflamm. 11, 205 (2014).
Menko, A. S. et al. Resident immune cells of the avascular lens: mediators of the injury and fibrotic response of the lens. FASEB J. 35, e21341 (2021).
Boneva, S. K. et al. Transcriptional profiling uncovers human hyalocytes as a unique innate immune cell population. Front. Immunol. 11, 567274 (2020).
Stachs, O., Guthoff, R. F. & Aumann, S. in High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics (ed. Bille, J. F.) 263–284 (Springer, 2019).
Chiang, J. C. B., Roy, M., Kim, J., Markoulli, M. & Krishnan, A. V. In-vivo corneal confocal microscopy: imaging analysis, biological insights and future directions. Commun. Biol. 6, 652 (2023).
Stepp, M. A. et al. Corneal epithelial ‘neuromas’: a case of mistaken identity? Cornea 39, 930–934 (2020).
Chinnery, H. R. et al. Identification of presumed corneal neuromas and microneuromas using laser-scanning in vivo confocal microscopy: a systematic review. Br. J. Ophthalmol. 106, 765–771 (2022).
Wu, M., Chinnery, H. R., De Silva, M. E. H. & Downie, L. E. Characterisation and clustering of corneal stromal-epithelial nerve penetration sites in healthy adults: a laser-scanning in vivo confocal microscopy study. Clin. Exp. Ophthalmol. 51, 746–749 (2023).
Chinnery, H. R., Zhang, X. Y., Wu, C. Y. & Downie, L. E. Corneal immune cell morphometry as an indicator of local and systemic pathology: a review. Clin. Exp. Ophthalmol. 49, 729–740 (2021).
Liu, Z., Kurokawa, K., Zhang, F., Lee, J. J. & Miller, D. T. Imaging and quantifying ganglion cells and other transparent neurons in the living human retina. Proc. Natl Acad. Sci. USA 114, 12803–12808 (2017).
Liu, Z., Tam, J., Saeedi, O. & Hammer, D. X. Trans-retinal cellular imaging with multimodal adaptive optics. Biomed. Opt. Express 9, 4246–4262 (2018).
Migacz, J. V. et al. Imaging of vitreous cortex hyalocyte dynamics using non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy in human subjects. Biomed. Opt. Express 13, 1755–1773 (2022).
Hammer, D. X. et al. Cellular-level visualization of retinal pathology in multiple sclerosis with adaptive optics. Invest. Ophthalmol. Vis. Sci. 64, 21 (2023).
Castanos, M. V. et al. Imaging of macrophage-like cells in living human retina using clinical OCT. Invest. Ophthalmol. Vis. Sci. 61, 48 (2020).
Joseph, A., Chu, C. J., Feng, G., Dholakia, K. & Schallek, J. Label-free imaging of immune cell dynamics in the living retina using adaptive optics. eLife 9, e60547 (2020).
Joseph, A., Power, D. & Schallek, J. Imaging the dynamics of individual processes of microglia in the living retina in vivo. Biomed. Opt. Express 12, 6157–6183 (2021).
Acknowledgements
The authors thank H. Horsnell, L. Alarcon-Martinez and K. Senthil for critical input. This work was supported by the Australian Research Council (DP230102105 to H.R.C., L.E.D. and S.N.M.). S.N.M. is a recipient of a National Health and Medical Research Council Investigator Fellowship (2017220).
Author information
Authors and Affiliations
Contributions
H.R.C., L.E.D. and S.N.M. conceived the article. M.W., H.R.C., L.E.D. and S.N.M. wrote the first draft of the manuscript. All authors contributed to additional writing and editing of the article. M.W. prepared display items under supervision from E.L.F., H.R.C., L.E.D. and S.N.M. All authors approved the submitted version of the article.
Corresponding authors
Ethics declarations
Competing interests
M.W., H.R.C, L.E.D and S.N.M have submitted an Australian Provisional Patent Application (2023901150) relating to the imaging method described in this article (intellectual property owned by institution). E.L.F. declares no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks John Forrester and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Age-related macular degeneration
-
(AMD). A degenerative condition of the central retina (macula) that is the leading cause of irreversible vision loss in those over 65 years of age. AMD is clinically classified into three main stages, based on severity: early, intermediate and late. Late AMD has two forms: neovascular and geographic atrophy.
- Experimental autoimmune uveoretinitis
-
A T cell-mediated autoimmune disease induced in preclinical (animal) models to affect the uvea and retina, which serves as a model for the intraocular inflammation evident in human autoimmune (non-infectious) uveitis.
- Ischaemic retinopathy
-
A retinal pathological state caused by lack of sufficient blood flow to match the metabolic needs of the retina. Common examples include experimental oxygen-induced retinopathy, human retinopathy of prematurity and diabetic retinopathy.
- Retinal degeneration
-
A group of heterogeneous conditions defined by the progressive death of retinal neural cells.
- Uveitis
-
An umbrella term for a group of conditions caused by inflammation of the uvea, comprising the iris, ciliary body and choroid. Causes of uveitis are heterogeneous, including infectious and non-infectious aetiologies.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, M., Fletcher, E.L., Chinnery, H.R. et al. Redefining our vision: an updated guide to the ocular immune system. Nat Rev Immunol 24, 896–911 (2024). https://doi.org/10.1038/s41577-024-01064-y
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41577-024-01064-y
This article is cited by
-
Role of CD4+ T cell-derived cytokines in the pathogenesis of uveitis
Clinical and Experimental Medicine (2025)
-
Seeing Through the Layers: Clinical, Immunological, and Diagnostic Advances in Ocular Herpesvirus Infections
Current Clinical Microbiology Reports (2025)