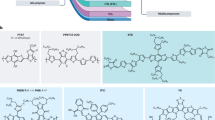
Abstract
The power conversion efficiencies of organic solar cells have now surpassed 20%, marking a considerable advance in performance. This progress raises an important question: which molecular or macromolecular modifications contribute most effectively to efficiency gains? Among these, halogenation — specifically fluorination and chlorination — has been a key driver of performance improvements, making it a particularly promising avenue for materials exploration. In this Perspective, we provide a comparative discussion of a broad range of non-halogenated and halogenated building blocks, acceptors and donors, evaluating the impact of halogenation on efficiency and scalability. We also examine critical challenges, including organic solar cell durability, large-scale manufacturability and the realistic costs associated with halogenation, positioning it as a central factor in performance optimization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
29 May 2025
In the version of the article initially published, in the Acknowledgements section, the US Office of Naval Research contract no. N00014-20-1-2110 (Georgia Institute of Technology) was incorrect and has now been amended to N00014-24-1-2110 in the HTML and PDF versions of the article.
References
Yi, J., Zhang, G., Yu, H. & Yan, H. Advantages, challenges and molecular design of different material-types used in organic solar cells. Nat. Rev. Mater. 9, 46–62 (2024).
Li, G. et al. What is the role of non-fullerene acceptor symmetry in polymer solar cell efficiency? Joule 7, 2152–2173 (2023).
Zhang, G. C. et al. Renewed prospects for organic photovoltaics. Chem. Rev. 122, 14180–14274 (2022).
Zhang, J. Q., Tan, H. S., Guo, X. G., Facchetti, A. & Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 3, 720–731 (2018).
Li, G., Zhu, R. & Yang, Y. Polymer solar cells. Nat. Photon. 6, 153–161 (2012).
Yan, C. et al. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 3, 18003 (2018).
Wang, Y. et al. The critical role of the donor polymer in the stability of high-performance non-fullerene acceptor organic solar cells. Joule 7, 810–829 (2023).
An, K. et al. Mastering morphology of non-fullerene acceptors towards long-term stable organic solar cells. Nat. Commun. 14, 2688 (2023).
Zhao, F., Wang, C. & Zhan, X. Morphology control in organic solar cells. Adv. Energy Mater. 8, 1703147 (2018).
Wang, G., Melkonyan, F. S., Facchetti, A. & Marks, T. J. All-polymer solar cells: recent progress, challenges, and prospects. Angew. Chem. Int. Ed. 58, 4129–4142 (2019).
Chen, L. X. Organic solar cells: recent progress and challenges. ACS Energy Lett. 4, 2537–2539 (2019).
Zhu, L. et al. Single-junction organic solar cells with over 19% efficiency enabled by a refined double-fibril network morphology. Nat. Mater. 21, 656–663 (2022).
Li, H. et al. Beyond fullerenes: design of nonfullerene acceptors for efficient organic photovoltaics. J. Am. Chem. Soc. 136, 14589–14597 (2014).
Lai, H. et al. Molecular skeletons modification induces distinctive aggregation behaviors and boosts the efficiency over 19% in organic solar cells. CCS Chem. 0, 1–14 (2024).
Lai, H., Deng, Z. & He, F. C-shaped A–D–A non-fullerene acceptor achieves efficient organic solar cells. Joule 8, 572–575 (2024).
Wu, J. et al. Towards a bright future: the versatile applications of organic solar cells. Mater. Rep. Energy 1, 100062 (2021).
Li, Y., Xu, G., Cui, C. & Li, Y. Flexible and semitransparent organic solar cells. Adv. Energy Mater. 8, 1701791 (2018).
Cui, Y., Hong, L. & Hou, J. Organic photovoltaic cells for indoor applications: opportunities and challenges. ACS Appl. Mater. Interfaces 12, 38815–38828 (2020).
Arai, R., Furukawa, S., Hidaka, Y., Komiyama, H. & Yasuda, T. High-performance organic energy-harvesting devices and modules for self-sustainable power generation under ambient indoor lighting environments. ACS Appl. Mater. Interfaces 11, 9259–9264 (2019).
Xiong, H. et al. General room-temperature Suzuki–Miyaura polymerization for organic electronics. Nat. Mater. 23, 695–702 (2024).
Li, G. P. et al. Non-fullerene acceptors with direct and indirect hexa-fluorination afford >17% efficiency in polymer solar cells. Energy Environ. Sci. 15, 645–659 (2022).
