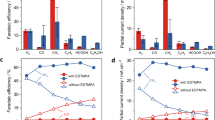
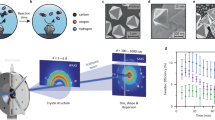
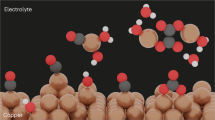
Abstract
Copper and copper-based catalysts can electrochemically convert CO2 into ethylene and higher alcohols, among other products, at room temperature and pressure. This approach may be suitable for the production of high-value compounds. However, such a promising reaction is heavily burdened by the instability of copper during CO2 reduction. To date, non-copper catalysts have also failed to supplant the activity and selectivity of copper, leaving CO2-to-C2 electrolysis in the balance. In this Perspective, we discuss copper catalyst instability from both the atomistic and the microstructure viewpoint. We motivate that increased fundamental understanding, material design and operational approaches, along with increased reporting of failure mechanisms, will contribute to overcoming the barriers to multi-year operation. Our narrative focuses on the copper catalyst reconstruction occurring during CO2 reduction as one of the major causes inducing loss of C2 activity. We conclude with a rational path forward towards longer operations of CO2-to-C2 electrolysis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
27 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41578-025-00825-y
References
Scott, A. Global plastics glut and weak regulations hurt European recyclers. C&EN https://cen.acs.org/business/economy/Global-plastics-glut-weak-regulations/102/i4 (2024).
Belsa, B. et al. Materials challenges on the path to gigatonne CO2 electrolysis. Nat. Rev. Mater. 9, 535–549 (2024).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Schreiber, M. W. Industrial CO2 electroreduction to ethylene: main technical challenges. Curr. Opin. Electrochem. 44, 101438 (2024).
Sisler, J. et al. Ethylene electrosynthesis: a comparative techno-economic analysis of alkaline vs membrane electrode assembly vs CO2–CO–C2H4 tandems. ACS Energy Lett. 6, 997–1002 (2021).
Popović, S. et al. Stability and degradation mechanisms of copper‐based catalysts for electrochemical CO2 reduction. Angew. Chem. Int. Ed. 59, 14736–14746 (2020).
Wu, H., Yu, H., Chow, Y., Webley, P. A. & Zhang, J. Toward durable CO2 electroreduction with Cu‐based catalysts via understanding their deactivation modes. Adv. Mater. 36, 2403217 (2024).
Lai, W. et al. Dynamic evolution of active sites in electrocatalytic CO2 reduction reaction: fundamental understanding and recent progress. Adv. Funct. Mater. 32, 2111193 (2022).
Lai, W., Qiao, Y., Wang, Y. & Huang, H. Stability issues in electrochemical CO2 reduction: recent advances in fundamental understanding and design strategies. Adv. Mater. 35, 2306288 (2023).
Yang, Y. et al. Operando studies reveal active Cu nanograins for CO2 electroreduction. Nature 614, 262–269 (2023).
Amirbeigiarab, R. et al. Atomic-scale surface restructuring of copper electrodes under CO2 electroreduction conditions. Nat. Catal. 6, 837–846 (2023).
Vavra, J. et al. Solution-based Cu+ transient species mediate the reconstruction of copper electrocatalysts for CO2 reduction. Nat. Catal. 7, 89–97 (2024).
Zhang, Q. et al. Atomic dynamics of electrified solid–liquid interfaces in liquid-cell TEM. Nature 630, 643–647 (2024).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Popovic, S., Bele, M. & Hodnik, N. Reconstruction of copper nanoparticles at electrochemical CO2 reduction reaction conditions occurs via two‐step dissolution/redeposition mechanism. ChemElectroChem 8, 2634–2639 (2021).
Kim, D., Kley, C. S., Li, Y. & Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl Acad. Sci. USA 114, 10560–10565 (2017).
