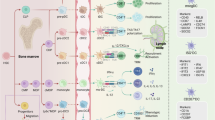
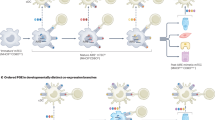
Abstract
In the context of adaptive immunity, T cells are activated by professional antigen-presenting cells (APCs) in a process that begins with peptide–MHC complexes on the APC being recognized by T cell receptor and CD3 co-receptor complexes on the T cell. This triggers a reorganization of T cell morphology, formation of an immune synapse, and the delivery of signals that ultimately culminate in nuclear activation. The interaction between T cells and APCs, such as dendritic cells (DCs), was originally viewed as a unidirectional information highway in which the APC instructs the T cell. It is now clear that bidirectional crosstalk occurs at the immune synapse and that T cells also shape APC functions. The concept of ‘DC licensing’ originally suggested an instructive role for T cells in modifying DC functions. More recent studies have provided important insight into the changes that occur in DCs during antigen-driven contacts with T cells at the immune synapse. In this Review, we discuss our current understanding of the bidirectional T cell–DC crosstalk that occurs at the IS and its relevance for immune responses and immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The review manuscript does not contain shared data. Images are accessible by request to the corresponding author.
References
Dustin, M. L. & Choudhuri, K. Signaling and polarized communication across the T cell immunological synapse. Annu. Rev. Cell Dev. Biol. 32, 303–325 (2016).
Huse, M. Mechanoregulation of lymphocyte cytotoxicity. Nat. Rev. Immunol. 25, 680–695 (2025).
Martin-Cofreces, N. B., Baixauli, F. & Sanchez-Madrid, F. Immune synapse: conductor of orchestrated organelle movement. Trends Cell Biol. 24, 61–72 (2014).
Cabeza-Cabrerizo, M., Cardoso, A., Minutti, C. M., Pereira da Costa, M. & Reis e Sousa, C. Dendritic cells revisited. Annu. Rev. Immunol. 39, 131–166 (2021).
Wulfing, C. & Davis, M. M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 282, 2266–2269 (1998).
Monks, C. R., Freiberg, B. A., Kupfer, H., Sciaky, N. & Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 (1998). This seminal paper describes the structure and segregation of receptors at the immune synapse by forming supramolecular activation clusters, a critical process for T cell communication and activation.
Dustin, M. L. et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell 94, 667–677 (1998).
Huang, H., Long, L., Zhou, P., Chapman, N. M. & Chi, H. mTOR signaling at the crossroads of environmental signals and T-cell fate decisions. Immunol. Rev. 295, 15–38 (2020).
Wulfing, C., Sjaastad, M. D. & Davis, M. M. Visualizing the dynamics of T cell activation: intercellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl Acad. Sci. USA 95, 6302–6307 (1998).
Montoya, M. C. et al. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat. Immunol. 3, 159–168 (2002). A description of the initial, exploratory antigen-independent contacts between T cells and APCs by promoting cell adhesion.
Li, J. & Springer, T. A. Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc. Natl Acad. Sci. USA 114, 4685–4690 (2017).
Cai, E. et al. Visualizing dynamic microvillar search and stabilization during ligand detection by T cells. Science 356, eaal3118 (2017).
Martin-Cofreces, N. B., Valpuesta, J. M. & Sanchez-Madrid, F. T cell asymmetry and metabolic crosstalk can fine-tune immunological synapses. Trends Immunol. 42, 649–653 (2021).
Hu, Y. S., Cang, H. & Lillemeier, B. F. Superresolution imaging reveals nanometer- and micrometer-scale spatial distributions of T-cell receptors in lymph nodes. Proc. Natl Acad. Sci. USA 113, 7201–7206 (2016).
Schamel, W. W. et al. Coexistence of multivalent and monovalent TCRs explains high sensitivity and wide range of response. J. Exp. Med. 202, 493–503 (2005).
Cai, E. et al. T cells use distinct topographical and membrane receptor scanning strategies that individually coalesce during receptor recognition. Proc. Natl Acad. Sci. USA 119, e2203247119 (2022).
Mittelbrunn, M. et al. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc. Natl Acad. Sci. USA 101, 11058–11063 (2004).
Gil, D., Schamel, W. W., Montoya, M., Sanchez-Madrid, F. & Alarcon, B. Recruitment of Nck by CD3 epsilon reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109, 901–912 (2002). The binding of a cognate antigen to the TCR promotes a conformational change in the CD3 complex that exposes proline-rich sequences in the CD3ε chain as a binding site for actin regulator NCK.
Malissen, B. & Bongrand, P. Early T cell activation: integrating biochemical, structural, and biophysical cues. Annu. Rev. Immunol. 33, 539–561 (2015).
Glassman, C. R., Parrish, H. L., Lee, M. S. & Kuhns, M. S. Reciprocal TCR-CD3 and CD4 engagement of a nucleating pMHCII stabilizes a functional receptor macrocomplex. Cell Rep. 22, 1263–1275 (2017).
Horkova, V. et al. Unique roles of co-receptor-bound LCK in helper and cytotoxic T cells. Nat. Immunol. 24, 174–185 (2022).
