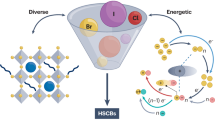
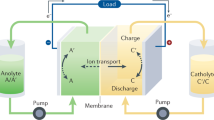
Abstract
Rapid developments in electric vehicles and portable electronic devices have fuelled demand for high-energy batteries. Along these lines, chalcogen-driven static conversion batteries (CSCBs), which operate by multielectron transfer, are attracting attention from academia and industry. Because of their high capacity and high voltage output, CSCBs are promising for efficient energy-storage applications. This Review surveys efforts to implement chalcogens with multivalent conversion as the high-energy redox-active component in various rechargeable batteries. First, we examine the evolution of CSCBs and summarize the merits and limitations of these batteries. Subsequently, we discuss state-of-the-art redox mechanisms, approaches for multivalent conversion activation, problems faced in using CSCBs and strategies for enhancing their performance. We also describe the potential of using chalcogens with multivalent conversion chemistry for halogen fixation in reversible multistage processes. Finally, we cover the challenges associated with the design of high-performance CSCBs and provide guidelines for their future design.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Zhang, C., Wei, Y.-L., Cao, P.-F. & Lin, M.-C. Energy storage system: current studies on batteries and power condition system. Renew. Sustain. Energy Rev. 82, 3091–3106 (2018).
Bolsen, T. Framing renewable energy. Nat. Energy 7, 1003–1004 (2022).
Larcher, D. & Tarascon, J.-M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2015).
Frondel, M., Ritter, N., Schmidt, C. M. & Vance, C. Economic impacts from the promotion of renewable energy technologies: the German experience. Energy Policy 38, 4048–4056 (2010).
Goodenough, J. B. Rechargeable batteries: challenges old and new. J. Solid State Electrochem. 16, 2019–2029 (2012).
Liang, Y. et al. A review of rechargeable batteries for portable electronic devices. InfoMat 1, 6–32 (2019).
Whittingham, M. S. Lithium batteries and cathode materials. Chem. Rev. 104, 4271 (2004).
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359 (2001).
Liu, M. et al. Aqueous rechargeable sodium ion batteries: developments and prospects. Mater. Today Energy 17, 100432 (2020).
Liang, Y., Lai, W.-H., Miao, Z. & Chou, S.-L. Nanocomposite materials for the sodium-ion battery: a review. Small 14, 1702514 (2018).
Zhang, Y., Liu, S., Ji, Y., Ma, J. & Yu, H. Emerging nonaqueous aluminum-ion batteries: challenges, status, and perspectives. Adv. Mater. 30, 1706310 (2018).
Yang, H. et al. The rechargeable aluminum battery: opportunities and challenges. Angew. Chem. Int. Ed. 58, 11978–11996 (2019).
Ma, L. et al. Realizing high zinc reversibility in rechargeable batteries. Nat. Energy 5, 743–749 (2020).
Lavine, M. S. Zinc can compete with lithium. Science 356, 392 (2017).
Aravindan, V., Gnanaraj, J., Lee, Y.-S. & Madhavi, S. Insertion-type electrodes for nonaqueous Li-ion capacitors. Chem. Rev. 114, 11619–11635 (2014).
Manthiram, A. A reflection on lithium-ion battery cathode chemistry. Nat. Commun. 11, 1550 (2020).
Liang, G., Mo, F., Ji, X. & Zhi, C. Non-metallic charge carriers for aqueous batteries. Nat. Rev. Mater. 6, 109–123 (2021).
Wang, H. et al. Recent advances in conversion-type electrode materials for post lithium-ion batteries. ACS Mater. Lett. 3, 956–977 (2021).
Yu, S.-H., Feng, X., Zhang, N., Seok, J. & Abruña, H. D. Understanding conversion-type electrodes for lithium rechargeable batteries. Acc. Chem. Res. 51, 273–281 (2018).
Boyjoo, Y. et al. Engineering nanoreactors for metal–chalcogen batteries. Energy Environ. Sci. 14, 540–575 (2021).
Wang, Y.-H. et al. Chalcogen cathode and its conversion electrochemistry in rechargeable Li/Na batteries. Sci. China Chem. 63, 1402–1415 (2020).
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Yu, X. & Manthiram, A. A progress report on metal–sulfur batteries. Adv. Funct. Mater. 30, 2004084 (2020).
Zhang, L. High-performance metal–chalcogen batteries. Batteries 9, 35 (2023).
Mu, P. et al. Crucial challenges and recent optimization progress of metal–sulfur battery electrolytes. Energy Fuels 35, 1966–1988 (2021).