Li, G. et al. Systematic merging of nonfullerene acceptor π-extension and tetrafluorination strategies affords polymer solar cells with >16% efficiency. J. Am. Chem. Soc. 143, 6123–6139 (2021).
Cui, Y. et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 10, 2515 (2019).
Liang, H. et al. A rare case of brominated small molecule acceptors for high-efficiency organic solar cells. Nat. Commun. 14, 4707 (2023).
Aldrich, T. J. et al. Fluorination effects on indacenodithienothiophene acceptor packing and electronic structure, end-group redistribution, and solar cell photovoltaic response. J. Am. Chem. Soc. 141, 3274–3287 (2019).
Fu, J. et al. 31% Binary organic solar cell and low non-radiative recombination enabled by non-monotonic intermediate state transition. Nat. Commun. 14, 1760 (2023).
Brinkmann, K. O. et al. Perovskite–organic tandem solar cells. Nat. Rev. Mater. 9, 202–217 (2024).
Wang, J. et al. Tandem organic solar cells with 20.6% efficiency enabled by reduced voltage losses. Natl Sci. Rev. 10, nwad085 (2023).
Yuan, J. et al. Single-junction organic solar cell with over 15% efficiency using fused-ring acceptor with electron-deficient core. Joule 3, 1140–1151 (2019).
Li, C. et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 6, 605–613 (2021).
Po, R., Bianchi, G., Carbonera, C. & Pellegrino, A. ‘All that glisters is not gold’: an analysis of the synthetic complexity of efficient polymer donors for polymer solar cells. Macromolecules 48, 453–461 (2015).
Moser, M., Wadsworth, A., Gasparini, N. & McCulloch, I. Challenges to the success of commercial organic photovoltaic products. Adv. Energy Mater. 11, 2100056 (2021).
Günther, M. et al. Models and mechanisms of ternary organic solar cells. Nat. Rev. Mater. 8, 456–471 (2023).
Sangwan, V. K. et al. Elucidating performance degradation mechanisms in non-fullerene acceptor solar cells. J. Mater. Chem. A 12, 21213–21229 (2024).
Eisner, F. D. et al. Hybridization of local exciton and charge-transfer states reduces nonradiative voltage losses in organic solar cells. J. Am. Chem. Soc. 141, 6362–6374 (2019).
Coropceanu, V., Chen, X.-K., Wang, T., Zheng, Z. & Brédas, J.-L. Charge-transfer electronic states in organic solar cells. Nat. Rev. Mater. 4, 689–707 (2019).
Shrotriya, V. et al. Accurate measurement and characterization of organic solar cells. Adv. Funct. Mater. 16, 2016–2023 (2006).
Zhu, W. et al. Quantitative relationships between film morphology, charge carrier dynamics, and photovoltaic performance in bulk-heterojunction binary vs. ternary acceptor blends. Energy Environ. Sci. 16, 1234–1250 (2023).
Machui, F. et al. Cost analysis of roll-to-roll fabricated ITO free single and tandem organic solar modules based on data from manufacture. Energy Environ. Sci. 7, 2792–2802 (2014).
Forti, G. et al. Recent advances in non-fullerene acceptors of the IDIC/ITIC families for bulk-heterojunction organic solar cells. Int. J. Mol. Sci. 21, 8085 (2020).
Zeng, A. et al. A chlorinated donor polymer achieving high-performance organic solar cells with a wide range of polymer molecular weight. Adv. Funct. Mater. 31, 2102413 (2021).
Hu, Z. et al. Design and synthesis of chlorinated benzothiadiazole-based polymers for efficient solar energy conversion. ACS Energy Lett. 2, 753–758 (2017).
Mo, D. et al. Chlorination of low-band-gap polymers: toward high-performance polymer solar cells. Chem. Mater. 29, 2819–2830 (2017).
Chen, H. et al. A chlorinated π-conjugated polymer donor for efficient organic solar cells. Joule 2, 1623–1634 (2018).
Zhang, Z. G. & Li, Y. F. Polymerized small-molecule acceptors for high-performance all-polymer solar cells. Angew. Chem. Int. Ed. 60, 4422–4433 (2021).
Sun, C. K. et al. A low cost and high performance polymer donor material for polymer solar cells. Nat. Commun. 9, 743 (2018).