Guy, C. et al. LAG3 associates with TCR-CD3 complexes and suppresses signaling by driving co-receptor-Lck dissociation. Nat. Immunol. 23, 757–767 (2022).
Lo, W. L. et al. Lck promotes Zap70-dependent LAT phosphorylation by bridging Zap70 to LAT. Nat. Immunol. 19, 733–741 (2018). LCK acts as an adaptor molecule to spread TCR signals upon antigen recognition by bridging ZAP70 kinase to LAT, a substrate for phosphorylation.
Hartl, F. A. et al. Noncanonical binding of Lck to CD3epsilon promotes TCR signaling and CAR function. Nat. Immunol. 21, 902–913 (2020).
Li, L. et al. Ionic CD3-Lck interaction regulates the initiation of T-cell receptor signaling. Proc. Natl Acad. Sci. USA 114, E5891–E5899 (2017).
Nika, K. et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010).
Murugesan, S. et al. Formin-generated actomyosin arcs propel T cell receptor microcluster movement at the immune synapse. J. Cell Biol. 215, 383–399 (2016).
Johnson, K. G., Bromley, S. K., Dustin, M. L. & Thomas, M. L. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc. Natl Acad. Sci. USA 97, 10138–10143 (2000).
Jung, Y., Wen, L., Altman, A. & Ley, K. CD45 pre-exclusion from the tips of T cell microvilli prior to antigen recognition. Nat. Commun. 12, 3872 (2021).
Lin, J. & Weiss, A. The tyrosine phosphatase CD148 is excluded from the immunologic synapse and down-regulates prolonged T cell signaling. J. Cell Biol. 162, 673–682 (2003).
Gomez-Moron, A. et al. Human T-cell receptor triggering requires inactivation of Lim kinase-1 by slingshot-1 phosphatase. Commun. Biol. 7, 918 (2024).
Sanchez-Blanco, C. et al. Protein tyrosine phosphatase PTPN22 regulates LFA-1 dependent Th1 responses. J. Autoimmun. 94, 45–55 (2018).
Dutta, D. et al. Recruitment of calcineurin to the TCR positively regulates T cell activation. Nat. Immunol. 18, 196–204 (2016).
Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352, 595–599 (2016).
Lillemeier, B. F. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 11, 90–96 (2010).
Ashouri, J. F., Lo, W. L., Nguyen, T. T. T., Shen, L. & Weiss, A. ZAP70, too little, too much can lead to autoimmunity. Immunol. Rev. 307, 145–160 (2022).
Rudd, C. E., Taylor, A. & Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 229, 12–26 (2009).
Leitner, J., Herndler-Brandstetter, D., Zlabinger, G. J., Grubeck-Loebenstein, B. & Steinberger, P. CD58/CD2 Is the primary costimulatory pathway in human CD28-CD8+ T cells. J. Immunol. 195, 477–487 (2015).
Williams, C. et al. CD28 and TCR differentially impact naive and memory T cell responses. Discov. Immunol. 4, kyaf006 (2025).
Sanchez-Lockhart, M., Graf, B. & Miller, J. Signals and sequences that control CD28 localization to the central region of the immunological synapse. J. Immunol. 181, 7639–7648 (2008).
Navarro, M. N. & Cantrell, D. A. Serine-threonine kinases in TCR signaling. Nat. Immunol. 15, 808–814 (2014).
Thauland, T. J., Koguchi, Y., Dustin, M. L. & Parker, D. C. CD28-CD80 interactions control regulatory T cell motility and immunological synapse formation. J. Immunol. 193, 5894–5903 (2014).
Vignali, D. A., Collison, L. W. & Workman, C. J. How regulatory T cells work. Nat. Rev. Immunol. 8, 523–532 (2008).
Ecker, M. et al. SNX9-induced membrane tubulation regulates CD28 cluster stability and signalling. eLife 11, e67550 (2022).
Matalon, O., Reicher, B. & Barda-Saad, M. Wiskott-Aldrich syndrome protein-dynamic regulation of actin homeostasis: from activation through function and signal termination in T lymphocytes. Immunol. Rev. 256, 10–29 (2013).
Trzupek, D. et al. Discovery of CD80 and CD86 as recent activation markers on regulatory T cells by protein-RNA single-cell analysis. Genome Med. 12, 55 (2020).
Zhao, Y. et al. cis-B7:CD28 interactions at invaginated synaptic membranes provide CD28 co-stimulation and promote CD8+ T cell function and anti-tumor. immunity. Immun. 56, 1187–1203.e1112 (2023).
Wang, X. D. et al. TCR-induced sumoylation of the kinase PKC-θ controls T cell synapse organization and T cell activation. Nat. Immunol. 16, 1195–1203 (2015).
Garcia-Ortiz, A. et al. eNOS S-nitrosylates β-actin on Cys374 and regulates PKC-θ at the immune synapse by impairing actin binding to profilin-1. PLoS Biol. 15, e2000653 (2017).
Trefny, M. P. et al. Deletion of SNX9 alleviates CD8 T cell exhaustion for effective cellular cancer immunotherapy. Nat. Commun. 14, 86 (2023).