Li, Y. et al. Electrochemically scrambled nanocrystals are catalytically active for CO2-to-multicarbons. Proc. Natl Acad. Sci. USA 117, 9194–9201 (2020).
Huang, J. et al. Potential-induced nanoclustering of metallic catalysts during electrochemical CO2 reduction. Nat. Commun. 9, 3117 (2018).
Grosse, P. et al. Dynamic transformation of cubic copper catalysts during CO2 electroreduction and its impact on catalytic selectivity. Nat. Commun. 12, 7329 (2021).
Li, Y. et al. Structure-sensitive CO2 electroreduction to hydrocarbons on ultrathin 5-fold twinned copper nanowires. Nano Lett. 17, 1312–1317 (2017).
Grosse, P. et al. Dynamic changes in the structure, chemical state and catalytic selectivity of Cu nanocubes during CO2 electroreduction: size and support effects. Angew. Chem. Int. Ed. 57, 6192–6197 (2018).
Choi, C. et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 3, 804–812 (2020).
Wang, X. et al. Morphology and mechanism of highly selective Cu(II) oxide nanosheet catalysts for carbon dioxide electroreduction. Nat. Commun. 12, 794 (2021).
Lei, Q. et al. Investigating the origin of enhanced C2+ selectivity in oxide-/hydroxide-derived copper electrodes during CO2 electroreduction. J. Am. Chem. Soc. 142, 4213–4222 (2020).
Raaijman, S. J., Arulmozhi, N. & Koper, M. T. M. Morphological stability of copper surfaces under reducing conditions. ACS Appl. Mater. Interfaces 13, 48730–48744 (2021).
Hochfilzer, D. et al. The importance of potential control for accurate studies of electrochemical CO reduction. ACS Energy Lett. 6, 1879–1885 (2021).
Vavra, J., Shen, T., Stoian, D., Tileli, V. & Buonsanti, R. Real‐time monitoring reveals dissolution/redeposition mechanism in copper nanocatalysts during the initial stages of the CO2 reduction reaction. Angew. Chem. Int. Ed. 133, 1367–1374 (2021).
Speck, F. D. & Cherevko, S. Electrochemical copper dissolution: a benchmark for stable CO2 reduction on copper electrocatalysts. Electrochem. Commun. 115, 106739 (2020).
Kim, Y.-G., Baricuatro, J. H., Javier, A., Gregoire, J. M. & Soriaga, M. P. The evolution of the polycrystalline copper surface, first to Cu(111) and then to Cu(100), at a fixed CO2RR potential: a study by operando EC-STM. Langmuir 30, 15053–15056 (2014).
Kim, Y. G. et al. Surface reconstruction of pure-Cu single-crystal electrodes under CO-reduction potentials in alkaline solutions: a study by seriatim ECSTM-DEMS. J. Electroanal. Chem. 780, 290–295 (2016).
Kim, Y. G., Baricuatro, J. H. & Soriaga, M. P. Surface reconstruction of polycrystalline Cu electrodes in aqueous KHCO3 electrolyte at potentials in the early stages of CO2 reduction. Electrocatalysis 9, 526–530 (2018).
Osowiecki, W. T. et al. Factors and dynamics of Cu nanocrystal reconstruction under CO2 reduction. ACS Appl. Energy Mater. 2, 7744–7749 (2019).
Wilde, P. et al. Is Cu instability during the CO2 reduction reaction governed by the applied potential or the local CO concentration? Chem. Sci. 12, 4028–4033 (2021).
Lee, S. H. et al. Oxidation state and surface reconstruction of Cu under CO2 reduction conditions from in situ X-ray characterization. J. Am. Chem. Soc. 143, 588–592 (2021).
Jung, H. et al. Electrochemical fragmentation of Cu2O nanoparticles enhancing selective C–C coupling from CO2 reduction reaction. J. Am. Chem. Soc. 141, 4624–4633 (2019).