Shi, F. et al. Advances in understanding and regulation of sulfur conversion processes in metal–sulfur batteries. J. Mater. Chem. A 10, 19412–19443 (2022).
Samsonov, G. V. Handbook of the Physicochemical Properties of the Elements (Springer, 2012).
Sudworth, J. & Tiley, A. Sodium Sulphur Battery (Springer, 1985).
Eftekhari, A. The rise of lithium–selenium batteries. Sustain. Energy Fuels 1, 14–29 (2017).
Mamantov, G. & Hvistendahl, J. Rechargeable high voltage low temperature molten, salt cell Na/β″-alumina/SCl3+ in AlCl3–NaCl. J. Electroanal. Chem. Interfacial Electrochem. 168, 451–466 (1984).
Peramunage, D. & Licht, S. A solid sulfur cathode for aqueous batteries. Science 261, 1029–1032 (1993).
Liu, Y. et al. Lithium–tellurium batteries based on tellurium/porous carbon composite. J. Mater. Chem. A 2, 12201–12207 (2014).
Wei, X. et al. An aqueous redox flow battery based on neutral alkali metal ferri/ferrocyanide and polysulfide electrolytes. J. Electrochem. Soc. 163, A5150 (2015).
Huang, X. et al. Rechargeable aluminum–selenium batteries with high capacity. Chem. Sci. 9, 5178–5182 (2018).
Zhang, X. F. et al. Rechargeable ultrahigh-capacity tellurium–aluminum batteries. Energy Environ. Sci. 12, 1918–1927 (2019).
Li, H. et al. Reversible electrochemical oxidation of sulfur in ionic liquid for high-voltage Al−S batteries. Nat. Commun. 12, 5714 (2021).
Chen, Z. et al. Tellurium with reversible six-electron transfer chemistry for high-performance zinc batteries. J. Am. Chem. Soc. 145, 20521–20529 (2023).
Chen, Z. et al. Zinc/selenium conversion battery: a system highly compatible with both organic and aqueous electrolytes. Energy Environ. Sci. 14, 2441–2450 (2021).
Si, J. et al. Deep multiphase conversion derived from NiTe2 nanosheets with preferred kinetics for highly reversible mild aqueous zinc–tellurium batteries. Adv. Energy Mater. 14, 2303982 (2024).
Li, H. et al. Superhalide-anion-motivator reforming-enabled bipolar manipulation toward longevous energy-type Zn||chalcogen batteries. Nano Lett. 24, 6465–6473 (2024).
Du, J. et al. A high-energy tellurium redox-amphoteric conversion cathode chemistry for aqueous zinc batteries. Adv. Mater. 36, 2313621 (2024).
Morag, A. et al. Unlocking four-electron conversion in tellurium cathodes for advanced magnesium-based dual-ion batteries. Angew. Chem. Int. Ed. 63, e202401818 (2024).
Qi, J. et al. Offense–defense-balanced strategy escorting tellurium oxidation conversion towards energetic and long-life Zn batteries. Adv. Energy Mater. 14, 2303616 (2024).
Yan, Z. et al. A reversible six-electron transfer cathode for advanced aqueous zinc batteries. Angew. Chem. Int. Ed. 62, e202312000 (2023).
Ma, W. et al. A twelve-electron conversion iodine cathode enabled by interhalogen chemistry in aqueous solution. Nat. Commun. 14, 5508 (2023).
Julien, C. M., Mauger, A., Zaghib, K. & Groult, H. Comparative issues of cathode materials for Li-ion batteries. Inorganics 2, 132–154 (2014).
Gupta, P., Pushpakanth, S., Haider, M. A. & Basu, S. Understanding the design of cathode materials for Na-ion batteries. ACS Omega 7, 5605–5614 (2022).
Zhu, Y.-H. et al. Recent progresses and prospects of cathode materials for non-aqueous potassium-ion batteries. Electrochem. Energy Rev. 1, 548–566 (2018).
Rashad, M., Asif, M., Wang, Y., He, Z. & Ahmed, I. Recent advances in electrolytes and cathode materials for magnesium and hybrid-ion batteries. Energy Storage Mater. 25, 342–375 (2020).
Wu, F., Yang, H., Bai, Y. & Wu, C. Paving the path toward reliable cathode materials for aluminum-ion batteries. Adv. Mater. 31, 1806510 (2019).
Yang, Q., Li, X., Chen, Z., Huang, Z. & Zhi, C. Cathode engineering for high energy density aqueous Zn batteries. Acc. Mater. Res. 3, 78–88 (2021).
Kim, J. et al. Organic batteries for a greener rechargeable world. Nat. Rev. Mater. 8, 54–70 (2023).