Wei, S. C. et al. Negative co-stimulation constrains T cell differentiation by imposing boundaries on possible cell states. Immunity 50, 1084–1098.e10 (2019).
Xu, X. et al. CTLA4 depletes T cell endogenous and trogocytosed B7 ligands via cis-endocytosis. J. Exp. Med. 220, e20221391 (2023).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2017).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017). PD-1 suppresses T cell function primarily by targeting CD28 phosphorylation through SHP2 and preventing co-stimulation.
Sanchez-Madrid, F. et al. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc. Natl Acad. Sci. USA 79, 7489–7493 (1982).
McArdel, S. L., Terhorst, C. & Sharpe, A. H. Roles of CD48 in regulating immunity and tolerance. Clin. Immunol. 164, 10–20 (2016).
Kaizuka, Y., Douglass, A. D., Vardhana, S., Dustin, M. L. & Vale, R. D. The coreceptor CD2 uses plasma membrane microdomains to transduce signals in T cells. J. Cell Biol. 185, 521–534 (2009).
Demetriou, P. et al. A dynamic CD2-rich compartment at the outer edge of the immunological synapse boosts and integrates signals. Nat. Immunol. 21, 1232–1243 (2020).
Delon, J., Stoll, S. & Germain, R. N. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol. Rev. 189, 51–63 (2002).
Cyster, J. G. Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102 (1999).
Friedman, R. S., Jacobelli, J. & Krummel, M. F. Surface-bound chemokines capture and prime T cells for synapse formation. Nat. Immunol. 7, 1101–1108 (2006).
Molon, B. et al. T cell costimulation by chemokine receptors. Nat. Immunol. 6, 465–471 (2005).
Laufer, J. M., Kindinger, I., Artinger, M., Pauli, A. & Legler, D. F. CCR7 is recruited to the immunological synapse, acts as co-stimulatory molecule and drives LFA-1 clustering for efficient T cell adhesion through ZAP70. Front. Immunol. 9, 3115 (2019).
Felce, J. H. et al. Single-molecule, super-resolution, and functional analysis of G protein-coupled receptor behavior within the T cell immunological synapse. Front. Cell Dev. Biol. 8, 608484 (2021).
Perez-Martinez, M. et al. F-actin-binding protein drebrin regulates CXCR4 recruitment to the immune synapse. J. Cell Sci. 123, 1160–1170 (2010).
Cascio, G. et al. CXCL12 regulates through JAK1 and JAK2 formation of productive immunological synapses. J. Immunol. 194, 5509–5519 (2015).
Kallikourdis, M. et al. The CXCR4 mutations in WHIM syndrome impair the stability of the T-cell immunologic synapse. Blood 122, 666–673 (2013).
Hickman, A. et al. LFA-1 activation enriches tumor-specific T cells in a cold tumor model and synergizes with CTLA-4 blockade. J. Clin. Invest. 132, e154152 (2022).
Geltink, R. I. K., Kyle, R. L. & Pearce, E. L. Unraveling the complex interplay between T cell metabolism and function. Annu. Rev. Immunol. 36, 461–488 (2018).
Schamel, W. W., Alarcon, B. & Minguet, S. The TCR is an allosterically regulated macromolecular machinery changing its conformation while working. Immunol. Rev. 291, 8–25 (2019).
Chen, Y. et al. Cholesterol inhibits TCR signaling by directly restricting TCR-CD3 core tunnel motility. Mol. Cell 82, 1278–1287.e5 (2022).
Wu, W., Shi, X. & Xu, C. Regulation of T cell signalling by membrane lipids. Nat. Rev. Immunol. 16, 690–701 (2016).
Howden, A. J. M. et al. Quantitative analysis of T cell proteomes and environmental sensors during T cell differentiation. Nat. Immunol. 20, 1542–1554 (2019).
Martin-Cofreces, N. B. et al. The chaperonin CCT controls T cell receptor-driven 3D configuration of centrioles. Sci. Adv. 6, eabb7242 (2020). CCT chaperonin helps the folding of de novo proteins induced by cognate TCR activation, such as tubulin, thereby regulating immune synapse asymmetric shape adoption and metabolic response.
Gomez-Moron, A. et al. Cytosolic protein translation regulates cell asymmetry and function in early TCR activation of human CD8+ T lymphocytes. Front. Immunol. 15, 1411957 (2024).
Araki, K. et al. Translation is actively regulated during the differentiation of CD8+ effector T cells. Nat. Immunol. 18, 1046–1057 (2017).
Ricciardi, S. et al. The translational machinery of human CD4+ T cells is poised for activation and controls the switch from quiescence to metabolic remodeling. Cell Metab. 28, 961 (2018).
Wolf, T. et al. Dynamics in protein translation sustaining T cell preparedness. Nat. Immunol. 21, 927–937 (2020).
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013). TCR activation promotes T cell proliferation and differentiation by upregulating specific amino acid transporters, such as system L-amino acid transporters, which increases the uptake of leucine, and subsequent activation of mTOR, translation and MYC expression.