Zhang, M. et al. Efficient and stable high-entropy organic photovoltaics. Joule 9, 101851 (2025).
Bannock, J. E., Xu, W. M., Baissas, T., Heeney, M. & de Mello, J. C. Rapid flow-based synthesis of poly(3-hexylthiophene) using 2-methyltetrahydrofuran as a bio-derived reaction solvent. Eur. Polym. J. 80, 240–246 (2016).
Xu, X., Zhang, G., Yu, L., Li, R. & Peng, Q. P3HT-based polymer solar cells with 8.25% efficiency enabled by a matched molecular acceptor and smart green-solvent processing technology. Adv. Mater. 31, 1906045 (2019).
Holliday, S. et al. High-efficiency and air-stable P3HT-based polymer solar cells with a new non-fullerene acceptor. Nat. Commun. 7, 11585 (2016).
Chandrasekaran, N., Kumar, A., Thomsen, L., Kabra, D. & McNeill, C. R. High performance as-cast P3HT:PCBM devices: understanding the role of molecular weight in high regioregularity P3HT. Mater. Adv. 2, 2045–2054 (2021).
Ansari, M. A., Mohiuddin, S., Kandemirli, F. & Malik, M. I. Synthesis and characterization of poly(3-hexylthiophene): improvement of regioregularity and energy band gap. RSC Adv. 8, 8319–8328 (2018).
Kim, Y. et al. A strong regioregularity effect in self-organizing conjugated polymer films and high-efficiency polythiophene: fullerene solar cells. Nat. Mater. 5, 197–203 (2006).
Sirringhaus, H. et al. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 401, 685–688 (1999).
Bronstein, H. A. & Luscombe, C. K. Externally initiated regioregular P3HT with controlled molecular weight and narrow polydispersity. J. Am. Chem. Soc. 131, 12894–12895 (2009).
Ballantyne, A. M. et al. The effect of poly(3-hexylthiophene) molecular weight on charge transport and the performance of polymer: fullerene solar cells. Adv. Funct. Mater. 18, 2373–2380 (2008).
Homyak, P. D. et al. Systematic fluorination of P3HT: synthesis of P(3HT-co-3H4FT)s by direct arylation polymerization, characterization, and device performance in OPVs. Macromolecules 49, 3028–3037 (2016).
Gohier, F., Frère, P. & Roncali, J. 3-Fluoro-4-hexylthiophene as a building block for tuning the electronic properties of conjugated polythiophenes. J. Org. Chem. 78, 1497–1503 (2013).
Koo, B., Sletten, E. M. & Swager, T. M. Functionalized poly(3-hexylthiophene)s via lithium–bromine exchange. Macromolecules 48, 229–235 (2015).
Fei, Z. P. et al. Influence of backbone fluorination in regioregular poly(3-alkyl-4-fluoro)thiophenes. J. Am. Chem. Soc. 137, 6866–6879 (2015).
Carsten, B., He, F., Son, H. J., Xu, T. & Yu, L. P. Stille polycondensation for synthesis of functional materials. Chem. Rev. 111, 1493–1528 (2011).
Pouliot, J. R., Grenier, F., Blaskovits, J. T., Beaupre, S. & Leclerc, M. Direct (hetero)arylation polymerization: simplicity for conjugated polymer synthesis. Chem. Rev. 116, 14225–14274 (2016).
Aldrich, T. J. et al. Suppressing defect formation pathways in the direct C–H arylation polymerization of photovoltaic copolymers. Macromolecules 51, 9140–9155 (2018).
Aldrich, T. J. et al. Stable postfullerene solar cells via direct C–H arylation polymerization. Morphology–performance relationships. Chem. Mater. 31, 4313–4321 (2019).
Zheng, Z. et al. PBDB-T and its derivatives: a family of polymer donors enables over 17% efficiency in organic photovoltaics. Mater. Today 35, 115–130 (2020).
Liu, Q. et al. 18% Efficiency organic solar cells. Sci. Bull. 65, 272–275 (2020).
Zhu, W. et al. Fluorine tuning of morphology, energy loss, and carrier dynamics in perylenediimide polymer solar cells. ACS Energy Lett. 4, 2695–2702 (2019).
Zhang, M., Guo, X., Ma, W., Ade, H. & Hou, J. A large-bandgap conjugated polymer for versatile photovoltaic applications with high performance. Adv. Mater. 27, 4655–4660 (2015).