Liu, C., Neale, Z. G. & Cao, G. Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 19, 109–123 (2016).
Hoekstra, F. S., Raijmakers, L., Donkers, M. & Bergveld, H. J. Comparison of battery electromotive-force measurement and modelling approaches. J. Energy Storage 56, 105910 (2022).
Liu, L., Zhu, J. & Zheng, L. An effective method for estimating state of charge of lithium-ion batteries based on an electrochemical model and nernst equation. IEEE Access. 8, 211738–211749 (2020).
Kirchev, A. in Electrochemical Energy Storage for Renewable Sources and Grid Balancing (eds Moseley, P. T. & Garche, J.) 411–435 (Elsevier, 2015).
Vogel, L., Wonner, P. & Huber, S. M. Chalcogen bonding: an overview. Angew. Chem. Int. Ed. 58, 1880–1891 (2019).
Bratsch, S. G. Standard electrode potentials and temperature coefficients in water at 298.15 K. J. Phys. Chem. Ref. Data 18, 1–21 (1989).
Bouroushian, M. Electrochemistry of Metal Chalcogenides (Springer, 2010).
Srimuk, P., Su, X., Yoon, J., Aurbach, D. & Presser, V. Charge-transfer materials for electrochemical water desalination, ion separation and the recovery of elements. Nat. Rev. Mater. 5, 517–538 (2020).
Li, W., Wang, K. & Jiang, K. A low cost aqueous Zn–S battery realizing ultrahigh energy density. Adv. Sci. 7, 2000761 (2020).
Chen, Z. et al. Highly reversible positive-valence conversion of sulfur chemistry for high-voltage zinc–sulfur batteries. Adv. Mater. 36, 2402898 (2024).
Gao, Y. et al. Low-cost polyanion-type sulfate cathode for sodium-ion battery. Adv. Energy Mater. 11, 2101751 (2021).
Luo, C. et al. Selenium@Mesoporous carbon composite with superior lithium and sodium storage capacity. ACS Nano 7, 8003–8010 (2013).
Ho, P. C., Wang, J. Z., Meloni, F. & Vargas-Baca, I. Chalcogen bonding in materials chemistry. Coord. Chem. Rev. 422, 213464 (2020).
Kolb, S., Oliver, G. A. & Werz, D. B. Chemistry evolves, terms evolve, but phenomena do not evolve: from chalcogen–chalcogen interactions to chalcogen bonding. Angew. Chem. Int. Ed. 59, 22306–22310 (2020).
Yang, Z. et al. Thermodynamic analysis and perspective of aqueous metal-sulfur batteries. Mater. Today 49, 184–200 (2021).
Zhang, T. et al. A rechargeable 6-electron Al–Se battery with high energy density. Energy Storage Mater. 41, 667–676 (2021).
Liu, Y. T., Liu, S., Li, G. R. & Gao, X. P. Strategy of enhancing the volumetric energy density for lithium–sulfur batteries. Adv. Mater. 33, 2003955 (2021).
Shin, H. et al. Recent progress in high donor electrolytes for lithium–sulfur batteries. Adv. Energy Mater. 10, 2001456 (2020).
Zeng, L. et al. Chalcogen-bridged coordination polymer for the photocatalytic activation of aryl halides. Nat. Commun. 14, 4002 (2023).
Kolb, S., Oliver, G. A. & Werz, D. B. in Comprehensive Inorganic Chemistry III 3rd edn (eds Reedijk, J. & Poeppelmeier, K. R.) 602–651 (Elsevier, 2023).
Jiao, H., Tian, D., Li, S., Fu, C. & Jiao, S. A rechargeable Al–Te battery. ACS Appl. Energy Mater. 1, 4924–4930 (2018).
Bian, Y. et al. Using an AlCl3/urea ionic liquid analog electrolyte for improving the lifetime of aluminum-sulfur batteries. ChemElectroChem 5, 3607–3611 (2018).
Mamantov, G. et al. SCl3+AlCl4−: improved synthesis and characterization. J. Inorg. Nucl. Chem. 41, 260–261 (1979).
Chen, Z. et al. Anion chemistry enabled positive valence conversion to achieve a record high-voltage organic cathode for zinc batteries. Chem 8, 2204–2216 (2022).
Hicks, J., Vasko, P., Goicoechea, J. M. & Aldridge, S. Synthesis, structure and reaction chemistry of a nucleophilic aluminyl anion. Nature 557, 92–95 (2018).
Pauling, L. The nature of the chemical bond — 1992. J. Chem. Educ. 69, 519 (1992).