Cibrian, D. et al. Targeting L-type amino acid transporter 1 in innate and adaptive T cells efficiently controls skin inflammation. J. Allergy Clin. Immunol. 145, 199–214.e11 (2020).
Cibrian, D. et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 17, 985–996 (2016).
Ogbechi, J. et al. LAT1 enables T cell activation under inflammatory conditions. J. Autoimmun. 138, 103031 (2023).
Sinclair, L. V. et al. Antigen receptor control of methionine metabolism in T cells. eLife 8, e44210 (2019).
Wu, J. et al. Asparagine enhances LCK signalling to potentiate CD8+ T-cell activation and anti-tumour responses. Nat. Cell Biol. 23, 75–86 (2021).
Hope, H. C. et al. Coordination of asparagine uptake and asparagine synthetase expression modulates CD8+ T cell activation. JCI Insight 6, e137761 (2021).
Woehrle, T. et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116, 3475–3484 (2010).
Wang, C. M., Ploia, C., Anselmi, F., Sarukhan, A. & Viola, A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 33, 1354–1364 (2014).
Mittelbrunn, M. & Sanchez-Madrid, F. Intercellular communication: diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 13, 328–335 (2012).
Vivar, O. I. et al. IFT20 controls LAT recruitment to the immune synapse and T-cell activation in vivo. Proc. Natl Acad. Sci. USA 113, 386–391 (2015).
Mittelbrunn, M. et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2, 282 (2011).
Gomez-Moron, A. et al. End-binding protein 1 regulates the metabolic fate of CD4+ T lymphoblasts and Jurkat T cells and the organization of the mitochondrial network. Front. Immunol. 14, 1197289 (2023).
Ruiz-Navarro, J. et al. Formin-like 1β phosphorylation at S1086 is necessary for secretory polarized traffic of exosomes at the immune synapse in Jurkat T lymphocytes. eLife 13, RP96942 (2024).
Blas-Rus, N. et al. Aurora A drives early signalling and vesicle dynamics during T-cell activation. Nat. Commun. 7, 11389 (2016).
Bustos-Moran, E., Blas-Rus, N., Martin-Cofreces, N. B. & Sanchez-Madrid, F. Microtubule-associated protein-4 controls nanovesicle dynamics and T cell activation. J. Cell Sci. 130, 1217–1223 (2017).
Torralba, D. et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 9, 2658 (2018). Extracellular vesicles enriched in mitochondrial DNA are released at the immune synapse by T cells and taken up by DCs, in which they activate an anti-viral programme through the cGAS–STING pathway.
Saliba, D. G. et al. Composition and structure of synaptic ectosomes exporting antigen receptor linked to functional CD40 ligand from helper T cells. eLife 8, e47528 (2019).
Stinchcombe, J. C. et al. Ectocytosis renders T cell receptor signaling self-limiting at the immune synapse. Science 380, 818–823 (2023).
Rodriguez-Fernandez, J. L., Riol-Blanco, L., Delgado-Martin, C. & Escribano-Diaz, C. The dendritic cell side of the immunological synapse: exploring terra incognita. Discov. Med. 8, 108–112 (2009).
Revy, P., Sospedra, M., Barbour, B. & Trautmann, A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat. Immunol. 2, 925–931 (2001).
Mittelbrunn, M. et al. Imaging of plasmacytoid dendritic cell interactions with T cells. Blood 113, 75–84 (2009).
Mempel, T. R., Henrickson, S. E. & Von Andrian, U. H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Benvenuti, F. et al. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J. Immunol. 172, 292–301 (2004).
de la Fuente, H. et al. Synaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanning. Mol. Biol. Cell 16, 3314–3322 (2005).
Turley, S. J. et al. Transport of peptide-MHC class II complexes in developing dendritic cells. Science 288, 522–527 (2000).
Benvenuti, F. et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science 305, 1150–1153 (2004).
Leithner, A. et al. Dendritic cell actin dynamics control contact duration and priming efficiency at the immunological synapse. J. Cell Biol. 220, e202006081 (2021).
Brossard, C. et al. Multifocal structure of the T cell – dendritic cell synapse. Eur. J. Immunol. 35, 1741–1753 (2005).
Blanchard, N. et al. Strong and durable TCR clustering at the T/dendritic cell immune synapse is not required for NFAT activation and IFN-γ production in human CD4+ T cells. J. Immunol. 173, 3062–3072 (2004).
Pulecio, J. et al. Cdc42-mediated MTOC polarization in dendritic cells controls targeted delivery of cytokines at the immune synapse. J. Exp. Med. 207, 2719–2732 (2010).
Vyas, J. M. et al. Tubulation of class II MHC compartments is microtubule dependent and involves multiple endolysosomal membrane proteins in primary dendritic cells. J. Immunol. 178, 7199–7210 (2007).
Rodriguez-Fernandez, J. L., Riol-Blanco, L. & Delgado-Martin, C. What is the function of the dendritic cell side of the immunological synapse. Sci. Signal. 3, re2 (2010).