Choi, W., Won, D. H. & Hwang, Y. J. Catalyst design strategies for stable electrochemical CO2 reduction reaction. J. Mater. Chem. A 8, 15341–15357 (2020).
Subramanian, S. et al. CO residence time modulates multi-carbon formation rates in a zero-gap Cu based CO2 electrolyzer. Energy Environ. Sci. 17, 6728–6738 (2024).
Gao, W., Xu, Y., Fu, L., Chang, X. & Xu, B. Experimental evidence of distinct sites for CO2-to-CO and CO conversion on Cu in the electrochemical CO2 reduction reaction. Nat. Catal. 6, 885–894 (2023).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Kim, J. Y. et al. Different distributions of multi-carbon products in CO2 and CO electroreduction under practical reaction conditions. Nat. Catal. 6, 1115–1124 (2023).
Varela, A. S., Ju, W., Reier, T. & Strasser, P. Tuning the catalytic activity and selectivity of Cu for CO2 electroreduction in the presence of halides. ACS Catal. 6, 2136–2144 (2016).
Li, Z., Wang, L., Sun, L. & Yang, W. Dynamic cation enrichment during pulsed CO2 electrolysis and the cation-promoted multicarbon formation. J. Am. Chem. Soc. 146, 23901–23908 (2024).
Wang, Y.-Q. et al. Alkali metal cations induce structural evolution on Au(111) during cathodic polarization. J. Am. Chem. Soc. 146, 27713–27724 (2024).
Liu, S. et al. Alkali cation-induced cathodic corrosion in Cu electrocatalysts. Nat. Commun. 15, 5080 (2024).
Martín, A. J., Mitchell, S., Mondelli, C., Jaydev, S. & Pérez-Ramírez, J. Unifying views on catalyst deactivation. Nat. Catal. 5, 854–866 (2022).
Otor, H. O., Steiner, J. B., García-Sancho, C. & Alba-Rubio, A. C. Encapsulation methods for control of catalyst deactivation: a review. ACS Catal. 10, 7630–7656 (2020).
Meier, J. C. et al. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 5, 44–67 (2014).
Okatenko, V. et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical CO2 reduction reaction. J. Am. Chem. Soc. 145, 5370–5383 (2023).
Lu, X. K., Lu, B., Li, H., Lim, K. & Seitz, L. C. Stabilization of undercoordinated Cu sites in strontium copper oxides for enhanced formation of C2+ products in electrochemical CO2 reduction. ACS Catal. 12, 6663–6671 (2022).
Zhang, L. et al. Oxophilicity-controlled CO2 electroreduction to C2+ alcohols over Lewis acid metal-doped Cuδ+ catalysts. J. Am. Chem. Soc. 145, 21945–21954 (2023).
Kim, J. Y. et al. Quasi-graphitic carbon shell-induced Cu confinement promotes electrocatalytic CO2 reduction toward C2+ products. Nat. Commun. 12, 3765 (2021).
Yoo, J. M., Shin, H., Chung, D. Y. & Sung, Y. E. Carbon shell on active nanocatalyst for stable electrocatalysis. Acc. Chem. Res. 55, 1278–1289 (2022).
Gao, D. et al. Enhancing CO2 electroreduction with the metal-oxide interface. J. Am. Chem. Soc. 139, 5652–5655 (2017).
Zhang, X. Y. et al. Selective methane electrosynthesis enabled by a hydrophobic carbon coated copper core-shell architecture. Energy Environ. Sci. 15, 234–243 (2022).
Das, S. et al. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 49, 2937–3004 (2020).
Esposito, D. V., Guilimondi, V., Vos, J. G. & Koper, M. T. M. in RSC Energy and Environment Series Ch. 7 (Royal Society of Chemistry, 2022).
Li, L., Shao, Q. & Huang, X. Amorphous oxide nanostructures for advanced electrocatalysis. Chem. Eur. J. 26, 3943–3960 (2020).