Zhang, S., Qin, Y., Zhu, J. & Hou, J. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv. Mater. 30, 1800868 (2018).
Ye, L. et al. Manipulating aggregation and molecular orientation in all-polymer photovoltaic cells. Adv. Mater. 27, 6046–6054 (2015).
Zhang, K.-N. et al. Multiple temporal-scale photocarrier dynamics induced by synergistic effects of fluorination and chlorination in highly efficient nonfullerene organic solar cells. Sol. RRL 4, 1900552 (2020).
Hu, R. et al. Charge photogeneration and recombination in ternary polymer solar cells based on compatible acceptors. J. Mater. Sci. 56, 14181–14195 (2021).
Zheng, Z. et al. A highly efficient non-fullerene organic solar cell with a fill factor over 0.80 enabled by a fine-tuned hole-transporting layer. Adv. Mater. 30, 1801801 (2018).
Son, H. J. et al. Synthesis of fluorinated polythienothiophene-co-benzodithiophenes and effect of fluorination on the photovoltaic properties. J. Am. Chem. Soc. 133, 1885–1894 (2011).
Kelly, M. A. et al. Incorporating fluorine substitution into conjugated polymers for solar cells: three different means, same results. J. Phys. Chem. C 121, 2059–2068 (2017).
Zhang, H. et al. Over 14% efficiency in organic solar cells enabled by chlorinated nonfullerene small-molecule acceptors. Adv. Mater. 30, e1800613 (2018).
Du, X. et al. Unraveling the microstructure-related device stability for polymer solar cells based on nonfullerene small-molecular acceptors. Adv. Mater. 32, 1908305 (2020).
Ding, P., Yang, D., Yang, S. & Ge, Z. Stability of organic solar cells: toward commercial applications. Chem. Soc. Rev. 53, 2350–2387 (2024).
Jia, T. et al. All-polymer solar cells with efficiency approaching 16% enabled using a dithieno[3′,2′:3,4;2′′,3′′:5,6]benzo[1,2-c][1,2,5]thiadiazole (fDTBT)-based polymer donor. J. Mater. Chem. A 9, 8975–8983 (2021).
Ma, X. et al. Approaching 18% efficiency of ternary organic photovoltaics with wide bandgap polymer donor and well compatible Y6: Y6-1O as acceptor. Natl Sci. Rev. 8, nwaa305 (2020).
Zhao, Q., Qu, J. & He, F. Chlorination: an effective strategy for high-performance organic solar cells. Adv. Sci. 7, 2000509 (2020).
Heumueller, T. et al. Morphological and electrical control of fullerene dimerization determines organic photovoltaic stability. Energy Environ. Sci. 9, 247–256 (2016).
Schroeder, B. C. et al. Enhancing fullerene-based solar cell lifetimes by addition of a fullerene dumbbell. Angew. Chem. Int. Ed. 53, 12870–12875 (2014).
Lin, Y. Z. et al. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 27, 1170–1174 (2015).
Fei, Z. et al. An alkylated indacenodithieno[3,2-b]thiophene-based nonfullerene acceptor with high crystallinity exhibiting single junction solar cell efficiencies greater than 13% with low voltage losses. Adv. Mater. 30, 1705209 (2018).
Swick, S. M. et al. Closely packed, low reorganization energy π-extended postfullerene acceptors for efficient polymer solar cells. Proc. Natl Acad. Sci. USA 115, E8341 (2018).
Swick, S. M. et al. Fluorinating π-extended molecular acceptors yields highly connected crystal structures and low reorganization energies for efficient solar cells. Adv. Energy Mater. 10, 2000635 (2020).
Wang, H. et al. Bromination: an alternative strategy for non-fullerene small molecule acceptors. Adv. Sci. 7, 1903784 (2020).
Cui, Y. et al. Organic photovoltaic cell with 17% efficiency and superior processability. Natl Sci. Rev. 7, 1239–1246 (2020).
Cui, Y. et al. Single-junction organic photovoltaic cells with approaching 18% efficiency. Adv. Mater. 32, 1908205 (2020).
Zou, Y. et al. Peripheral halogenation engineering controls molecular stacking to enable highly efficient organic solar cells. Energy Environ. Sci. 15, 3519–3533 (2022).
Li, D. et al. Halogenated nonfused ring electron acceptor for organic solar cells with a record efficiency of over 17%. Adv. Mater. 36, 2310362 (2024).