Foster, N., Turnbull, E. L. & Macpherson, G. Migrating lymph dendritic cells contain intracellular CD40 that is mobilized to the immunological synapse during interactions with antigen-specific T lymphocytes. J. Immunol. 189, 5632–5637 (2012).
Fooksman, D. R., Shaikh, S. R., Boyle, S. & Edidin, M. Cutting edge: phosphatidylinositol 4,5-bisphosphate concentration at the APC side of the immunological synapse is required for effector T cell function. J. Immunol. 182, 5179–5182 (2009).
Gutierrez-Vazquez, C., Villarroya-Beltri, C., Mittelbrunn, M. & Sanchez-Madrid, F. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol. Rev. 251, 125–142 (2013).
Fernandez-Delgado, I., Calzada-Fraile, D. & Sanchez-Madrid, F. Immune regulation by dendritic cell extracellular vesicles in cancer immunotherapy and vaccines. Cancers 12, 3558 (2020).
Cespedes, P. F. et al. T-cell trans-synaptic vesicles are distinct and carry greater effector content than constitutive extracellular vesicles. Nat. Commun. 13, 3460 (2022).
Benvenuti, F. The dendritic cell synapse: a life dedicated to T cell activation. Front. Immunol. 7, 70 (2016).
Alcaraz-Serna, A. et al. Immune synapse instructs epigenomic and transcriptomic functional reprogramming in dendritic cells. Sci. Adv. 7, eabb9965 (2021). DCs reprogramme their gene expression through epigenetic DNA marks after synaptic contacts with T cells, which leads to changes such as enhanced chemotactic cell motility.
Calzada-Fraile, D. et al. Immune synapse formation promotes lipid peroxidation and MHC-I upregulation in licensed dendritic cells for efficient priming of CD8+ T cells. Nat. Commun. 14, 6772 (2023). Immune synaptic contacts with CD4+ T cells modify the metabolic fate of DCs by increasing lipid peroxidation, which fosters antigen loading in MHC class I and cross-presentation to CD8+ T cells.
Hole, C. R. et al. Induction of memory-like dendritic cell responses in vivo. Nat. Commun. 10, 2955 (2019).
Guarda, G. et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat. Immunol. 8, 743–752 (2007).
Hou, W. S. & Van Parijs, L. A Bcl-2-dependent molecular timer regulates the lifespan and immunogenicity of dendritic cells. Nat. Immunol. 5, 583–589 (2004).
Ma, D. Y. & Clark, E. A. The role of CD40 and CD154/CD40L in dendritic cells. Semin. Immunol. 21, 265–272 (2009).
Riol-Blanco, L. et al. Immunological synapse formation inhibits, via NF-κB and FOXO1, the apoptosis of dendritic cells. Nat. Immunol. 10, 753–760 (2009).
Giladi, A. et al. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol. 38, 629–637 (2020). Description of ‘sequencing physically interacting cells’ (PIC-seq) technology to characterize pathways involved in intercellular interaction through omics.
Pasqual, G. et al. Monitoring T cell-dendritic cell interactions in vivo by intercellular enzymatic labelling. Nature 553, 496–500 (2018). Characterization of a CD40–CD40L-based technology to detect ISs in vivo between DCs with CD4+ T cells in LIPSTIC mice.
Ge, Y. et al. Enzyme-mediated intercellular proximity labeling for detecting cell-cell interactions. J. Am. Chem. Soc. 141, 1833–1837 (2019).
Pasqual, G., Chudnovskiy, A. & Victora, G. D. Monitoring the interaction between dendritic cells and T cells in vivo with LIPSTIC. Methods Mol. Biol. 2618, 71–80 (2023).
Campos Canesso, M. C. et al. Identification of antigen-presenting cell-T cell interactions driving immune responses to food. Science 387, eado5088 (2025).
Chudnovskiy, A. et al. Proximity-dependent labeling identifies dendritic cells that drive the tumor-specific CD4+ T cell response. Sci. Immunol. 9, eadq8843 (2024).
Nakandakari-Higa, S. et al. Universal recording of immune cell interactions in vivo. Nature 627, 399–406 (2024).
Wu, R. & Murphy, K. M. DCs at the center of help: origins and evolution of the three-cell-type hypothesis. J. Exp. Med. 219, e20211519 (2022).
Ferris, S. T. et al. cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature 584, 624–629 (2020).
Gerner, M. Y., Casey, K. A. & Mescher, M. F. Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses. J. Immunol. 181, 155–164 (2008).
Joffre, O. P., Segura, E., Savina, A. & Amigorena, S. Cross-presentation by dendritic cells. Nat. Rev. Immunol. 12, 557–569 (2012).
Castellino, F. & Germain, R. N. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu. Rev. Immunol. 24, 519–540 (2006).
Borst, J., Ahrends, T., Babala, N., Melief, C. J. M. & Kastenmuller, W. CD4+ T cell help in cancer immunology and immunotherapy. Nat. Rev. Immunol. 18, 635–647 (2018).
Smith, C. M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol. 5, 1143–1148 (2004).
Bedoui, S., Heath, W. R. & Mueller, S. N. CD4+ T-cell help amplifies innate signals for primary CD8+ T-cell immunity. Immunol. Rev. 272, 52–64 (2016).