Pacchioni, G. & Freund, H.-J. Controlling the charge state of supported nanoparticles in catalysis: lessons from model systems. Chem. Soc. Rev. 47, 8474–8502 (2018).
Xu, A. et al. Copper/alkaline earth metal oxide interfaces for electrochemical CO2-to-alcohol conversion by selective hydrogenation. Nat. Catal. 5, 1081–1088 (2022).
Varandili, S. B. et al. Synthesis of Cu/CeO2-x nanocrystalline heterodimers with interfacial active sites to promote CO2 electroreduction. ACS Catal. 9, 5035–5046 (2019).
Lee, C. W. et al. Metal-oxide interfaces for selective electrochemical C-C coupling reactions. ACS Energy Lett. 4, 2241–2248 (2019).
Wang, Y. et al. Single-atomic Cu with multiple oxygen vacancies on ceria for electrocatalytic CO2 reduction to CH4. ACS Catal. 8, 7113–7119 (2018).
Hemmingson, S. L. & Campbell, C. T. Trends in adhesion energies of metal nanoparticles on oxide surfaces: understanding support effects in catalysis and nanotechnology. ACS Nano 11, 1196–1203 (2017).
Campbell, C. T. & Mao, Z. Chemical potential of metal atoms in supported nanoparticles: dependence upon particle size and support. ACS Catal. 7, 8460–8466 (2017).
Albertini, P. P. et al. Hybrid oxide coatings generate stable Cu catalysts for CO2 electroreduction. Nat. Mater. 23, 680–687 (2024).
Chen, H. Q. et al. Origin of metal-support interactions for selective electrochemical CO2 reduction into C1 and C2+ products. ACS Catal. 14, 11794–11802 (2024).
Li, H. et al. Facet-selective deposition of ultrathin Al2O3 on copper nanocrystals for highly stable CO2 electroreduction to ethylene. Angew. Chem. Int. Ed. 60, 24838–24843 (2021).
Zhao, T. et al. Tailoring the catalytic microenvironment of Cu2O with SiO2 to enhance C2+ product selectivity in CO2 electroreduction. ACS Catal. 13, 4444–4453 (2023).
Monai, M. et al. Restructuring of titanium oxide overlayers over nickel nanoparticles during catalysis. Science 380, 644–651 (2023).
Paolucci, C. et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 357, 898–903 (2017).
Jung, S., Pizzolitto, C., Biasi, P., Dauenhauer, P. J. & Birol, T. Programmable catalysis by support polarization: elucidating and breaking scaling relations. Nat. Commun. 14, 7795 (2023).
He, J. et al. Stabilizing copper for CO2 reduction in low-grade electrolyte. Inorg. Chem. 57, 14624–14631 (2018).
Han, Z. et al. Steering surface reconstruction of copper with electrolyte additives for CO2 electroreduction. Nat. Commun. 13, 3158 (2022).
Kim, D. et al. Selective CO2 electrocatalysis at the pseudocapacitive nanoparticle/ordered-ligand interlayer. Nat. Energy 5, 1032–1042 (2020).
Yu, S. et al. Nanoparticle assembly induced ligand interactions for enhanced electrocatalytic CO2 conversion. J. Am. Chem. Soc. 143, 19919–19927 (2021).
Parada, W. A., Sajevic, U., Mammadzada, R., Nikolaienko, P. & Mayrhofer, K. J. J. Tethered alkylammonium dications as electrochemical interface modifiers: chain length effect on CO2 reduction selectivity at industry-relevant current density. ACS Appl. Mater. Interfaces 16, 30107–30116 (2024).
Tao, Z., Wu, Z., Wu, Y. & Wang, H. Activating copper for electrocatalytic CO2 reduction to formate via molecular interactions. ACS Catal. 10, 9271–9275 (2020).
Zhang, H. et al. Creating polycrystalline Cu catalysts via capping agents and electrochemical treatment for CO2 reduction to C2H4. Energy Fuels 37, 529–538 (2023).