Cai, Y. et al. A well-mixed phase formed by two compatible non-fullerene acceptors enables ternary organic solar cells with efficiency over 18%. Adv. Mater. 33, 2101733 (2021).
He, C. et al. Manipulating the D:A interfacial energetics and intermolecular packing for 19.2% efficiency organic photovoltaics. Energy Environ. Sci. 15, 2537–2544 (2022).
Cai, G. et al. Computational chemistry-assisted design of a non-fullerene acceptor enables 17.4% efficiency in high-boiling-point solvent processed binary organic solar cells. J. Mater. Chem. A 10, 21061–21071 (2022).
Wang, Y. et al. Enhancing efficiency and stability through halogenation of dimerized acceptors in quasiplanar heterojunction organic solar cells. ACS Mater. Lett. 6, 2506–2514 (2024).
Qin, F. et al. Conjugated versus nonconjugated polymerized small-molecule acceptors. Photovoltaic response and mechanical properties. ACS Energy Lett. 8, 4733–4745 (2023).
Su, N. et al. High-efficiency all-polymer solar cells with poly-small-molecule acceptors having π-extended units with broad near-IR absorption. ACS Energy Lett. 6, 728–738 (2021).
Du, J. et al. Polymerized small molecular acceptor based all-polymer solar cells with an efficiency of 16.16% via tuning polymer blend morphology by molecular design. Nat. Commun. 12, 5264 (2021).
Wang, H. et al. Oligomeric acceptor: a ‘two-in-one’ strategy to bridge small molecules and polymers for stable solar devices. Angew. Chem. Int. Ed. 61, e202201844 (2022).
Yu, H. et al. A difluoro-monobromo end group enables high-performance polymer acceptor and efficient all-polymer solar cells processable with green solvent under ambient condition. Adv. Funct. Mater. 31 (2021).
Yan, H. et al. A high-mobility electron-transporting polymer for printed transistors. Nature 457, 679–686 (2009).
Wang, H., Wang, X., Fan, P., Yang, X. & Yu, J. Enhanced power conversion efficiency of P3HT:PC71BM bulk heterojunction polymer solar cells by doping a high-mobility small organic molecule. Int. J. Photoenergy 2015, 982064 (2015).
Pan, J. et al. π-extended nonfullerene acceptors for efficient organic solar cells with a high open-circuit voltage of 0.94 V and a low energy loss of 0.49 eV. ACS Appl. Mater. Interfaces 13, 22531–22539 (2021).
Zhang, X. et al. Systematically controlling acceptor fluorination optimizes hierarchical morphology, vertical phase separation, and efficiency in non-fullerene organic solar cells. Adv. Energy Mater. 12, 2102172 (2022).
Chen, Z. et al. Iodinated electron acceptor with significantly extended exciton diffusion length for efficient organic photovoltaic cells. Angew. Chem. Int. Ed. 63, e202317892 (2024).
Yu, H. et al. Fluorinated end group enables high-performance all-polymer solar cells with near-infrared absorption and enhanced device efficiency over 14%. Adv. Energy Mater. 11, 2003171 (2021).
Sun, C. et al. Achieving fast charge separation and low nonradiative recombination loss by rational fluorination for high-efficiency polymer solar cells. Adv. Mater. 31, 1905480 (2019).
Xu, X. et al. High-performance all-polymer solar cells based on fluorinated naphthalene diimide acceptor polymers with fine-tuned crystallinity and enhanced dielectric constants. Nano Energy 45, 368–379 (2018).
Acknowledgements
The authors gratefully acknowledge the support of US Office of Naval Research under contract nos N00014-24-1-2110 (Georgia Institute of Technology) and N00014-24-1-2109 (Northwestern University), the Qatar National Research Foundation under grant NPRP12S-0304-190227/02-484761 and the Northwestern University Materials Research Science and Engineering Center Award under NSF grant DMR-DMR-2308691.
Author information
Authors and Affiliations
Contributions
G.L. assembled and researched data for the paper. G.L., M.A.-H., A.F. and T.J.M. drafted and revised the paper. All authors contributed substantially to discussion of the content and reviewed and/or edited the paper before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Frédéric Laquai and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, G., Al-Hashimi, M., Facchetti, A. et al. Decoding the halogenation cost-performance paradox in organic solar cells. Nat Rev Mater 10, 617–631 (2025). https://doi.org/10.1038/s41578-025-00804-3
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41578-025-00804-3