Bedenikovic, G., Crouse, J. & Oxenius, A. T-cell help dependence of memory CD8+ T-cell expansion upon vaccinia virus challenge relies on CD40 signaling. Eur. J. Immunol. 44, 115–126 (2013).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003).
Sun, J. C., Williams, M. A. & Bevan, M. J. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5, 927–933 (2004).
Matthews, K. E. et al. Increasing the survival of dendritic cells in vivo does not replace the requirement for CD4+ T cell help during primary CD8+ T cell responses. J. Immunol. 179, 5738–5747 (2007).
Dingjan, I. et al. Lipid peroxidation causes endosomal antigen release for cross-presentation. Sci. Rep. 6, 22064 (2016).
Canton, J. et al. The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens. Nat. Immunol. 22, 140–153 (2020). DNGR1 priming leads to damage of phagosomes and release of antigens to the cytosol that are processed and loaded onto MHC class I molecules for cross-presentation to cytotoxic CD8+ T cells.
Henry, C. M., Castellanos, C. A. & Reis e Sousa, C. DNGR-1-mediated cross-presentation of dead cell-associated antigens. Semin. Immunol. 66, 101726 (2023).
Gonzales, G. A. et al. The pore-forming apolipoprotein APOL7C drives phagosomal rupture and antigen cross-presentation by dendritic cells. Sci. Immunol. 9, eadn2168 (2024).
Xiong, P. et al. Regulation of expression and trafficking of perforin-2 by LPS and TNF-α. Cell Immunol. 320, 1–10 (2017).
Rodriguez-Silvestre, P. et al. Perforin-2 is a pore-forming effector of endocytic escape in cross-presenting dendritic cells. Science 380, 1258–1265 (2023). Perforin-2 allows endocytic escape of antigens to the cytosol for proteasome processing and loading onto MHC class I molecules in cross-presenting DCs; this helps to initiate anti-viral and anti-tumour immune responses.
Al-Alwan, M. M., Rowden, G., Lee, T. D. & West, K. A. Fascin is involved in the antigen presentation activity of mature dendritic cells. J. Immunol. 166, 338–345 (2001).
Chen, J. et al. Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy. J. Exp. Med. 214, 327–338 (2017).
Eickhoff, S. et al. Robust anti-viral immunity requires multiple distinct T cell-dendritic cell interactions. Cell 162, 1322–1337 (2015).
Hor, J. L. et al. Spatiotemporally distinct interactions with dendritic cell subsets facilitates CD4+ and CD8+ T cell activation to localized viral infection. Immunity 43, 554–565 (2015).
Allan, R. S. et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25, 153–162 (2006).
Yewdall, A. W., Drutman, S. B., Jinwala, F., Bahjat, K. S. & Bhardwaj, N. CD8+ T cell priming by dendritic cell vaccines requires antigen transfer to endogenous antigen presenting cells. PLoS One 5, e11144 (2010).
Ruhland, M. K. et al. Visualizing synaptic transfer of tumor antigens among dendritic cells. Cancer Cell 37, 786–799.e5 (2020).
Brandi, P. et al. Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections. Cell Rep. 38, 110184 (2022).
Del Fresno, C. et al. The Bacterial mucosal immunotherapy MV130 protects against SARS-CoV-2 infection and improves COVID-19 vaccines immunogenicity. Front. Immunol. 12, 748103 (2021).
Cheever, M. A. & Higano, C. S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 17, 3520–3526 (2011).
Heras-Murillo, I., Adan-Barrientos, I., Galan, M., Wculek, S. K. & Sancho, D. Dendritic cells as orchestrators of anticancer immunity and immunotherapy. Nat. Rev. Clin. Oncol. 21, 257–277 (2024).
Wculek, S. K. et al. Dendritic cells in cancer immunology and immunotherapy. Nat. Rev. Immunol. 20, 7–24 (2019).
Tanyi, J. L. et al. Personalized cancer vaccine effectively mobilizes antitumor T cell immunity in ovarian cancer. Sci. Transl. Med. 10, eaao5931 (2018).
Li, Q. et al. A dendritic cell vaccine for both vaccination and neoantigen-reactive T cell preparation for cancer immunotherapy in mice. Nat. Commun. 15, 10419 (2024).
Adamik, J. et al. Immuno-metabolic dendritic cell vaccine signatures associate with overall survival in vaccinated melanoma patients. Nat. Commun. 14, 7211 (2023). For DC vaccines, the administration of DCs with increased levels of mitochondrial respiration and fatty acid oxidation (as opposed to highly glycolytic cells) was associated with better patient survival in an NCT01622933 phase I study.
Wieland, A. et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 597, 274–278 (2021).
Fu, C. et al. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl Acad. Sci. USA 117, 23730–23741 (2020).
Cao, Y. et al. Dendritic cell-mimicking nanoparticles promote mRNA delivery to lymphoid organs. Adv. Sci. 10, e2302423 (2023). The coating of ionizable lipid nanoparticles with DC membranes favours their accumulation in lymphoid organs upon intramuscular or subcutaneous injection and promotes their further adsorption by DCs.