Dong, L. et al. Surfactant-modified electrode–electrolyte interface for steering CO2 electrolysis on Cu electrodes. AIChE J. 70, e18271 (2024).
Noh, H., Park, Y., Bhadouria, A. & Tackett, B. M. Effects of electrochemical active surface area of Cu on electrochemical CO2 reduction in acidic electrolyte using Cu nanoparticles on surfactant-treated carbon. J. Catal. 437, 115662 (2024).
Banerjee, S., Han, X. & Thoi, V. S. Modulating the electrode-electrolyte interface with cationic surfactants in carbon dioxide reduction. ACS Catal. 9, 5631–5637 (2019).
Pankhurst, J. R., Iyengar, P., Loiudice, A., Mensi, M. & Buonsanti, R. Metal-ligand bond strength determines the fate of organic ligands on the catalyst surface during the electrochemical CO2 reduction reaction. Chem. Sci. 11, 9296–9302 (2020).
Xu, L. & Xu, S. Investigation of pyridine as a cocatalyst for the CO2 reduction reaction on the Cu2O cathode surface. ACS Catal. 14, 9554–9564 (2024).
Lee, S. A., Yang, J. W., Choi, S. & Jang, H. W. Nanoscale electrodeposition: dimension control and 3D conformality. Exploration 1, 20210012 (2021).
Kok, J., de Ruiter, J., van der Stam, W. & Burdyny, T. Interrogation of oxidative pulsed methods for the stabilization of copper electrodes for CO2 electrolysis. J. Am. Chem. Soc. 146, 19509–19520 (2024).
Iglesias et al. Non-invasive current collectors for improved current-density distribution during CO2 electrolysis on super-hydrophobic electrodes. Nat. Commun. 14, 6579 (2023).
Filippi, M. et al. Scale-up of PTFE-based gas diffusion electrodes using an electrolyte-integrated polymer-coated current collector approach. ACS Energy Lett. 9, 1361–1368 (2024).
Yang, K., Kas, R., Smith, W. A. & Burdyny, T. Role of the carbon-based gas diffusion layer on flooding in a gas diffusion electrode cell for electrochemical CO2 reduction. ACS Energy Lett. 6, 33–40 (2021).
Zhang, Y.-J., Sethuraman, V., Michalsky, R. & Peterson, A. A. Competition between CO2 reduction and H2 evolution on transition-metal electrocatalysts. ACS Catal. 4, 3742–3748 (2014).
Niu, Z.-Z. et al. Hierarchical copper with inherent hydrophobicity mitigates electrode flooding for high-rate CO2 electroreduction to multicarbon products. J. Am. Chem. Soc. 143, 8011–8021 (2021).
Sahin, B. et al. Fine-tuned combination of cell and electrode designs unlocks month-long stable low temperature Cu-based CO2 electrolysis. J. CO2 Util. 82, 102766 (2024).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Obasanjo, C. A. et al. In situ regeneration of copper catalysts for long-term electrochemical CO2 reduction to multiple carbon products. J. Mater. Chem. A 10, 20059–20070 (2022).
Gabardo, C. M. et al. Continuous carbon dioxide electroreduction to concentrated multi-carbon products using a membrane electrode assembly. Joule 3, 2777–2791 (2019).
Tan, Y. C., Lee, K. B., Song, H. & Oh, J. Modulating local CO2 concentration as a general strategy for enhancing C−C coupling in CO2 electroreduction. Joule 4, 1104–1120 (2020).
García De Arquer, F. P. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Wang, Y. et al. Catalyst synthesis under CO2 electroreduction favours faceting and promotes renewable fuels electrosynthesis. Nat. Catal. 3, 98–106 (2019).
Ma, S. et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 301, 219–228 (2016).
Cao, Y. et al. Surface hydroxide promotes CO2 electrolysis to ethylene in acidic conditions. Nat. Commun. 14, 2387 (2023).