Michmerhuizen, A., Rose, K., Annankra, W. & Vander Griend, D. A. Radius ratio rule rescue. J. Chem. Educ. 94, 1480–1485 (2017).
Yang, Z. Z. & Davidson, E. R. Evaluation of a characteristic atomic radius by an ab initio method. Int. J. Quantum Chem. 62, 47–53 (1997).
Rahm, M., Hoffmann, R. & Ashcroft, N. Atomic and ionic radii of elements 1–96. Chem. Eur. J. 22, 14625–14632 (2016).
Sun, L. et al. Ionic liquid-based redox active electrolytes for supercapacitors. Adv. Funct. Mater. 32, 2203611 (2022).
Huang, Z. et al. Manipulating anion intercalation enables a high-voltage aqueous dual ion battery. Nat. Commun. 12, 3106 (2021).
Li, Z., Lv, W., Wu, G. & Zhang, W. Hollow nanotubes carbon@tellurium for high-performance Al–Te batteries. Electrochim. Acta 401, 139498 (2022).
Zhu, Y.-h et al. Decoupled aqueous batteries using pH-decoupling electrolytes. Nat. Rev. Chem. 6, 505–517 (2022).
Liang, Y. & Yao, Y. Designing modern aqueous batteries. Nat. Rev. Mater. 8, 109–122 (2023).
Huang, Z. et al. Anion chemistry in energy storage devices. Nat. Rev. Chem. 7, 616–631 (2023).
Eshetu, G. G. et al. Comprehensive insights into the reactivity of electrolytes based on sodium ions. ChemSusChem 9, 462–471 (2016).
Saha, S. Anion-induced electron transfer. Acc. Chem. Res. 51, 2225–2236 (2018).
Krossing, I. & Raabe, I. Relative stabilities of weakly coordinating anions: a computational study. Chem. Eur. J. 10, 5017–5030 (2004).
Liu, G., Sun, Q., Li, Q., Zhang, J. & Ming, J. Electrolyte issues in lithium–sulfur batteries: development, prospect, and challenges. Energy Fuels 35, 10405–10427 (2021).
Zhu, N., Zhang, K., Wu, F., Bai, Y. & Wu, C. Ionic liquid-based electrolytes for aluminum/magnesium/sodium-ion batteries. Energy Mater. Adv. 2021, 9204217 (2021).
Di Pietro, M. E. & Mele, A. Deep eutectics and analogues as electrolytes in batteries. J. Mol. Liq. 338, 116597 (2021).
Wang, Y. et al. Lean-water hydrogel electrolyte for zinc ion batteries. Nat. Commun. 14, 3890 (2023).
Smith, L. & Dunn, B. Opening the window for aqueous electrolytes. Science 350, 918–918 (2015).
Liang, T., Hou, R., Dou, Q., Zhang, H. & Yan, X. The applications of water‐in‐salt electrolytes in electrochemical energy storage devices. Adv. Funct. Mater. 31, 2006749 (2021).
Suo, L. et al. ‘Water-in-salt’ electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Zhang, C. et al. Tailoring the linking patterns of polypyrene cathodes for high-performance aqueous Zn dual-ion batteries. Energy Environ. Sci. 14, 462–472 (2021).
Zhao, J. et al. ‘Water-in-deep eutectic solvent’ electrolytes enable zinc metal anodes for rechargeable aqueous batteries. Nano Energy 57, 625–634 (2019).
Hao, J. et al. Boosting zinc electrode reversibility in aqueous electrolytes by using low‐cost antisolvents. Angew. Chem. Int. Ed. 60, 7366–7375 (2021).
Bi, H. et al. A universal approach to aqueous energy storage via ultralow‐cost electrolyte with super‐concentrated sugar as hydrogen‐bond‐regulated solute. Adv. Mater. 32, 2000074 (2020).
Jaumaux, P. et al. Non-flammable liquid and quasi-solid electrolytes toward highly-safe alkali metal-based batteries. Adv. Funct. Mater. 31, 2008644 (2021).
Ibrahim, M. A. A. & Safy, M. E. A. A new insight for chalcogen bonding based on point-of-charge approach. Phosphorus Sulfur Silicon Relat. Elem. 194, 444–454 (2019).
Yan, W., Zheng, M., Xu, C. & Chen, F.-E. Harnessing noncovalent interaction of chalcogen bond in organocatalysis: from the catalyst point of view. Green Synth. Catal. 2, 329–336 (2021).
Teng, Q., Ng, P. S., Leung, J. N. & Huynh, H. V. Donor strengths determination of pnictogen and chalcogen ligands by the Huynh electronic parameter and its correlation to sigma Hammett constants. Chem. A Eur. J. 25, 13956–13963 (2019).