Gu, X., Erb, U., Buchler, M. W. & Zoller, M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int. J. Cancer 136, E74–E84 (2014).
Carrasco-Padilla, C. et al. T cell activation and effector function in the human Jurkat T cell model. Methods Cell Biol. 178, 25–41 (2023).
Villarroya-Beltri, C. et al. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980 (2013).
Garcia-Martin, R. et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 601, 446–451 (2022).
Liu, D. et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature 517, 214–218 (2014).
Zaretsky, I. et al. ICAMs support B cell interactions with T follicular helper cells and promote clonal selection. J. Exp. Med. 214, 3435–3448 (2022).
Crotty, S. T follicular helper cell biology: a decade of discovery and diseases. Immunity 50, 1132–1148 (2019).
Victora, G. D. & Nussenzweig, M. C. Germinal centers. Annu. Rev. Immunol. 30, 429–457 (2012).
Krautler, N. J. et al. Differentiation of germinal center B cells into plasma cells is initiated by high-affinity antigen and completed by Tfh cells. J. Exp. Med. 214, 1259–1267 (2017).
Calado, D. P. et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat. Immunol. 13, 1092–1100 (2012).
Dominguez-Sola, D. et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat. Immunol. 13, 1083–1091 (2012).
Luo, W., Weisel, F. & Shlomchik, M. J. B cell receptor and CD40 signaling are rewired for synergistic induction of the c-Myc transcription factor in germinal center B cells. Immunity 48, 313–326.e5 (2018).
Luo, W. et al. IL-21R signal reprogramming cooperates with CD40 and BCR signals to select and differentiate germinal center B cells. Sci. Immunol. 8, eadd1823 (2023).
Papa, I. et al. TFH-derived dopamine accelerates productive synapses in germinal centres. Nature 547, 318–323 (2017).
Fernandez-Messina, L. et al. Transfer of extracellular vesicle-microRNA controls germinal center reaction and antibody production. EMBO Rep. 21, e48925 (2020).
Carisey, A. F., Mace, E. M., Saeed, M. B., Davis, D. M. & Orange, J. S. Nanoscale dynamism of actin enables secretory function in cytolytic cells. Curr. Biol. 28, 489–502.e9 (2018).
de Jesus, M. et al. Single-cell topographical profiling of the immune synapse reveals a biomechanical signature of cytotoxicity. Sci. Immunol. 9, eadj2898 (2024).
Zheng, X. et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat. Immunol. 24, 802–813 (2023).
Balint, S. et al. Supramolecular attack particles are autonomous killing entities released from cytotoxic T cells. Science 368, 897–901 (2020).
Cassioli, C. et al. Activation-induced thrombospondin-4 works with thrombospondin-1 to build cytotoxic supramolecular attack particles. Proc. Natl Acad. Sci. USA 122, e2413866122 (2025).
Ambrose, A. R., Hazime, K. S., Worboys, J. D., Niembro-Vivanco, O. & Davis, D. M. Synaptic secretion from human natural killer cells is diverse and includes supramolecular attack particles. Proc. Natl Acad. Sci. USA 117, 23717–23720 (2020).
Dosil, S. G. et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. eLife 11, e76319 (2022).
Lisci, M. et al. Mitochondrial translation is required for sustained killing by cytotoxic T cells. Science 374, eabe9977 (2021).
Nunez-Andrade, N. et al. HDAC6 regulates the dynamics of lytic granules in cytotoxic T lymphocytes. J. Cell Sci. 129, 1305–1311 (2016).
Bonnet, V. et al. Cancer-on-a-chip model shows that the adenomatous polyposis coli mutation impairs T cell engagement and killing of cancer spheroids. Proc. Natl Acad. Sci. USA 121, e2316500121 (2024).
Cazaux, M. et al. Single-cell imaging of CAR T cell activity in vivo reveals extensive functional and anatomical heterogeneity. J. Exp. Med. 216, 1038–1049 (2019).
Gad, A. Z. et al. Molecular dynamics at immune synapse lipid rafts influence the cytolytic behavior of CAR T cells. Sci. Adv. 11, eadq8114 (2025).
Xu, X. et al. Phase separation of chimeric antigen receptor promotes immunological synapse maturation and persistent cytotoxicity. Immunity 57, 2755–2771.e8 (2024).
Gudipati, V. et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 21, 848–856 (2020).
Martin-Otal, C. et al. Phosphatidylserine as a tumor target for CAR-T cell therapy. J. Immunother. Cancer 13, e009468 (2025).
Xiao, Q. et al. Size-dependent activation of CAR-T cells. Sci. Immunol. 7, eabl3995 (2022).
Diez-Alonso, L. et al. Engineered T cells secreting anti-BCMA T cell engagers control multiple myeloma and promote immune memory in vivo. Sci. Transl. Med. 16, eadg7962 (2024).