Papangelakis, P. et al. Carbon‐efficient CO2 electrolysis to ethylene with nanoporous hydrophobic copper. Adv. Energy Mater. 14, 2400763 (2024).
Sun, M., Cheng, J. & Yamauchi, M. Gas diffusion enhanced electrode with ultrathin superhydrophobic macropore structure for acidic CO2 electroreduction. Nat. Commun. 15, 491 (2024).
Ma, Z. et al. CO2 electroreduction to multicarbon products in strongly acidic electrolyte via synergistically modulating the local microenvironment. Nat. Commun. 13, 7596 (2022).
Zhang, G. et al. Efficient CO2 electroreduction on facet-selective copper films with high conversion rate. Nat. Commun. 12, 5745 (2021).
Liu, W. et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 13, 1877 (2022).
Lv, J. et al. A highly porous copper electrocatalyst for carbon dioxide reduction. Adv. Mater. 30, 1803111 (2018).
Han, J. et al. Structuring Cu membrane electrode for maximizing ethylene yield from CO2 electroreduction. Adv. Mater. 36, 2313926 (2024).
She, X. et al. Pure-water-fed, electrocatalytic CO2 reduction to ethylene beyond 1,000 h stability at 10 A. Nat. Energy 9, 81–91 (2024).
Rufer, S., Nitzsche, M. P., Garimella, S., Lake, J. R. & Varanasi, K. K. Hierarchically conductive electrodes unlock stable and scalable CO2 electrolysis. Nat. Commun. 15, 9429 (2024).
Wang, H. et al. Synergistic enhancement of electrocatalytic CO2 reduction to C2 oxygenates at nitrogen-doped nanodiamonds/Cu interface. Nat. Nanotechnol. 15, 131–137 (2020).
Yao, K. et al. In situ copper faceting enables efficient CO2/CO electrolysis. Nat. Commun. 15, 1749 (2024).
Wang, J. et al. Fastening Br− ions at copper–molecule interface enables highly efficient electroreduction of CO2 to ethanol. ACS Energy Lett. 6, 437–444 (2021).
Ma, W. et al. Electrocatalytic reduction of CO2 to ethylene and ethanol through hydrogen-assisted C–C coupling over fluorine-modified copper. Nat. Catal. 3, 478–487 (2020).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
Abdinejad, M. et al. Eliminating redox-mediated electron transfer mechanisms on a supported molecular catalyst enables CO2 conversion to ethanol. Nat. Catal. 7, 1109–1119 (2024).
Rabinowitz, J. A., Ripatti, D. S., Mariano, R. G. & Kanan, M. W. Improving the energy efficiency of CO electrolysis by controlling Cu domain size in gas diffusion electrodes. ACS Energy Lett. 7, 4098–4105 (2022).
Patwardhan, A. V. & Sharma, M. M. Kinetics of absorption of carbon monoxide in aqueous solutions of sodium hydroxide and aqueous calcium hydroxide slurries. Ind. Eng. Chem. Res. 28, 5–9 (1989).
Xu, Q. et al. Identifying and alleviating the durability challenges in membrane-electrode-assembly devices for high-rate CO electrolysis. Nat. Catal. 6, 1042–1051 (2023).
Wang, L. et al. Electrochemically converting carbon monoxide to liquid fuels by directing selectivity with electrode surface area. Nat. Catal. 2, 702–708 (2019).
Schellekens, M. P., Raaijman, S. J., T. M. Koper, M. & Corbett, P. J. Temperature-dependent selectivity for CO electroreduction on copper-based gas-diffusion electrodes at high current densities. Chem. Eng. J. 483, 149105 (2024).
Nguyen, T. N. et al. Catalyst regeneration via chemical oxidation enables long-term electrochemical carbon dioxide reduction. J. Am. Chem. Soc. 144, 13254–13265 (2022).