Zhang, J. Y. et al. Sulfides organic polymer: novel cathode active material for rechargeable lithium batteries. J. Power Sources 168, 278–281 (2007).
Zeng, Z. et al. Pro-aromatic and anti-aromatic π-conjugated molecules: an irresistible wish to be diradicals. Chem. Soc. Rev. 44, 6578–6596 (2015).
Mo, Y. The resonance energy of benzene: a revisit. J. Phys. Chem. A 113, 5163–5169 (2009).
Healy, E. F. Organic chemistry as representation. Found. Chem. 23, 59–68 (2021).
Lu, Y. & Chen, J. Prospects of organic electrode materials for practical lithium batteries. Nat. Rev. Chem. 4, 127–142 (2020).
Speer, M. E. et al. Thianthrene-functionalized polynorbornenes as high-voltage materials for organic cathode-based dual-ion batteries. Chem. Commun. 51, 15261–15264 (2015).
Schon, T. B., McAllister, B. T., Li, P.-F. & Seferos, D. S. The rise of organic electrode materials for energy storage. Chem. Soc. Rev. 45, 6345–6404 (2016).
Janoschka, T., Hager, M. D. & Schubert, U. S. Powering up the future: radical polymers for battery applications. Adv. Mater. 24, 6397–6409 (2012).
Cui, F. et al. Activating selenium cathode chemistry for aqueous zinc-ion batteries. Adv. Mater. 35, 2306580 (2023).
Wang, H. et al. Electrochemically stable sodium metal–tellurium/carbon nanorods batteries. Adv. Energy Mater. 9, 1903046 (2019).
Dong, S. et al. Tellurium: a high-volumetric-capacity potassium-ion battery electrode material. Adv. Mater. 32, 1908027 (2020).
Chen, Z. et al. Tellurium: a high-performance cathode for magnesium ion batteries based on a conversion mechanism. ACS Nano 16, 5349–5357 (2022).
Chen, Z. et al. Aqueous zinc–tellurium batteries with ultraflat discharge plateau and high volumetric capacity. Adv. Mater. 32, 2001469 (2020).
Li, Z., Yuan, L., Yi, Z., Liu, Y. & Huang, Y. Confined selenium within porous carbon nanospheres as cathode for advanced Li–Se batteries. Nano Energy 9, 229–236 (2014).
Huang, X. et al. Rechargeable K–Se batteries based on metal-organic-frameworks-derived porous carbon matrix confined selenium as cathode materials. J. Colloid Interface Sci. 539, 326–331 (2019).
Liu, S. et al. An advanced high energy-efficiency rechargeable aluminum–selenium battery. Nano Energy 66, 104159 (2019).
Zhang, Z. et al. Novel design concepts of efficient Mg-ion electrolytes toward high-performance magnesium–selenium and magnesium–sulfur batteries. Adv. Energy Mater. 7, 1602055 (2017).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Li, S. et al. High performance room temperature sodium–sulfur battery by eutectic acceleration in tellurium-doped sulfurized polyacrylonitrile. ACS Appl. Energy Mater. 2, 2956–2964 (2019).
Zhao, X. et al. High performance potassium–sulfur batteries and their reaction mechanism. J. Mater. Chem. A 8, 10875–10884 (2020).
Li, R. et al. Achieving high-energy-density magnesium/sulfur battery via a passivation-free Mg–Li alloy anode. Energy Storage Mater. 50, 380–386 (2022).
Yang, M. et al. Boosting cathode activity and anode stability of Zn–S batteries in aqueous media through cosolvent-catalyst synergy. Angew. Chem. Int. Ed. 61, e202212666 (2022).
Angell, M. et al. High Coulombic efficiency aluminum-ion battery using an AlCl3-urea ionic liquid analog electrolyte. Proc. Natl Acad. Sci. USA 114, 834–839 (2017).
Ye, H. & Li, Y. Room-temperature metal–sulfur batteries: what can we learn from lithium–sulfur? InfoMat 4, e12291 (2022).
Chen, Z. et al. Metal–tellurium batteries: a rising energy storage system. Small Struct. 1, 2000005 (2020).
Lin, P., Jin, P., Hong, J. & Wang, Z. Battery voltage and state of power prediction based on an improved novel polarization voltage model. Energy Rep. 6, 2299–2308 (2020).
Yuan, B. et al. Study on the relationship between open-circuit voltage, time constant and polarization resistance of lithium-ion batteries. J. Electrochem. Soc. 169, 060513 (2022).
Liu, D. et al. A durable ZnS cathode for aqueous Zn–S batteries. Nano Energy 101, 107474 (2022).