Acknowledgements
We thank A. Ramiro, D. Sancho, J. M. Serrador and E. Martín-Gayo for critical reading of the manuscript. This work was supported by CIBER Cardiovascular from the Instituto de Salud Carlos III (Fondo de Investigación Sanitaria del Instituto de Salud Carlos III with co-funding from FEDER) to F.S.-M. and N.B.M.-C.; La Caixa Banking Foundation (LCF/PR/HR23/52430018) to F.S.-M. and N.B.M.-C.; AECC grant PRYCO223002PEIN to F.S.-M.; grants PID2023-147805NB-I00, TERINMUN/CPP2021-008385 and RECYTSEA/PLEC2022-009298 to F.S.-M. and PID2022–141895OB-I00 to N.B.M.-C. funded by MCIN/AEI/10.13039/501100011033, Next Generation EU and “ERDF A way of making Europe”; and Comunidad de Madrid (grant n° S2022/BMD-7209-INTEGRAMUNE-CM) to N.B.M.-C.
Author information
Authors and Affiliations
Contributions
N.B.M.-C. and D.C.-F. contributed to the writing, revision and figure design. F.S.-M. contributed to the writing and revision of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks Johnathan Canton, Michael Dustin, Gerone A. Gonzales and Susana Minguet for their contribution to the peer review of this work.
Additional information
We wish to dedicate this article to Diego Calzada-Fraile, who devoted invaluable time to studying immune synapses. Unfortunately, he passed away while this article was under revision. Diego possessed an innate talent and tireless thirst for knowledge, which he exhibited both in his scientific pursuits and in his personal life.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Aryl hydrocarbon receptor (AhR)
-
Cytosolic transcription factor, member of the basic helix–loop–helix/Per-Arnt-Sim (bHLH/PAS) protein family, that regulates gene expression upon translocation to the nucleus in response to aromatic (aryl) hydrocarbons.
- cDC1s
-
Classical type 1 dendritic cells, a subset of dendritic cells characterized by their ability to cross-present antigens.
- cDC2s
-
Classical type 2 dendritic cells, a subset of dendritic cells characterized by their ability to activate CD4+ T cell responses.
- Corolla shape
-
A compartment of the immune synapse where CD2 accumulates in the T cell membrane during its interaction with CD58 in trans, located at the external part of the cell–cell contact with the antigen-presenting cell.
- Cross-presentation
-
A process in which professional antigen-presenting cells present exogenously derived antigenic peptides on MHC class I molecules. The latter are typically loaded with peptides derived from intracellular proteins. Cross-presentation activates CD8+ cytotoxic T lymphocytes to clear infections and tumours bearing these antigens.
- Exhausted T cells
-
A T cell state that is associated with the upregulation of negative regulatory molecules and antigen unresponsiveness. It typically occurs following chronic T cell receptor stimulation with antigen.
- Immunoreceptor tyrosine-based activation motifs (ITAMs)
-
Conserved tandem sequences of four amino acids located in the cytoplasmic tails of immune signalling receptors, such as CD3ε and CD3ζ; they contain a tyrosine that can be phosphorylated.
- Inside-out signalling
-
An intracellular communication process that alters the conformation and function of transmembrane receptors and has a specific effect on their interaction with extracellular ligands.
- Licensing
-
A process in which dendritic cells interaction with activated CD4+ T cells causes the dendritic cells to acquire characteristics that enable them to promote the activation of cytotoxic T lymphocytes.
- Memory T cells
-
Typically long-lived T cells that have previously been activated by cognate antigen. They respond rapidly to subsequent encounters with antigen and can differentiate into effector T cells.
- Microdomains
-
Specialized, small regions within the plasma membrane characterized by the enrichment in specific lipids and proteins, thereby facilitating the formation of distinct functional subdomains.
- Naive T cells
-
T cells that have developed in and exited the thymus but have not yet been activated via their T cell receptor by cognate antigen in the periphery.
- Plasmacytoid DCs
-
A specialized subset of dendritic cells (DCs) that shows lymphoid characteristics and can produce type I interferons. They have been shown to be involved in viral responses and in immune tolerance.
- Regulatory T (Treg) cells
-
A specialized subset of CD4+ T cells that suppresses the activity of other T cells to maintain peripheral tolerance and to limit inflammation. Treg cells also modulate the functions of other cells, including dendritic cells.
- SRC homology 3 (SH3)
-
A small protein domain that binds poly-proline sequences and has a crucial role in protein–protein interactions in cell signalling pathways.
- Sumoylation
-
A reversible post-translational modification of proteins due to the covalent addition of a SUMO (small ubiquitin-related modifier) protein to a lysine residue in a target protein.
- Supported-lipid bilayers
-
Lipidic chemical platforms formed on solid surfaces that have been developed to mimic cell-membrane surfaces by integrating a diverse array of isolated molecules, frequently recombinant proteins or antibodies against bioactive molecules.
- WAVE regulatory complex
-
A complex comprising five components that is essential for proper actin cytoskeletal dynamics and remodelling in eukaryotic cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martín-Cófreces, N.B., Calzada-Fraile, D. & Sánchez-Madrid, F. How crosstalk at the immune synapse shapes T cell and dendritic cell biologys. Nat Rev Immunol (2026). https://doi.org/10.1038/s41577-025-01262-2
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41577-025-01262-2