Blake, J. W., Padding, J. T. & Haverkort, J. W. Analytical modelling of CO2 reduction in gas-diffusion electrode catalyst layers. Electrochim. Acta 393, 138987 (2021).
Johnson, E. F., Boutin, E., Liu, S. & Haussener, S. Pathways to enhance electrochemical CO2 reduction identified through direct pore-level modeling. EES Catal. 1, 704–719 (2023).
Ding, P. et al. Elucidating the roles of Nafion/solvent formulations in copper-catalyzed CO2 electrolysis. ACS Catal. 13, 5336–5347 (2023).
Jännsch, Y. et al. Pulsed potential electrochemical CO2 reduction for enhanced stability and catalyst reactivation of copper electrodes. Electrochem. Commun. 121, 106861 (2020).
Speck, F. D., Zagalskaya, A., Alexandrov, V. & Cherevko, S. Periodicity in the electrochemical dissolution of transition metals. Angew. Chem. Int. Ed. 60, 13343–13349 (2021).
Cherevko, S. Electrochemical dissolution of noble metals native oxides. J. Electroanal. Chem. 787, 11–13 (2017).
Lian, Z., Dattila, F. & López, N. Stability and lifetime of diffusion-trapped oxygen in oxide-derived copper CO2 reduction electrocatalysts. Nat. Catal. 7, 401–411 (2024).
Burdyny, T. Using pseudo-steady-state operation to redefine stability in CO2 electrolysis. Nat. Chem. Eng. https://doi.org/10.1038/s44286-025-00210-0 (2025).
Sassenburg, M., Kelly, M., Subramanian, S., Smith, W. A. & Burdyny, T. Zero-gap electrochemical CO2 reduction cells: challenges and operational strategies for prevention of salt precipitation. ACS Energy Lett. 8, 321–331 (2023).
Garg, S. et al. How alkali cations affect salt precipitation and CO2 electrolysis performance in membrane electrode assembly electrolyzers. Energy Environ. Sci. 16, 1631–1643 (2023).
Cofell, E. R., Nwabara, U. O., Bhargava, S. S., Henckel, D. E. & Kenis, P. J. A. Investigation of electrolyte-dependent carbonate formation on gas diffusion electrodes for CO2 electrolysis. ACS Appl. Mater. Interfaces 13, 15132–15142 (2021).
Kong, X. et al. Understanding the effect of *CO coverage on C–C coupling toward CO2 electroreduction. Nano Lett. 22, 3801–3808 (2022).
Luo, Y. et al. Cobalt phthalocyanine promoted copper catalysts toward enhanced electro reduction of CO2 to C2: synergistic catalysis or tandem catalysis? J. Energy Chem. 92, 499–507 (2024).
Acknowledgements
J.K. and T.B. acknowledge the Dutch Research Council (NWO) for providing the FlexEChem Grant (NWA.1237.18.002) via the NWA-themed call ‘Opslag en conversie’. P.P.A. acknowledges the NCCR Catalysis, a National Centre of Competence in Research programme funded by the Swiss National Science Foundation (grant number 180544). J.L. acknowledges the Swiss National Science Foundation for the financial support from grant number 200021_219715/1.
Author information
Authors and Affiliations
Contributions
T.B. and R.B. conceptualized the article. J.K., P.P.A., J.L., R.B. and T.B. researched data for the article. All authors wrote the initial draft and contributed to the discussions of its content. The manuscript was revised in a collaborative manner.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Materials thanks Feng Jiao, Ruquan Ye and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kok, J., Albertini, P.P., Leemans, J. et al. Overcoming copper stability challenges in CO2 electrolysis. Nat Rev Mater 10, 550–563 (2025). https://doi.org/10.1038/s41578-025-00815-0
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41578-025-00815-0
This article is cited by
-
Scaling electrocatalysts for reduction of CO2 or CO to multicarbon products
Nature Reviews Materials (2026)
-
Recovering lost performance
Nature Energy (2025)