Yao, W. et al. ZnS–SnS@ NC heterostructure as robust lithiophilicity and sulfiphilicity mediator toward high-rate and long-life lithium–sulfur batteries. ACS Nano 15, 7114–7130 (2021).
Dong, S., Liu, H., Hu, Y. & Chong, S. Cathode materials for rechargeable lithium–sulfur batteries: current progress and future prospects. ChemElectroChem 9, e202101564 (2022).
Jiao, Y. et al. Challenges and advances on low-temperature rechargeable lithium–sulfur batteries. Nano Res. 16, 8082–8096 (2023).
Ma, L., Lv, Y., Wu, J., Chen, Y. & Jin, Z. Recent advances in emerging non-lithium metal–sulfur batteries: a review. Adv. Energy Mater. 11, 2100770 (2021).
Shi, F. et al. Stable liquid-sulfur generation on transition-metal dichalcogenides toward low-temperature lithium–sulfur batteries. ACS Nano 16, 14412–14421 (2022).
Shi, F. et al. Unlocking liquid sulfur chemistry for fast-charging lithium–sulfur batteries. Nano Lett. 23, 7906–7913 (2023).
Zhou, G. et al. Supercooled liquid sulfur maintained in three-dimensional current collector for high-performance Li–S batteries. Sci. Adv. 6, eaay5098 (2020).
Pang, Q. et al. Tuning the electrolyte network structure to invoke quasi-solid state sulfur conversion and suppress lithium dendrite formation in Li–S batteries. Nat. Energy 3, 783–791 (2018).
Zhang, J. et al. Four-electron transfer reaction endows high capacity for aqueous Cu–Se battery. Adv. Energy Mater. 12, 2103998 (2022).
Zhao, J. et al. A high-durability aqueous Cu–S battery assisted by pre-copper electrochemistry. Nano Res. 16, 9553–9560 (2023).
He, S. et al. Rechargeable Al–chalcogen batteries: status, challenges, and perspectives. Adv. Energy Mater. 11, 2100769 (2021).
Zhang, L. & Liu, Y. Aqueous zinc–chalcogen batteries: emerging conversion-type energy storage systems. Batteries 9, 62 (2023).
Yang, Y., Zheng, G. & Cui, Y. Nanostructured sulfur cathodes. Chem. Soc. Rev. 42, 3018–3032 (2013).
Yuan, H. et al. A review of functional binders in lithium–sulfur batteries. Adv. Energy Mater. 8, 1802107 (2018).
Fu, Y. et al. Understanding of low‐porosity sulfur electrode for high‐energy lithium–sulfur batteries. Adv. Energy Mater. 13, 2203386 (2023).
Chen, Z. et al. Conversion‐type nonmetal elemental tellurium anode with high utilization for mild/alkaline zinc batteries. Adv. Mater. 33, 2105426 (2021).
Jain, R. et al. Nanostructuring versus microstructuring in battery electrodes. Nat. Rev. Mater. 7, 736–746 (2022).
Tang, Y., Zhang, Y., Li, W., Ma, B. & Chen, X. Rational material design for ultrafast rechargeable lithium-ion batteries. Chem. Soc. Rev. 44, 5926–5940 (2015).
Chen, H. et al. Monodispersed sulfur nanoparticles for lithium–sulfur batteries with theoretical performance. Nano Lett. 15, 798–802 (2015).
Zhou, J. et al. The impact of the particle size of a metal–organic framework for sulfur storage in Li–S batteries. J. Mater. Chem. A 3, 8272–8275 (2015).
Azaceta, E. et al. Particle atomic layer deposition as an effective way to enhance Li–S battery energy density. Mater. Today Energy 18, 100567 (2020).
Xie, Z. et al. Ultrathin 2D nonlayered tellurium nanosheets: facile liquid-phase exfoliation, characterization, and photoresponse with high performance and enhanced stability. Adv. Funct. Mater. 28, 1705833 (2018).
Yang, L. et al. Research progress on improving the sulfur conversion efficiency on the sulfur cathode side in lithium–sulfur batteries. Ind. Eng. Chem. Res. 59, 20979–21000 (2020).
Liu, T., Zhang, L., Cheng, B. & Yu, J. Hollow carbon spheres and their hybrid nanomaterials in electrochemical energy storage. Adv. Energy Mater. 9, 1803900 (2019).
Wang, J., Wan, J., Yang, N., Li, Q. & Wang, D. Hollow multishell structures exercise temporal–spatial ordering and dynamic smart behaviour. Nat. Rev. Chem. 4, 159–168 (2020).
Liu, J., Wickramaratne, N. P., Qiao, S. Z. & Jaroniec, M. Molecular-based design and emerging applications of nanoporous carbon spheres. Nat. Mater. 14, 763–774 (2015).
Deng, C., Wang, Z., Feng, L., Wang, S. & Yu, J. Electrocatalysis of sulfur and polysulfides in Li–S batteries. J. Mater. Chem. A 8, 19704–19728 (2020).
Zhang, Y. et al. Nanostructured metal chalcogenides for energy storage and electrocatalysis. Adv. Funct. Mater. 27, 1702317 (2017).
Zhou, L. et al. Sulfur reduction reaction in lithium–sulfur batteries: mechanisms, catalysts, and characterization. Adv. Energy Mater. 12, 2202094 (2022).
Zou, Y. et al. Toward high-performance lithium–sulfur batteries: efficient anchoring and catalytic conversion of polysulfides using P-doped carbon foam. ACS Appl. Mater. Interfaces 13, 50093–50100 (2021).
Al Salem, H., Babu, G., V. Rao, C. & Arava, L. M. R. Electrocatalytic polysulfide traps for controlling redox shuttle process of Li–S batteries. J. Am. Chem. Soc. 137, 11542–11545 (2015).
Li, H. et al. FeCo alloy catalysts promoting polysulfide conversion for advanced lithium–sulfur batteries. J. Energy Chem. 49, 339–347 (2020).
Shi, Z. et al. Boosting dual-directional polysulfide electrocatalysis via bimetallic alloying for printable Li–S batteries. Adv. Funct. Mater. 31, 2006798 (2021).
Fang, D. et al. Synergistic regulation of polysulfides conversion and deposition by MOF-derived hierarchically ordered carbonaceous composite for high-energy lithium–sulfur batteries. Adv. Funct. Mater. 29, 1900875 (2019).
Wang, F. et al. Single-atom electrocatalysts for lithium sulfur batteries: progress, opportunities, and challenges. ACS Mater. Lett. 2, 1450–1463 (2020).
Wang, J. et al. Single-atom catalyst boosts electrochemical conversion reactions in batteries. Energy Storage Mater. 18, 246–252 (2019).
Shen, Z. et al. Cation-doped ZnS catalysts for polysulfide conversion in lithium–sulfur batteries. Nat. Catal. 5, 555–563 (2022).
Zhao, M., Chen, X., Li, X. Y., Li, B. Q. & Huang, J. Q. An organodiselenide comediator to facilitate sulfur redox kinetics in lithium–sulfur batteries. Adv. Mater. 33, 2007298 (2021).
Meini, S., Elazari, R., Rosenman, A., Garsuch, A. & Aurbach, D. The use of redox mediators for enhancing utilization of Li2S cathodes for advanced Li–S battery systems. J. Phys. Chem. Lett. 5, 915–918 (2014).
Gerber, L. C. H. et al. Three-dimensional growth of Li2S in lithium–sulfur batteries promoted by a redox mediator. Nano Lett. 16, 549–554 (2016).
Liu, Y.-H. et al. A polymer organosulfur redox mediator for high-performance lithium-sulfur batteries. Energy Storage Mater. 46, 313–321 (2022).
Chen, H. et al. Catalytic materials for lithium-sulfur batteries: mechanisms, design strategies and future perspective. Mater. Today 52, 364–388 (2022).
Zhang, M. et al. Adsorption‐catalysis design in the lithium‐sulfur battery. Adv. Energy Mater. 10, 1903008 (2020).
Fan, J., Xiao, Q., Fang, Y., Li, L. & Yuan, W. A rechargeable Zn/graphite dual-ion battery with an ionic liquid-based electrolyte. Ionics 25, 1303–1313 (2019).
Lu, H. et al. The enhanced performance of lithium sulfur battery with ionic liquid-based electrolyte mixed with fluorinated ether. Ionics 25, 2685–2691 (2019).
Chu, W. et al. A low-cost deep eutectic solvent electrolyte for rechargeable aluminum-sulfur battery. Energy Storage Mater. 22, 418–423 (2019).
Pan, H., Cheng, Z., He, P. & Zhou, H. A review of solid-state lithium–sulfur battery: ion transport and polysulfide chemistry. Energy Fuels 34, 11942–11961 (2020).
Jones, S. D. et al. Design of polymeric zwitterionic solid electrolytes with superionic lithium transport. ACS Cent. Sci. 8, 169–175 (2022).
Jackson, D. T. & Nelson, P. N. Preparation and properties of some ion selective membranes: a review. J. Mol. Struct. 1182, 241–259 (2019).
Dai, C. et al. Enabling fast-charging selenium-based aqueous batteries via conversion reaction with copper ions. Nat. Commun. 13, 1863 (2022).
Li, X., Qin, Z., Deng, Y., Wu, Z. & Hu, W. Development and challenges of biphasic membrane‐less redox batteries. Adv. Sci. 9, 2105468 (2022).
Chen, A. et al. An immiscible phase-separation electrolyte and interface ion transfer electrochemistry enable zinc/lithium hybrid batteries with a 3.5 V-class operating voltage. Energy Environ. Sci. 16, 4054–4064 (2023).
Kim, W.-Y. et al. Demixing the miscible liquids: toward biphasic battery electrolytes based on the kosmotropic effect. Energy Environ. Sci. 15, 5217–5228 (2022).
Krebs, B. & Ahlers, F.-P. in Advances in Inorganic Chemistry Vol. 35 (Sykes, A.G. & Wilkinson, G.) 235–317 (Elsevier, 1990).
Kim, J. T. & Jorné, J. The kinetics of a chlorine graphite electrode in the zinc‐chlorine battery. J. Electrochem. Soc. 124, 1473 (1977).
Kralik, D. & Jorne, J. Hydrogen evolution and zinc nodular growth in the zinc chloride battery. J. Electrochem. Soc. 127, 2335 (1980).
Zhu, G. et al. High-capacity rechargeable Li/Cl2 batteries with graphite positive electrodes. J. Am. Chem. Soc. 144, 22505–22513 (2022).
Fan, X. et al. High power- and energy-density supercapacitors through the chlorine respiration mechanism. Angew. Chem. Int. Ed. 135, e202215342 (2023).
Liu, H. et al. A zinc–dual-halogen battery with a molten hydrate electrolyte. Adv. Mater. 32, 2004553 (2020).
Guo, Q. et al. Reversible insertion of I–Cl interhalogen in a graphite cathode for aqueous dual-ion batteries. ACS Energy Lett. 6, 459–467 (2021).
Holleck, G. L. The reduction of chlorine on carbon in AlCl3 – KCl – NaCl melts. J. Electrochem. Soc. 119, 1158 (1972).
Udachin, K. A., Alavi, S. & Ripmeester, J. A. Water–halogen interactions in chlorine and bromine clathrate hydrates: an example of multidirectional halogen bonding. J. Phys. Chem. C 117, 14176–14182 (2013).
Küpper, F. C. et al. Commemorating two centuries of iodine research: an interdisciplinary overview of current research. Angew. Chem. Int. Ed. 50, 11598–11620 (2011).
Yang, C. et al. Aqueous Li-ion battery enabled by halogen conversion–intercalation chemistry in graphite. Nature 569, 245–250 (2019).
Lantelme, F., Alexopoulos, H., Devilliers, D. & Chemla, M. A gas electrode: behavior of the chlorine injection electrode in fused alkali chlorides. J. Electrochem. Soc. 138, 1665 (1991).
Kim, K.-i et al. Reversible insertion of Mg-Cl superhalides in graphite as a cathode for aqueous dual-ion batteries. Angew. Chem. Int. Ed. 59, 19924–19928 (2020).
Skinner, H. A revision of some bond-energy values and the variation of bond-energy with bond-length. Trans. Faraday Soc. 41, 645–662 (1945).
Wang, W. & Hobza, P. Origin of the X−Hal (Hal = Cl, Br) bond-length change in the halogen-bonded complexes. J. Phys. Chem. A 112, 4114–4119 (2008).
Juárez‐Pérez, E. J. et al. A unique case of oxidative addition of interhalogens IX (X=Cl, Br) to organodiselone ligands: nature of the chemical bonding in asymmetric I–Se–X polarised hypervalent systems. Chem. Eur. J. 17, 11497–11514 (2011).
Sanderson, R. T. Electronegativity and bond energy. J. Am. Chem. Soc. 105, 2259–2261 (1983).
Chen, Z. et al. Selenium-anchored chlorine redox chemistry in aqueous zinc dual-ion batteries. Adv. Mater. 36, 2309330 (2024).
Acknowledgements
This research was supported by the National Key R&D Program of China under Project 2019YFA0705104 and the RGC Collaborative Research Fund under C1002-21G.
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation and reviewing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Guanjie He, Zheng-Long Xu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Z., Zhi, C. Chalcogens for high-energy batteries. Nat Rev Mater 10, 268–284 (2025). https://doi.org/10.1038/s41578-025-00773-7
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41578-025-00773-7
This article is cited by
-
Hierarchical-porous V-MOF cathodes enabling high-performance aqueous zinc-ion hybrid batteries
Science China Materials (2025)